
Shifting paradigms in the treatment of small renal masses
Introduction
According to the World Health Organization’s GLOBOCAN report, kidney cancer accounted for an estimated 435,000 new cases and 156,000 cancer-specific deaths globally in 2022. The highest age-standardized incidence rates are observed in developed countries, likely reflecting greater exposure to known risk factors and the increased utilization of diagnostic imaging, which has led to the incidental detection of many cases (1). Kidney cancer incidence increases steadily with age, although the median age at diagnosis varies by region (1).
Small renal masses (SRMs), defined as tumors ≤4 cm (T1a), account for approximately 48–66% of newly diagnosed renal tumors (2). Surgical resection, preferably in a nephron-sparing fashion when feasible, has been considered the gold standard treatment. As the field evolves, alternative approaches are redefining the balance between oncological control, patient safety, and overtreatment minimization, particularly given that over 90% of SRMs are low-grade, with a substantial proportion being non-malignant (3). While partial nephrectomy (PN) remains the gold standard, boasting excellent 5-year and 10-year cancer-specific mortality rates of 1–3% and less than 10%, respectively (3), along with definitive pathological diagnosis, its invasiveness, complications, potential impact on kidney function, and a notable rate of benign histology, reported in up to 27% of cases (4), particularly in smaller tumors (5), have spurred the pursuit of non-surgical options.
Most guidelines still include non-surgical alternatives for patients with advanced age, significant comorbidities, or reduced renal function. However, considering the generally favorable tumor biology of SRMs, these non-surgical treatments not only maintain safety but also open the door for non-urologist stakeholders to effectively manage a significant portion of these patients.
This manuscript examines the latest evidence on non-surgical alternatives for SRMs, with a particular focus on how tumor size influences decision-making.
Current evidence on surgical alternatives
Active surveillance (AS)
AS for SRMs involves monitoring tumors while deferring treatment, preserving the option for cure when necessary, and avoiding overtreatment through predefined intervention triggers. Notably, these triggers have not yet been precisely defined by level 1 evidence. AS is considered oncologically safe for SRMs, as approximately 40% are benign, growth rates are low (0.10–0.27 cm/year), and less than 1% of malignant cases metastasize (6). Additionally, although delayed intervention (DI) rates range from 7% to 44%, most salvage interventions will still be nephron-sparing (6). Long-term follow-up (21.7–57.6 months) has shown low metastasis rates (0–3.1%) and cancer-specific mortality of 0–4% (6).
Due to the limited and predominantly retrospective evidence supporting AS, the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) Registry was established in 2009 (7). This multi-center, prospective cohort study aims to evaluate the outcomes of patients managed with AS compared to those undergoing primary intervention (PI) for newly diagnosed SRMs. Initially developed at Johns Hopkins University (Baltimore, MD), the study expanded to include Columbia University Medical Center (New York, NY) and Beth Israel Deaconess Medical Center (Boston, MA), and it is now recognized as the largest prospective program of its kind worldwide (7). Intervention triggers include tumor growth >0.5 cm/year, maximum diameter >4 cm, or metastatic progression. The DISSRM study’s latest data, with 15 years of follow-up, provide comprehensive evidence for the safety of AS. After a median follow-up of 4.73 years, the crossover rate to intervention was 15.15%, and cancer-specific survival (CSS) at 12 years was comparable between AS and PI (99.3% vs. 99.8%, P=0.43). However, overall survival (OS) was higher with PI at 3, 6, 9, and 12 years (7), probably due to selection bias.
A decision-analytic Markov model by Su et al. [2022] found that AS with timely DI is the most cost-effective strategy for 65-year-old patients with incidental SRMs (8). This is supported by a 10-year all-cause mortality of 22–24% in both active modalities and AS, alongside the very low annual metastatic incidence of 0.35%. Arguably, definitive treatment might be more suitable for younger patients due to the need for prolonged follow-up compared to a single intervention, despite the risk of overtreatment.
To assess the feasibility of AS in younger patients, Metcalf et al. [2021] conducted a subgroup analysis of individuals under 60 years old (n=224) from the DISSRM trial (9). Among those with an initial tumor size ≤2 cm, 15.1% transitioned to intervention, compared to 33.3% with tumors measuring 2–4 cm. At 7 years, OS was comparable between PI and AS (94.0% vs. 90.8%, P=0.2), with CSS at 100% for both groups. There were no significant differences in the use of minimally invasive or nephron-sparing procedures between primary and DIs. Additionally, 5-year recurrence-free survival rates were 96.0% for PI and 100% for DI (P=0.6) (9).
How does tumor size matter?
Tumor size is a crucial predictor of malignancy in AS, with malignancy rates increasing from 53.8% for masses under 1 cm to 80.1% for those between 3 and 4 cm (10). Additionally, metastatic risk, virtually 0% for tumors smaller than 1 cm, escalates with increasing mass diameter, with a hazard ratio (HR) of approximately 1.24 for each additional centimeter (11). Pallauf et al. [2025] conducted a secondary analysis of the DISSRM database to assess progression risks for SRMs based on initial and surveillance tumor sizes, using criteria of tumor growth >0.5 cm or transitioning to DI (12). A clear correlation was observed between tumor size at diagnosis and the risk of tumor growth and DI. Specifically, for tumor growth, HRs were 1.74 for tumors ≥2 cm and 2.4 for those ≥2.9 cm. For DI, the HRs were 2.4 and 3.41, respectively.
HRs were even more pronounced when considering tumor size, while patients were already under surveillance. When accounting for changes in tumor size and SRM category during AS, after more than five years of AS, the risk of DI for SRMs ≥2 cm was 12.8 times greater [HR: 12.8, 95% confidence interval (CI): 5.6–29; P<0.001] than for SRMs <2 cm. For SRMs ≥2.9 cm, the risk of DI was 13.5 times greater (HR: 13.5, 95% CI: 7.39–24.5; P<0.001) than for SRMs <2.9 cm.
Overall, data from the long-term DISSRM follow-up by tumor size shows that after a median follow-up of 4.73 years, the likelihood of transitioning to intervention correlated with initial tumor size: <2 cm (10.28%), 2 to <3 cm (16.25%), and ≥3 cm (25.76%) (12).
Driven by the low malignancy potential and the ability to safely salvage growing tumors with nephron-sparing surgery, National Comprehensive Cancer Network (NCCN) (13) and American Urological Association (AUA) (3) guidelines recommend AS for T1a tumors, particularly those ≤2 cm or predominantly cystic tumors ≤4 cm, as well as for patients with significant comorbidities. Notably, SRMs ≤2 cm represent the first quartile in the National Cancer Database, indicating AS may be appropriate for at least 25% of newly diagnosed patients (14).
As more data become available on the safety of the AS approach, specific groups of patients, such as those with long-term stable tumor size or smaller lesions, could potentially be followed up with negligible risk by primary care providers (PCPs). A more protocolized approach could facilitate this transition.
Here, it is important to distinguish that in the case of elderly or frail patients with limited life expectancy and incidental, asymptomatic SRMs, renal cell carcinoma (RCC)-specific mortality remains low regardless of management strategy, supporting the Watchful Waiting approach where symptomatic progression due to advanced disease becomes the trigger for intervention. A summary of potential risks and treatment recommendations based on tumor diameter is presented in Table 1.
Table 1
| Parameter | <1 cm | 1–2 cm | 2–3 cm | 3–4 cm |
|---|---|---|---|---|
| Malignancy risk (13) | 62.5% | 81% | 17% | 13% |
| High grade pathology (13) | 0% | 16% | 17% | 73% |
| Metastatic potential (9) | 0% | 0% | 0.2% | 1.8% |
| Guidelines-based approach (11)e | ||||
| Surgerya | + | + | ++ | ++ |
| Active surveillanceb | ++ | ++ | + | + |
| Thermal ablationc | ++ | ++ | ++ | + |
| SBRTd | + | + | + | + |
+, ++. a, the gold standard for lesions <2 cm and may be preferred for younger patients with anticipated long follow-up and low risk of procedural morbidity; b, suitable for <2 cm cystic renal masses and comorbid patients with SRMs or cystic masses; c, thermal ablation (e.g., cryosurgery, radiofrequency ablation, microwave ablation) is an option for managing clinical stage T1 renal lesions and is suitable for renal masses ≤3 cm. Mostly in comorbid/poor surgical candidates; d, SBRT may be considered for non-optimal surgical candidates with stage I kidney cancer (category 2B); e, based on the 3.2025 NCCN guidelines. NCCN, National Comprehensive Cancer Network; SBRT, stereotactic body radiation therapy; SRMs, small renal masses.
Thermal ablative (TA) techniques
TA serves as an effective alternative to surgical tumor removal by destroying tumor cells using either heat [radiofrequency ablation (RFA), microwave ablation] or freezing energy (cryoablation). Since its introduction by Rossi et al. [1996], TA has been utilized for over two decades, consistently demonstrating excellent oncological outcomes for SRMs. Current evidence on cT1a renal tumors grossly shows comparable complication rates, oncological efficacy, and renal functional outcomes across all modalities (15). Consequently, the choice of technique primarily depends on equipment availability and the team’s expertise, with minor adjustments on a case-by-case basis. For instance, cryoablation is often favored for centrally located tumors or those adjacent to the ureter due to slightly better margin control, while RFA may be limited by the heat-sink effect (reducing efficacy near large blood vessels) (16). However, RFA is associated with a lower risk of hemorrhage due to renal fracture, a complication unique to cryoablation (16), although this consideration is beyond the scope of this manuscript.
Treatment efficacy
One of the longest follow-up studies, conducted by the MD Anderson Cancer Center group, involved 243 SRMs with a median tumor size of 2.5 cm (68.6% RCC) (17). They reported 15-year local recurrence-free survival and disease-free survival (DFS) rates of 96.5% and 88.6%, respectively; local recurrence in 3.1% of cases within the ablation zone and 4.5% elsewhere in the kidney. They report 15-year metastasis-free survival and CSS rates of 100%. Another long-term single-center group reported 10-year disease-free, cancer-specific, and OS rates of 82%, 94%, and 49%, respectively (18).
A large systematic review by Chan et al. [2022], including approximately 75,000 patients, compared TA with PN for SRMs (19). The key findings included comparable local recurrence-free survival to PN for patients with over five years of follow-up (HR: 1.54, 95% CI: 0.88–2.71), similar CSS, metastasis-free survival, and DFS, but worse OS for TA patients (HR: 1.64, 95% CI 1.39–1.95), likely due to selection bias, as TA is often used for frail or patients with many comorbidities that would render them inappropriate for surgery.
The European Association of Urology (EAU) guidelines panel conducted a systematic review of comparative studies [including >50 patients from 2000–2019 (20), totaling 16,780 patients] on TA vs. PN for SRMs, mainly concluding that long-term oncological effectiveness compared to PN remains unclear due to retrospective observational data and high bias.
Procedural success rates and perioperative complications
TA has a high success rate (>95%) and relatively low high-grade complication rates (17-19). A review of complications from 573 renal ablation procedures at a single institution showed an overall complication rate of 11.3% and a major complication (defined as Clavien-Dindo II–IV) rate of 6.6% (21). The most common complications associated with cryoablation were bleeding and hematuria, which were linked to advanced age, larger tumor size, a greater number of cryoprobes, and a central tumor position. Among patients treated with RF ablation, nerve and ureteral injuries were the most frequent complications (21). Here as well, high-quality data comparing complication rates between TA and PN are lacking, and systematic reviews have grossly shown similar major complication risks. Overall, in TA as well, proximity to the collecting system, ureteral, and bowel structures might pose challenges. The heat sink effect is special in RFA, where proximity to vessels might make treatment less effective. In contrast, hemorrhage due to renal fracture is a complication unique to cryoablation and is seen with breakage at the junction of the ice ball formation.
How does tumor size matter?
Tumor size is a crucial predictor of the efficacy of TA, as it influences the likelihood of incomplete ablation and local recurrence. In the case of cryoablation, Tanagho et al. [2012] identified a tumor size greater than 2.5 cm as a significant predictor of local recurrence in a multivariate analysis (22). A recent series of SRMs treated with percutaneous cryoablation reported a 7.7% rate of local recurrence; disease progression increased by 32% for each 1-cm increase in tumor size (HR: 1.32, P<0.001). In RFA, Gervais et al. found that RFA was 100% effective for tumors smaller than 3 cm, but only 81% effective for tumors larger than 3 cm (23). Best et al. reported a 5-year DFS rate of 95% for tumors less than 3 cm, compared to 79% for larger tumors (24). Others demonstrated a 10-year DFS rate dropping to 68% when tumors >3 cm were ablated by RFA (18). Finally, complication rates were linked to larger tumor size (25). These findings have influenced the EAU guidelines, which recommend RFA for tumors less than 3 cm and cryoablation for tumors less than 4 cm (26). In contrast, the AUA and NCCN guidelines do not provide a specific size threshold but suggest that tumors ≤3 cm are suitable for thermal ablation (3,27).
Stereotactic body radiotherapy (SBRT)
RCC was historically considered radioresistant to conventional fractionation (<5 Gy per fraction) (28). However, data on the use of SBRT have been accumulating in recent years, with several rigorously conducted prospective clinical trials potentially making renal mass treatment entirely non-invasive.
A recent systematic review by Siva et al. [2024] encompassing 822 patients from 36 studies found a median local control rate of 94.1% (range, 70–100%) with 5-year progression-free survival of 80.5% (95% CI: 72–92%)—although reported by the minority of the studies (29). The 5-year OS was found to be 77.2% (95% CI: 65–89%). SBRT is generally safe, with a systematic review and meta-analysis of 26 studies (involving 372 patients and 383 primary tumors) reporting a grade 3–4 toxicity rate of 1.5%. The decline in estimated post-treatment was modest, with a mean decrease of –7.7 mL/min. Further, the authors pointed out that in SBRT, biopsy does not change patient outcomes and might not be required (29). In comparison to other ablation modalities, data on SBRT are considered less robust, and the NCCN guidelines suggest SBRT for non-optimal surgical candidates with stage I kidney cancer with category 2B level of evidence (13).
Multidisciplinary leadership in SRM management
SRMs are primarily managed by urologists, but emerging alternatives supported by high-quality data are expanding treatment options, potentially reshaping the future role of urologists in SRM management. Tan et al. [2020] analyzed renal TA trends among American urologists using the American Board of Urology case logs from 2013 to 2018 (30). Out of 54,075 renal procedures performed by 6,200 urologists, only 3.5% were thermal ablations and 1.2% were renal biopsies, with a declining trend largely due to decreased use of laparoscopic techniques.
However, urologists are expected to play a pivotal role in histotripsy, an innovative non-invasive treatment currently under clinical trials. Their expertise in ultrasound-based minimally and non-invasive procedures, as seen in localized prostate disease and extracorporeal shockwave lithotripsy, positions them well to lead in this emerging field. Histotripsy is a non-invasive ultrasound therapy that uses focused sound waves to create microscopic bubbles in the targeted tissue. These bubbles rapidly expand and collapse, generating mechanical forces that break down the tissue without heat. This process, known as cavitation, precisely destroys tumors while preserving surrounding structures. Currently, in early-phase trials for SRMs, histotripsy has shown efficacy in in vivo kidney studies and clinical experience in the treatment of liver masses (31). Two clinical trials are investigating its safety and effectiveness: HOPE4KIDNEY (NCT05820087) and Investigational System (NCT05432232), both sponsored by HistoSonics Inc. (32). Expected to be performed by urologists, histotripsy avoids heat damage, is unaffected by blood flow, and selectively spares critical structures like the collecting system and blood vessels.
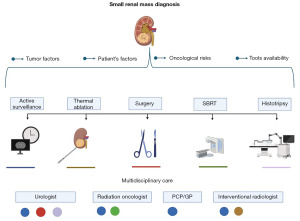
On the other end of the spectrum, there is limited data on AS of SRMs managed by general practitioners (GPs) or family doctors. Chang et al. [2025] highlighted a Genitourinary Fellowship for Family Medicine Physicians, where trainees gained competencies in procedures like stent insertion and on-call management (33). As kidney cancer incidence is projected to rise by approximately 60% by 2045, it is reasonable to anticipate broader involvement of GPs in SRM management, including AS—particularly for more stable, smaller tumors, watchful waiting, and shared decision-making processes. A visual representation of key factors to consider in the management of SRMs, including treatment options and the importance of multidisciplinary care, is provided in Figure 1.
Finally, as the field is moving toward non-invasiveness, advancements in diagnosis and prediction are expected to be supported by advanced biomarkers for better treatment selection. RCC is known to shed relatively little circulating tumor DNA (ctDNA) into the bloodstream compared to other cancer types, such as urothelial and prostate cancer. This is likely due to lower baseline cell turnover, differences in the tumor microenvironment, and less direct interaction with circulating blood (34).
Kidney injury molecule-1 (KIM-1) has long been known to be more sensitive in this context (35). A recent study by Xu et al. [2024] (35) assessed the relationship between pKIM-1, surgical pathology, and clinical outcomes, demonstrating its added value to current clinical nomograms. Specifically, pKIM-1 is associated with malignant pathology, worse metastasis-free survival, and increased risk of death. The evolving landscape of SRM management, driven by emerging treatments and the involvement of various stakeholders, underscores the need for collaborative, patient-centered approaches to optimize outcomes.
Conclusions
SRM management is evolving with an expanding array of tailored treatment options, demonstrating feasibility, effectiveness, and safety with appropriate patient selection. A diverse range of stakeholders is driving a patient-specific approach to minimize invasiveness and reduce overtreatment. As non-invasive technologies emerge, continued urologist engagement is crucial. However, improved selection methods are needed, as biomarkers for kidney tumors remain in their infancy.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-213/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: casting doubt on the efficacy of early intervention. Urology 2001;57:1013-5. [Crossref] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, Todd Bishoff J, Bass EB, Cadeddu JA, et al. AUA Renal mass 2021. 2021;(January 1999):1–63.
- Baio R, Molisso G, Caruana C, et al. "To Be or Not to Be Benign" at Partial Nephrectomy for Presumed RCC Renal Masses: Single-Center Experience with 195 Consecutive Patients. Diseases 2023;11:27. [Crossref] [PubMed]
- Baio R, Molisso G, Caruana C, et al. "Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?": A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy. Bioengineering (Basel) 2023;10:794. [Crossref] [PubMed]
- Campi R, Sessa F, Corti F, et al. Triggers for delayed intervention in patients with small renal masses undergoing active surveillance: a systematic review. Minerva Urol Nefrol 2020;72:389-407. [Crossref] [PubMed]
- Alkatib KY, Leff MA, Guzzo TJ, et al. Active surveillance versus primary intervention for clinical T1a kidney tumors: contemporary results after fourteen-year experience of the DISSRM prospective comparative study. Urologic Oncology: Seminars and Original Investigations 2024;42:S72-3.
- Su ZT, Patel HD, Huang MM, et al. Active Surveillance versus Immediate Intervention for Small Renal Masses: A Cost-Effectiveness and Clinical Decision Analysis. J Urol 2022;208:794-803. [Crossref] [PubMed]
- Metcalf MR, Cheaib JG, Biles MJ, et al. Outcomes of Active Surveillance for Young Patients with Small Renal Masses: Prospective Data from the DISSRM Registry. J Urol 2021;205:1286-93. [Crossref] [PubMed]
- Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003;170:2217-20. [Crossref] [PubMed]
- Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol 2009;182:41-5. [Crossref] [PubMed]
- Pallauf M, Rezaee M, Elias R, et al. Tumour size is associated with growth rates of >0.5 cm/year and delayed intervention in small renal masses in patients on active surveillance. BJU Int 2025;135:860-8. [Crossref] [PubMed]
- Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw 2009;7:618-30. [Crossref] [PubMed]
- Carbunaru S, Rich JM, Neshatvar Y, et al. Differences in the treatment patterns of small renal masses: A disaggregated analyses by race/ethnicity. Urol Oncol 2024;42:453.e1-8. [Crossref] [PubMed]
- Huang RS, Chow R, Benour A, et al. Comparative efficacy and safety of ablative therapies in the management of primary localised renal cell carcinoma: a systematic review and meta-analysis. Lancet Oncol 2025;26:387-98. [Crossref] [PubMed]
- Ramanathan R, Leveillee RJ. Ablative therapies for renal tumors. Ther Adv Urol 2010;2:51-68. [Crossref] [PubMed]
- Abdelsalam ME, Awad A, Baiomy A, et al. Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience. Cancers (Basel) 2023;15:909. [Crossref] [PubMed]
- Johnson BA, Sorokin I, Cadeddu JA. Ten-Year Outcomes of Renal Tumor Radio Frequency Ablation. J Urol 2019;201:251-8. [Crossref] [PubMed]
- Chan VW, Abul A, Osman FH, et al. Ablative therapies versus partial nephrectomy for small renal masses - A systematic review and meta-analysis. Int J Surg 2022;97:106194. [Crossref] [PubMed]
- Abu-Ghanem Y, Fernández-Pello S, Bex A, et al. Limitations of Available Studies Prevent Reliable Comparison Between Tumour Ablation and Partial Nephrectomy for Patients with Localised Renal Masses: A Systematic Review from the European Association of Urology Renal Cell Cancer Guideline Panel. Eur Urol Oncol 2020;3:433-52. [Crossref] [PubMed]
- Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012;23:48-54. [Crossref] [PubMed]
- Tanagho YS, Roytman TM, Bhayani SB, et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology 2012;80:307-14. [Crossref] [PubMed]
- Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 2005;185:64-71. [Crossref] [PubMed]
- Best SL, Park SK, Youssef RF, et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol 2012;187:1183-9. [Crossref] [PubMed]
- Salagierski M, Wojciechowska A, Zając K, et al. The Role of Ablation and Minimally Invasive Techniques in the Management of Small Renal Masses. Eur Urol Oncol 2018;1:395-402. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Bedke J, et al. EAU Guidelines on Renal Cell Carcinoma. European Association of Urology; 2023:1-100.
- Clinical N, Guidelines P, Guidelines N. Kidney Cancer. 2025;
- Ali M, Mooi J, Lawrentschuk N, et al. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur Urol 2022;82:613-22. [Crossref] [PubMed]
- Siva S, Louie AV, Kotecha R, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol 2024;25:e18-28. [Crossref] [PubMed]
- Tan WP, Schulman AA, Barton GJ, et al. Renal Thermal Ablation Trends of American Urologists. J Endourol 2020;34:409-16. [Crossref] [PubMed]
- Sandilos G, Butchy MV, Koneru M, et al. Histotripsy - hype or hope? Review of innovation and future implications. J Gastrointest Surg 2024;28:1370-5. [Crossref] [PubMed]
- Clinicaltrials.gov. Histosonics [Internet]. Available online: https://clinicaltrials.gov/search?cond=histosonics
- Chang R, Yang J, Taormina F, et al. Genitourinary Fellowship for Family Medicine Physicians. Urology 2025;S0090-4295(25)00191-8.
- Chavarriaga J. ASCO GU 2025: Evolution of ctDNA Detection with Next Generation Technology. UroToday; 2025. Available online: https://www.urotoday.com/conference-highlights/asco-gu-2025/asco-gu-2025-bladder-cancer/158294-asco-gu-2025-evolution-of-ctdna-detection-with-next-generation-technology.html
- Xu W, Gaborieau V, Niman SM, et al. Plasma Kidney Injury Molecule-1 for Preoperative Prediction of Renal Cell Carcinoma Versus Benign Renal Masses, and Association With Clinical Outcomes. J Clin Oncol 2024;42:2691-701. [Crossref] [PubMed]


