
Migrasome-related lncRNAs predict prognosis and immune response of clear cell renal cell carcinoma
Highlight box
Key findings
• In this study, a model of 13 migrasome-related long non-coding RNAs (lncRNAs) was established to predict the prognosis of clear cell renal cell carcinoma (ccRCC) patients, which showed good predictive ability for the prognosis and immune response of ccRCC patients.
What is known and what is new?
• Migrasomes are vesicular structures formed during cell migration and play a role in intercellular communication. Migrasomes and their associated lncrnas play important roles in cancer progression and prognosis.
• This study establishes the first prognostic model of ccRCC based on migrasome-related lncRNAs and reveals their relationship with immune responses, providing new insights into the personalized treatment of ccRCC patients and the underlying mechanisms of ccRCC progression.
What is the implication, and what should change now?
• The 13-lncRNA signature shows promise as a clinical tool for guiding personalized treatment decisions in ccRCC management.
• Future research should involve validating these findings in independent cohorts, performing functional analyses on the identified lncRNAs, and integrating the results with advanced imaging techniques.
Introduction
Clear cell carcinoma is the dominant histological subtype of kidney malignancy as a significant subset of urological neoplasms (1). Despite the advent of new therapeutic modalities, patients with clear cell renal cell carcinoma (ccRCC) still experience unsatisfactory clinical outcomes (2,3). This underscores the urgent need for novel prognostic biomarkers to stratify ccRCC patients and provide better individualized treatment. Long non-coding RNAs (lncRNAs) are transcripts exceeding 200 nucleotides in length, exhibiting minimal protein-coding potential and exerting a pivotal regulatory influence on diverse cellular processes (4,5). For instance, HCG18 can facilitate the proliferation and migration of ccRCC cells (6), whereas NEAT1 has been linked to the epithelial-mesenchymal transition and poor prognosis (7). These findings emphasize the potential importance of lncRNAs in ccRCC pathogenesis and as prognostic biomarkers.
In recent years, a novel organelle, the migrasome, has emerged as a subject of considerable interest within the field of cancer research (8,9). Migrasomes are vesicular structures formed during cell migration and play a role in intercellular communication (10). While studies on migrasomes in ccRCC are limited, research in other cancer types has revealed intriguing possibilities. In hepatocellular carcinoma, migrasomes promote metastasis by enhancing cancer cell invasion and angiogenesis (11). In pancreatic cancer, cancer-derived migrasomes can facilitate disease progression by suppressing the immune microenvironment (12).
The present study aimed to identify biomarkers for treatment stratification and prognosis in ccRCC. We sought to screen migrasome-related lncRNAs and analyze their biological functions and prognostic significance in ccRCC patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-728/rc).
Methods
Data sources
Gene expression profiles, somatic mutation information, and characteristics of individuals with ccRCC were acquired from The Cancer Genome Atlas (TCGA) database. The data included 542 tumor tissue samples and 72 control samples. Migrasome-related genes were obtained from Migrasome-associated studies and GeneCards (version 5.21; 20th August 2024) database (relevance score >1) (8,13,14). A total of eleven migrasome-related genes were identified: ITGB1, ITGA5, EOGT, CPQ, PIGK, NDST1, TSPAN4, TSPAN7, EPCIP, PKD2, and PKD1. TCGA gene annotation was used to distinguish lncRNAs in the expression matrix. Clinical and expression data were obtained from TCGA database. Missing data, if present, were handled as follows: (I) samples with missing survival information were excluded from the survival analysis. (II) For clinical covariates with missing values, we applied multiple imputation using the Multivariate Imputation by Chained Equations (MICE) package in R to minimize bias. (III) Gene expression data were processed by removing features with excessive missing values (>20%) and imputing remaining gaps using the K-nearest neighbor (KNN) algorithm. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Identification of migrasomerelated lncRNAs
TCGA gene annotation was used to distinguish lncRNAs in the expression matrix. A Pearson correlation analysis was conducted to identify co-expressed lncRNAs with migrasome-related genes, using a threshold of |R| >0.5 and P<0.001. This analysis was performed using the cor() function in R with the Pearson method, and statistical significance was assessed with the Hmisc package in R.
Construction and validation of prognostic models
Patients were randomized into two equal cohorts (15,16). The univariate Cox analysis, least absolute shrinkage and selection operator (LASSO) regression, and multivariate Cox regression were used to identify prognostically significant lncRNAs that formed the basis of a risk-scoring model. We assessed multicollinearity using the variance inflation factor (VIF). VIF scores were calculated using the car R package, and a threshold of VIF <5 was used to confirm that multicollinearity was not a concern. A risk score was calculated for each patient, with the median score serving as the threshold for high- and low-risk stratification. The prognostic effect of risk model was verified using Kaplan-Meier survival analysis. Differences between groups were assessed using the Log-rank test. To account for multiple comparisons, P-values were adjusted using the false discovery rate (FDR) correction. For multiple hypothesis testing, P-values were adjusted using the FDR correction to control for type I errors. The Benjamini-Hochberg (BH) method was applied. Overall survival (OS) data were obtained from TCGA database. In TCGA, OS is defined as the time from diagnosis to death from any cause (all-cause mortality). Since TCGA does not provide specific information on cancer-specific mortality, our analysis follows the standard practice of considering all reported death events as OS events. This approach is consistent with previous large-scale bioinformatics studies using TCGA data for prognostic model development. Its predictive power was evaluated in conjunction with clinical parameters through the use of univariate and multivariate Cox regression analyses. Receiver operating characteristic (ROC) curves were used for OS prediction accuracy, and principal component analysis (PCA) was used for risk group distinction. Model performance was also further validated by progression-free survival (PFS) analysis and Concordance index. A nomogram was constructed by combining the risk score with clinical characteristics in order to predict the 1-, 3-, and 5-year survival rates of patients with ccRCC. Clinical staging analysis was used to evaluate the applicability of the model to patients at different stages.
Univariate and multivariate Cox regression analyses were performed to identify prognostic lncRNAs. To ensure the validity of the Cox proportional hazards model, we tested the proportional hazards assumption using the Schoenfeld residuals test. No significant violations were detected, confirming the appropriateness of the model. To reduce overfitting and identify key prognostic lncRNAs, we employed the LASSO regression using 10-fold cross-validation to determine the optimal λ (lambda) value. The optimal model was selected based on the minimum mean cross-validated error. Additionally, the final prognostic model was validated using an independent testing cohort, Kaplan-Meier survival analysis, time-dependent ROC curves, and concordance index (C-index) to assess its predictive accuracy and robustness. To ensure the robustness and generalizability of our prognostic model, we employed 10-fold cross-validation instead of simple random splitting. The dataset was randomly divided into 10 equal subsets, with 9 subsets used for training and 1 for validation in each iteration. A prognostic model incorporating 13 migrasome-related lncRNAs was developed using multivariate Cox regression analysis. For each patient, a risk score was computed using the following formula: where betai represents the Cox regression coefficient for lncRNAi.
Gene enrichment analysis and immune correlation analysis
We used the clusterProfiler R (4.14.6) package to analyse gene functions and pathways, including Gene Ontology (GO) terms, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, and gene set enrichment analysis (GSEA) (17,18). For GO and KEGG pathway enrichment analysis, we applied the following parameters: P.adjust method = “BH”, P value cutoff =0.05, and Q-value cutoff =0.05. For GSEA, we utilized the “c6.all.v2024.1.Hs.symbols.gmt” gene set from the GSEA website, with the following parameters: nPerm =1,000, minGSSize =10, maxGSSize =500, and P value cutoff =0.05.
For GSEA, we employed the “c6.all.v2024.1.Hs.symbols.gmt” gene set from the GSEA website. Immune cell infiltration was assessed using the “CIBERSORT” package (19) (version 1.04) for immune cell deconvolution. The LM22 signature matrix, which includes the expression profiles of 22 human immune cell subtypes, was used as the reference dataset. The immune function between risk groups was assessed using single-sample GSEA scores. Additionally, the tumor immune dysfunction and exclusion (TIDE) algorithm was applied in the ccRCC expression matrix to evaluate immune escape potential and predict immunotherapy response. The analysis was conducted using the TIDE web application https://tide.nki.nl/, employing default parameters as specified on the website.
A comparison of TIDE scores across risk groups was conducted to elucidate differences in immune evasion mechanisms (20). To ensure an unbiased division of data for model development and validation, we randomly split the dataset into a training cohort (50%) and a testing cohort (50%). The caret package in R was used for stratified random sampling to maintain a balanced distribution of survival status across both groups. The stratification was performed using the createDataPartition() function, which ensures that the proportion of events (survival/death) is similar in both training and testing sets. The training cohort was used for feature selection and model construction, while the testing cohort was used for independent validation of the prognostic model. This randomization approach helps reduce potential selection bias and enhances the robustness of the model.
Tumor mutation burden (TMB) and drug sensitivity analysis
The “maftools” package was employed to calculate TMB scores (21). Differences in TMB between risk groups were visualized using violin plots. TMB was calculated as the total number of somatic mutations per megabase (mut/Mb) of the exonic coding region. The mutation data were obtained from TCGA, and TMB scores were computed using the maftools R package. Only non-synonymous somatic mutations were considered in the calculation to ensure biological relevance. The mutational landscape across the cohort was illustrated using waterfall plots. To predict half-maximal inhibitory concentration values, we utilized the “oncoPredict” package (version 1.2) (22,23). This package leverages large-scale pharmacogenomic datasets to build predictive models. Specifically, we employed the Genomics of Drug Sensitivity in Cancer (GDSC) version 2 dataset as the training reference, which includes gene expression profiles and corresponding drug response data for various cancer cell lines. Differential drug sensitivity between risk groups was assessed using the “limma” package. The “ggplot2” package was used for mapping.
Statistical analyses
Statistical analyses were conducted using R 4.3.3 and RStudio (Version 4.2.1). Results with a two-sided P value <0.05 were considered statistically significant. In figures, significance levels are represented as follows: P<0.05 (*), P<0.01 (**), and P<0.001 (***).
Results
Identification of migrasomerelated lncRNAs
The expression levels of 16,876 lncRNAs and 11 migrasome-related genes were obtained from the TCGA database, leading to the identification of 728 migrasome-related lncRNAs. A Sankey diagram was constructed to illustrate the associations between these lncRNAs and migrasome-related genes (Figure 1). The patient cohort was randomly divided into a training group (n=267) and a testing group (n=266). Table 1 presents the detailed characteristics of these groups. Notably, all clinical traits exhibited P values greater than 0.05, indicating comparable distributions between the two groups.
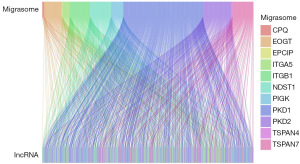
Table 1
| Covariates | Type | Total | Test | Train | P value |
|---|---|---|---|---|---|
| Age (years) | ≤65 | 349 (65.48) | 170 (63.91) | 179 (67.04) | 0.50 |
| >65 | 184 (34.52) | 96 (36.09) | 88 (32.96) | ||
| Gender | Female | 188 (35.27) | 89 (33.46) | 99 (37.08) | 0.43 |
| Male | 345 (64.73) | 177 (66.54) | 168 (62.92) | ||
| Grade | G1 | 14 (2.63) | 6 (2.26) | 8 (3.0) | 0.85 |
| G2 | 229 (42.96) | 113 (42.48) | 116 (43.45) | ||
| G3 | 206 (38.65) | 102 (38.35) | 104 (38.95) | ||
| G4 | 76 (14.26) | 41 (15.41) | 35 (13.11) | ||
| Unknown | 8 (1.5) | 4 (1.5) | 4 (1.5) | ||
| Stage | Stage I | 267 (50.09) | 134 (50.38) | 133 (49.81) | 0.99 |
| Stage II | 57 (10.69) | 28 (10.53) | 29 (10.86) | ||
| Stage III | 123 (23.08) | 60 (22.56) | 63 (23.6) | ||
| Stage IV | 83 (15.57) | 42 (15.79) | 41 (15.36) | ||
| Unknown | 3 (0.56) | 2 (0.75) | 1 (0.37) | ||
| T | T1 | 273 (51.22) | 138 (51.88) | 135 (50.56) | 0.78 |
| T2 | 69 (12.95) | 33 (12.41) | 36 (13.48) | ||
| T3 | 180 (33.77) | 88 (33.08) | 92 (34.46) | ||
| T4 | 11 (2.06) | 7 (2.63) | 4 (1.5) | ||
| M | M0 | 422 (79.17) | 209 (78.57) | 213 (79.78) | >0.99 |
| M1 | 79 (14.82) | 39 (14.66) | 40 (14.98) | ||
| Unknown | 32 (6.0) | 18 (6.77) | 14 (5.24) | ||
| N | N0 | 240 (45.03) | 116 (43.61) | 124 (46.44) | >0.99 |
| N1 | 16 (3.0) | 8 (3.01) | 8 (3.0) | ||
| Unknown | 277 (51.97) | 142 (53.38) | 135 (50.56) |
Data are presented as n (%). Grade: histopathological differentiation according to ISUP/WHO 2022 renal tumor classification. Stage: TNM staging per AJCC 8th edition criteria.
Construction of the ccRCC prognostic model
Univariate Cox regression analysis identified 267 lncRNAs with prognostic significance in ccRCC (Table S1). To prevent overfitting, LASSO regression was performed (Figure 2A,2B). Subsequently, multivariate Cox regression analysis identified 13 migrasome-related lncRNAs with significant prognostic value in ccRCC. KEGG pathway analysis further revealed the correlation between these lncRNAs and ccRCC (Figure S1). A heatmap was generated to visualize the relationships between these 13 lncRNAs and the 11 migrasome-related genes (Figure 2C). A prognostic model was then established using the identified lncRNAs, with the risk score calculated as follows:

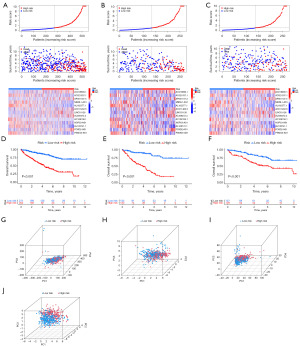
Risk score = Σ(ExplncRNAi × βlncRNAi), where i ranges from 1 to 13. Based on the median risk score, patients in the training cohort were classified into high- and low-risk groups. Patients in the high-risk group exhibited significantly shorter survival times (Figure 3A-3C). Kaplan-Meier survival analysis confirmed that patients in the low-risk group had significantly longer survival (P<0.001, Figure 3D-3F). PCA further demonstrated that the risk-associated lncRNAs effectively distinguished between the two groups (Figure 3G-3J).
Prognostic evaluation and nomogram construction
The prognostic performance of the developed ccRCC model was comprehensively assessed. As shown in the forest plots (Figure 4A,4B), the risk score derived from the lncRNA-based model was identified as an independent prognostic factor (P<0.001) in both univariate and multivariate analyses. In addition, patient age, tumor grade, and disease stage were also significant independent prognostic factors (P<0.001). ROC curve analysis demonstrated that the risk score exhibited superior prognostic accuracy compared to other clinical variables, with an area under the curve (AUC) of 0.794 (Figure 4C). The time-dependent ROC curves (Figure 4D) confirmed the sustained predictive power of the model at 1, 3, and 5 years, with AUC values of 0.796, 0.771, and 0.794, respectively. The time-dependent concordance index (C-index) plot (Figure 4E) indicated that the risk score achieved a higher predictive accuracy over a 10-year period compared to other clinical factors. Furthermore, PFS was significantly longer in the low-risk group than in the high-risk group (Figure 4F).
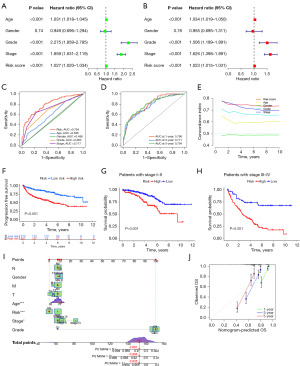
Stratified analysis using Kaplan-Meier curves (Figure 4G,4H) revealed a significant association between risk classification and OS, which remained consistent across different disease stages. Patients in the high-risk group exhibited markedly reduced survival probabilities compared to those in the low-risk group, both in early- and advanced-stage ccRCC (P<0.001). This highlights the broad applicability of the model across different disease stages. To facilitate individualized risk assessment, a nomogram was constructed by integrating risk scores with key clinical variables, including age, gender, tumor grade, and stage (Figure 4I). The calibration plot (Figure 4J) demonstrated a strong correlation between the nomogram-predicted and observed OS probabilities at 1, 3, and 5 years. Decision curve analysis further demonstrated the clinical utility of the prognostic model, showing consistent net benefits across threshold probabilities compared to alternative strategies (Figure S2). These findings validate the prognostic utility of the developed risk score model and its potential role in risk stratification for ccRCC patients.
Gene enrichment analysis
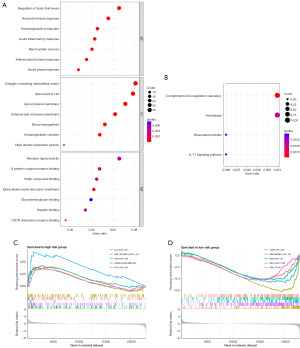
GO analysis indicated that the identified lncRNAs were primarily involved in lipid metabolism, immune responses, and various cellular binding activities (Figure 5A). KEGG pathway analysis further highlighted the involvement of complement and coagulation cascades in ccRCC progression (Figure 5B). The high-risk group showed enrichment in immune-related and cell cycle pathways, suggesting a propensity for more aggressive tumor characteristics (Figure 5C). In contrast, the low-risk group exhibited enrichment in multiple signaling pathways, including KRAS-related genes in kidney tissue (Figure 5D). These findings suggest potential molecular targets for further investigation and therapeutic development in ccRCC.
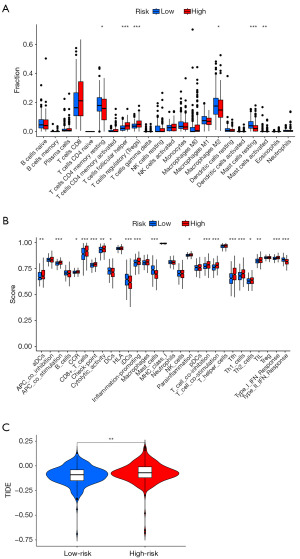
Immune correlation analysis
The migrasome-related lncRNA prognostic model revealed a significantly higher infiltration of immunosuppressive cells, particularly follicular helper T cells and regulatory T cells (Tregs), in the high-risk group (Figure 6A). Analysis of immune functions showed that the high-risk group had significantly higher scores for immune checkpoints, T cell co-inhibition, follicular helper T cells, T helper 1 (Th1), and T helper 2 (Th2) responses (Figure 6B), indicating an enhanced immunosuppressive function. Moreover, TIDE analysis revealed significantly higher TIDE scores in the high-risk group (P=0.006), suggesting a higher likelihood of immune evasion and potential resistance to immune checkpoint inhibitors (Figure 6C). These findings suggest that the migrasome-related lncRNA signature is not only a prognostic marker but also correlates with distinct immune profiles, which may influence treatment responses and patient outcomes in ccRCC. A comprehensive correlation analysis between the 13 prognostic lncRNAs and 22 immune cell types revealed distinct patterns of association (Figure S3).
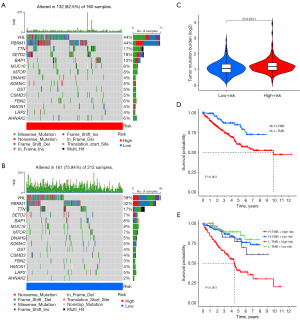
TMB and drug sensitivity analysis
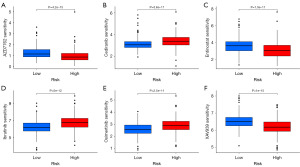
Analysis of somatic mutations and TMB in ccRCC patients identified distinct mutational patterns associated with the migrasome-related lncRNA prognostic model. The waterfall plot for the high-risk group (Figure 7A) revealed a high mutation frequency, with 82.5% of 160 samples exhibiting alterations. The most frequently mutated genes were VHL and PBRM1, each occurring at a frequency of 44%. Other common mutations included SETD2 (18%) and TTN (17%). In contrast, the low-risk group exhibited a lower overall mutation rate (75.94% of 212 samples), with VHL (39%) and PBRM1 (32%) being the most frequently altered genes (Figure 7B). A violin plot (Figure 7C) demonstrated a significantly higher TMB in the high-risk group (P=0.001). Furthermore, survival analysis showed that patients with a high TMB had significantly worse survival outcomes (P<0.001, Figure 7D). Combined analysis of TMB and risk score classification (Figure 7E) revealed that the worst prognosis was observed in the high-TMB + high-risk group, while the best survival probability was noted in the low-TMB + low-risk group (P<0.001). These results suggest that integrating TMB with the migrasome-related lncRNA signature enhances prognostic accuracy and could help guide personalized treatment strategies for ccRCC patients. Finally, drug sensitivity analysis was performed to explore potential therapeutic options. The low-risk group demonstrated significantly higher sensitivity to AZD7762, Entinostat, and XAV939, whereas the high-risk group exhibited increased sensitivity to Cediranib, Ibrutinib, and Osimertinib (P<1×10-10, Figure 8A-8F). These findings provide insights into potential treatment strategies tailored to risk stratification in ccRCC.
Discussion
ccRCC is a challenging malignancy with poor outcomes, largely due to its difficulty in early diagnosis, and drug resistance (24-26). Recent research has highlighted the potential role of migrasomes in cancer progression and treatment (27,28). Concurrently, lncRNAs have emerged as pivotal participants in the diverse biological processes of ccRCC, including its development, progression, and therapeutic response (29,30). Our study highlights the significant association between the migrasome-related lncRNA prognostic model and the immune microenvironment in ccRCC. The high-risk group exhibited increased infiltration of immunosuppressive cells, including follicular helper T cells and regulatory T cells, along with heightened immune checkpoint activity and T cell co-inhibition. This immunosuppressive state may contribute to weakened anti-tumor immune responses and poorer survival outcomes. Moreover, the elevated TIDE scores observed in the high-risk group indicate a greater potential for immune evasion, suggesting a lower likelihood of response to immune checkpoint inhibitors. These findings underscore the importance of migrasome-related lncRNAs in shaping the tumor immune landscape and highlight their potential in guiding personalized immunotherapy strategies for ccRCC patients. Our findings suggest that migrasome-related lncRNAs may contribute to immune evasion in ccRCC. The high-risk group exhibited an immunosuppressive tumor microenvironment (TME), which aligns with known mechanisms such as PD-L1 upregulation and T cell exhaustion. The increased immune checkpoint activity and higher TIDE scores further indicate a greater potential for immune escape and resistance to immunotherapy. These results highlight the relevance of migrasome-related lncRNAs in modulating immune evasion pathways and their potential role in optimizing immunotherapy strategies for ccRCC.
Our investigation introduces an innovative prognostic framework for ccRCC, which leverages the expression profiles of lncRNAs associated with migrasome. Our findings indicate that the 13-lncRNA signature can potentially predict patient outcomes. The superior performance of our risk score model compared to traditional clinical factors underscores the importance of molecular markers in prognostication. The prognostic model demonstrated robust discriminatory power in delineating subgroups with divergent survival trajectories, thereby underscoring its potential value in clinical decision-making and risk assessment. Furthermore, the model’s predictive capacity across different disease stages suggests its broad applicability. Huang et al. [2024] demonstrated that FOXD2-AS1 promotes the malignant progression of ccRCC by binding to MYC and activating EGLN3 (31). This mechanism suggests that FOXD2-AS1 may serve as a potential therapeutic target. Additionally, Pan et al. [2023] found that LINC00941 plays a crucial role in ccRCC prognosis assessment by promoting malignant cellular behavior and can be used to predict patient survival (32).
Some evidence suggests that the roles of the lncRNAs we identified are consistent in ccRCC and other cancers. AC0118690.1, previously associated with poor prognosis in hepatocellular carcinoma, was found to be relevant in our ccRCC cohort (33). Conversely, AL109741.1 and AL162377.1 have been reported to have tumor-suppressive effects in ccRCC, correlated with better prognosis (32,34). AL162377.1, in particular, has been demonstrated to augment anti-tumour immunity via the miR-21-5p/SYDE2 axis (34). FOXD2-AS1, identified in our signature, has been previously linked to a poor prognosis in ccRCC, which aligns with our findings (35). While LINC01126 has been primarily studied in prostate cancer (36), as it can drives castration resistance, its role in ccRCC warrants further investigation. Interestingly, MBNL1-AS1, another lncRNA in our signature, has demonstrated tumor-suppressive roles in a number of cancers. It has been shown to repress gastric cancer progression via the TGF-β pathway (37), inhibit breast cancer stem-like properties (38), and attenuate colorectal cancer proliferation (39). Its identification in our ccRCC prognostic model suggests a potential role in renal cancer biology that merits further exploration. PSMG3-AS1, associated with better prognosis in ccRCC in a previous study (40), was also identified in our signature, thereby supporting its potential as a favorable prognostic marker. In short, the identified lncRNAs may serve as potential biomarkers for personalized treatment strategies.
The immune correlation analysis indicated that high-risk patients had increased infiltration of immunosuppressive cells, enhanced immunosuppressive function, and increased immune escape potential. This association provides novel insights into the interplay between migrasome-related lncRNAs and the immune landscape within the ccRCC TME. Our finding suggests that the identified lncRNAs may influence immune evasion mechanisms, which could explain the poorer outcomes in high-risk patients. Furthermore, the differential drug sensitivity patterns between risk groups could inform treatment selection and the development of novel therapies. In recent years, advancements in medical imaging technology have played a significant role in disease diagnosis and treatment. For example, SPECT/CT and PET-CT have the advantages of faster acquisition time and better image quality in diagnosis (41). For ccRCC, these novel imaging technologies contribute to more accurate early diagnosis, tumor classification, and treatment monitoring (41,42). The integration of these imaging techniques may potentially optimize prognostic assessment and personalized therapeutic strategies for ccRCC, ultimately leading to enhanced patient survival outcomes.
Despite the promising results, this study has some limitations. First, our prognostic model was developed and validated using data exclusively from TCGA, and no independent external cohort was used for validation. While TCGA provides a large and well-characterized dataset, external validation in independent patient cohorts is crucial to confirm the generalizability and robustness of the proposed model. In future studies, we plan to validate our findings using independent datasets or prospective clinical cohorts to further enhance the reliability of our prognostic signature. Second, our study did not account for the spatial heterogeneity of the TME, which plays a crucial role in ccRCC immunology and progression. Tumor heterogeneity can lead to diverse immune infiltration patterns and distinct therapeutic responses, which were not captured in our bulk RNA-sequencing-based approach. Future research integrating spatial transcriptomics and single-cell sequencing will be essential to provide a more comprehensive understanding of the interactions between lncRNAs and the spatial architecture of the TME in ccRCC. Thirdly, due to the unavailability of detailed surgical approach data in TCGA, potential confounding effects of surgical intervention were excluded from our analysis. Additionally, the migrasome-related genes are not fully understood. Last but not least, further functional studies need to elucidate the mechanisms by which these lncRNAs impact the ccRCC progression and immune responses.
Future research should focus on experimental validation of the identified lncRNAs and exploration of their potential as therapeutic targets. Integration of this molecular signature with other omics data and clinical parameters could provide new ideas for further refining prognostic accuracy and treatment strategies for ccRCC patients.
Conclusions
Our study establishes a migrasome-associated lncRNA signature as a promising prognostic tool for ccRCC, thereby providing new perspectives for individualized treatment of ccRCC. Furthermore, the 13 lncRNAs may provide potential therapeutic targets for ccRCC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-728/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-728/prf
Funding: This research was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-728/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rose TL, Kim WY. Renal Cell Carcinoma: A Review. JAMA 2024;332:1001-10. [Crossref] [PubMed]
- Zhu H, Wang X, Lu S, et al. Metabolic reprogramming of clear cell renal cell carcinoma. Front Endocrinol (Lausanne) 2023;14:1195500. [Crossref] [PubMed]
- Meng L, Collier KA, Wang P, et al. Emerging Immunotherapy Approaches for Advanced Clear Cell Renal Cell Carcinoma. Cells 2023;13:34. [Crossref] [PubMed]
- Nemeth K, Bayraktar R, Ferracin M, et al. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet 2024;25:211-32. [Crossref] [PubMed]
- Zhang H, Yu L, Chen J, et al. Role of Metabolic Reprogramming of Long non-coding RNA in Clear Cell Renal Cell Carcinoma. J Cancer 2022;13:691-705. [Crossref] [PubMed]
- Du Z, Wang B, Tan F, et al. The regulatory role of LncRNA HCG18 in various cancers. J Mol Med (Berl) 2023;101:351-60. [Crossref] [PubMed]
- Ning L, Li Z, Wei D, et al. LncRNA, NEAT1 is a prognosis biomarker and regulates cancer progression via epithelial-mesenchymal transition in clear cell renal cell carcinoma. Cancer Biomark 2017;19:75-83. [Crossref] [PubMed]
- Qin Y, Yang J, Liang C, et al. Pan-cancer analysis identifies migrasome-related genes as a potential immunotherapeutic target: A bulk omics research and single cell sequencing validation. Front Immunol 2022;13:994828. [Crossref] [PubMed]
- Deng S, Wu Y, Huang S, et al. Novel insights into the roles of migrasome in cancer. Discov Oncol 2024;15:166. [Crossref] [PubMed]
- Jiang D, He J, Yu L. The migrasome, an organelle for cell-cell communication. Trends Cell Biol 2025;35:205-16. [Crossref] [PubMed]
- Zhang K, Zhu Z, Jia R, et al. CD151-enriched migrasomes mediate hepatocellular carcinoma invasion by conditioning cancer cells and promoting angiogenesis. J Exp Clin Cancer Res 2024;43:160. [Crossref] [PubMed]
- Zhang R, Liu Q, Peng J, et al. CXCL5 overexpression predicts a poor prognosis in pancreatic ductal adenocarcinoma and is correlated with immune cell infiltration. J Cancer 2020;11:2371-81. [Crossref] [PubMed]
- Zhao X, Lei Y, Zheng J, et al. Identification of markers for migrasome detection. Cell Discov 2019;5:27. [Crossref] [PubMed]
- Jiang D, Jiang Z, Lu D, et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat Cell Biol 2019;21:966-77. [Crossref] [PubMed]
- Zhao Z, Liu H, Zhou X, et al. Necroptosis-Related lncRNAs: Predicting Prognosis and the Distinction between the Cold and Hot Tumors in Gastric Cancer. J Oncol 2021;2021:6718443. [Crossref] [PubMed]
- Zhou Q, Sun Q, Shen Q, et al. Development and implementation of a prognostic model for clear cell renal cell carcinoma based on heterogeneous TLR4 expression. Heliyon 2024;10:e25571. [Crossref] [PubMed]
- Yu G, Wang LG, Yan GR, et al. DOSE: an R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics 2015;31:608-9. [Crossref] [PubMed]
- Xu S, Hu E, Cai Y, et al. Using clusterProfiler to characterize multiomics data. Nat Protoc 2024;19:3292-320. [Crossref] [PubMed]
- Chen B, Khodadoust MS, Liu CL, et al. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 2018;1711:243-59. [Crossref] [PubMed]
- Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 2018;24:1550-8. [Crossref] [PubMed]
- Mayakonda A, Lin DC, Assenov Y, et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 2018;28:1747-56. [Crossref] [PubMed]
- Geeleher P, Cox NJ, Huang RS. Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome Biol 2014;15:R47. [Crossref] [PubMed]
- Maeser D, Gruener RF, Huang RS. oncoPredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief Bioinform 2021;22:bbab260. [Crossref] [PubMed]
- Nejati R, Wei S, Uzzo RG, et al. Monosomy of Chromosome 9 Is Associated With Higher Grade, Advanced Stage, and Adverse Outcome in Clear-cell Renal Cell Carcinoma. Clin Genitourin Cancer 2020;18:56-61. [Crossref] [PubMed]
- Wang Z, Zheng Z, Wang B, et al. Characterization of a G2M checkpoint-related gene model and subtypes associated with immunotherapy response for clear cell renal cell carcinoma. Heliyon 2024;10:e29289. [Crossref] [PubMed]
- Aweys H, Lewis D, Sheriff M, et al. Renal Cell Cancer - Insights in Drug Resistance Mechanisms. Anticancer Res 2023;43:4781-92. [Crossref] [PubMed]
- Zheng Y, Lang Y, Qi B, et al. TSPAN4 and migrasomes in atherosclerosis regression correlated to myocardial infarction and pan-cancer progression. Cell Adh Migr 2023;17:14-9. [Crossref] [PubMed]
- Zhang Y, Guo W, Bi M, et al. Migrasomes: From Biogenesis, Release, Uptake, Rupture to Homeostasis and Diseases. Oxid Med Cell Longev 2022;2022:4525778. [Crossref] [PubMed]
- Rysz J, Konecki T, Franczyk B, et al. The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma. Int J Mol Sci 2022;24:643. [Crossref] [PubMed]
- Zhang Q, Ren H, Ge L, et al. A review on the role of long non-coding RNA and microRNA network in clear cell renal cell carcinoma and its tumor microenvironment. Cancer Cell Int 2023;23:16. [Crossref] [PubMed]
- Huang Z, Liu B, Li X, et al. FOXD2-AS1 Binding to MYC Activates EGLN3 to Affect the Malignant Progression of Clear Cell Renal Cell Carcinoma. J Biochem Mol Toxicol 2024;38:e70083. [Crossref] [PubMed]
- Pan H, Wei W, Fu G, et al. LINC00941 Promotes Cell Malignant Behavior and Is One of Five Costimulatory Molecule-Related lncRNAs That Predict Prognosis in Renal Clear Cell Carcinoma. Medicina (Kaunas) 2023;59:187. [Crossref] [PubMed]
- He J, Li W, Zhao W, et al. Potential of lncRNAs to regulate cuproptosis in hepatocellular carcinoma: Establishment and validation of a novel risk model. Heliyon 2024;10:e24453. [Crossref] [PubMed]
- Cui Y, Wu J, Zhou Z, et al. Two novel lncRNAs AF111167.2 and AL162377.1 targeting miR-21-5p mediated down expression of SYDE2 correlates with poor prognosis and tumor immune infiltration of ccRCC. Heliyon 2022;8:e11079. [Crossref] [PubMed]
- Bai Z, Lu J, Chen A, et al. Identification and Validation of Cuproptosis-Related LncRNA Signatures in the Prognosis and Immunotherapy of Clear Cell Renal Cell Carcinoma Using Machine Learning. Biomolecules 2022;12:1890. [Crossref] [PubMed]
- Cai Y, Chen M, Gong Y, et al. Androgen-repressed lncRNA LINC01126 drives castration-resistant prostate cancer by regulating the switch between O-GlcNAcylation and phosphorylation of androgen receptor. Clin Transl Med 2024;14:e1531. [Crossref] [PubMed]
- Su J, Chen D, Ruan Y, et al. LncRNA MBNL1-AS1 represses gastric cancer progression via the TGF-β pathway by modulating miR-424-5p/Smad7 axis. Bioengineered 2022;13:6978-95. [Crossref] [PubMed]
- Ding Y, Li Y, Duan Y, et al. LncRNA MBNL1-AS1 Represses Proliferation and Cancer Stem-Like Properties of Breast Cancer through MBNL1-AS1/ZFP36/CENPA Axis. J Oncol 2022;2022:9999343. [Crossref] [PubMed]
- Chen WS, Zhang X, Zhao ZF, et al. MBNL1 AS1 attenuates tumor cell proliferation by regulating the miR 29c 3p/BVES signal in colorectal cancer. Oncol Rep 2023;50:191. [Crossref] [PubMed]
- Cheng G, Liu D, Liang H, et al. A cluster of long non-coding RNAs exhibit diagnostic and prognostic values in renal cell carcinoma. Aging (Albany NY) 2019;11:9597-615. [Crossref] [PubMed]
- Tataru OS, Marchioni M, Crocetto F, et al. Molecular Imaging Diagnosis of Renal Cancer Using (99m)Tc-Sestamibi SPECT/CT and Girentuximab PET-CT-Current Evidence and Future Development of Novel Techniques. Diagnostics (Basel) 2023;13:593. [Crossref] [PubMed]
- Milella M, Rutigliano M, Lasorsa F, et al. The Role of MUC1 in Renal Cell Carcinoma. Biomolecules 2024;14:315. [Crossref] [PubMed]


