
Impact of suction ureteral access sheath in ureteroscopy/retrograde intrarenal surgery: a systematic review and meta-analysis
Highlight box
Key findings
• Using suction ureteral access sheaths (SUASs) during endoscopic stone surgery improves short-term stone-free rates (SFRs), shortens surgical time, and decreases overall complication rates.
What is known and what is new?
• Our study demonstrates that the use of SUASs during retrograde intrarenal surgery (RIRS) improves the SFR on the first postoperative day compared to the standard approach without SUASs. Additionally, ureteroscopy/RIRS with SUASs showed favorable perioperative outcomes, including operation time and complications. Short of well-designed controlled trials, these data represent the highest level of evidence supporting the use of SUASs in standard endoscopic stone surgery.
What is the implication, and what should change now?
• SUASs during RIRS improve the short-term SFR compared to the standard approach without SUASs. RIRS with SUASs showed favorable perioperative outcomes, including operation time and complications.
Introduction
The incidence of urolithiasis is steadily increasing worldwide (1). In response to the growing demand for less invasive and more effective interventions, current treatment strategies for urolithiasis focus on endourological procedures such as ureteroscopy (URS), retrograde intrarenal surgery (RIRS), and percutaneous nephrolithotomy (PCNL). Guidelines recommend URS as the primary treatment option for ureteral stones, while RIRS is suggested as a treatment option for kidney stones regardless of size, either as a primary or secondary treatment (2,3). Although both URS and RIRS are widely adopted, 22% of patients experience residual stones and 6.1% experience infectious complications with non-negligible rates of sepsis requiring intensive care unit (ICU) admission after RIRS (4). Even though URS is generally effective and safe for most cases, patients with larger stones or more complex situations can suffer from complications (5).
The continuous technological progress in endoscopic equipment has contributed to the improvement of outcomes of URS/RIRS. For instance, the suction device which is primarily employed during PCNL facilitates stone removal. This device reduces both the temperature and pressure in the renal pelvis, leading to a decreased risk of infection and improved surgical outcomes (6). Recent studies have suggested that the use of suction sheaths during PCNL significantly improves the stone-free rate (SFR) and operation time, as well as reduces the risk of complications (7). Recently, suction ureteral access sheaths (SUASs) were developed to improve outcomes of URS/RIRS. However, to date, there are no robust evidence regarding the potential impact of SUASs during URS/RIRS. Therefore, we conducted this systematic review and meta-analysis to evaluate the efficacy and safety of the use of SUASs during URS/RIRS compared to URS/RIRS without SUASs. We hypothesized that the use of SUASs during URS/RIRS improves perioperative outcomes of SUASs by a statistically and clinically significant margin. We present this article in accordance with the AMSTAR2 checklist and the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-138/rc) (8,9).
Methods
We registered the study protocol in the International Prospective Register of Systemic Reviews database (PROSPERO: CRD42024538956).
Study selection and characteristics
In April 2024, we conducted a comprehensive search of MEDLINE (via PubMed), Scopus, and Web of Science to identify studies assessing the impact of SUASs on clinical outcomes during URS/RIRS compared to the standard approach without SUASs. The detailed search strategy is provided in Appendix 1. Two investigators independently screened titles and abstracts to identify eligible studies. Any disagreements were resolved through discussion until a consensus was reached between authors.
Inclusion and exclusion criteria
To address our clinical question, we applied the PICO framework (10). We included studies assessing patients with urinary stones (Patients) who underwent URS/RIRS with the use of SUASs (Interventions), compared to those who underwent URS/RIRS without the use of SUASs (Comparison). Our primary endpoint was the SFR, with secondary endpoints including operation time, hospital stay, and complications (Outcome). Our exclusion criteria were as follows: reviews, editorial comments, replies to authors, meeting abstracts, single-arm studies, and articles not published in English. Two authors (S.K. and A.M.) screened independently. If disagreements were occurred during the article selection process, they were resolved through discussion between the two authors.
Data extraction
Two authors independently extracted details including the first author’s name, publication year, study design, follow-up protocol, patient characteristics (such as age, sex, and sample size), stone characteristics, and perioperative outcomes. The extracted outcomes included immediate SFR, follow-up SFR, operation time, duration of hospital stay, and complication rate. In this study, immediate residual and follow-up residual stone rates were assessed on postoperative day one and at one month. To minimize the effect of the location of urinary stones, we divided patients into two groups as follows: kidney and ureteral stone subgroups. Any conflicts that arose during the data extraction process were resolved through discussion between the two authors.
Risk of bias assessment
Each study was evaluated independently by two authors using the Risk-of-Bias version 2 (RoB2) tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (11) for randomized controlled trials (RCTs) and the Risk Of Bias in Non-randomized Studies—of Interventions tool (ROBINS-I) (12) for non-randomized studies (Figures S1,S2).
Statistical analyses
Meta-analysis
We conducted meta-analyses using R statistical software 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). For our calculations, we followed the methods recommended by the working group of the Cochrane Collaboration (13). Due to the diversity of clinical characteristics of the included studies, we employed random-effect models. To compare SFR and complication rate, we calculated risk ratios (RRs) with 95% confidence intervals (CIs). For assessing operation time and the duration of hospital stay, we calculated the mean differences (MDs) with 95% CIs. These calculations were performed using the “meta” package in R. Forest plots were utilized to visually compare RR and MDs between the SUAS and non-SUAS groups. In our analysis, the threshold for statistical significance was set at P<0.05. At least two studies were required to perform a meta-analysis. We evaluated the presence of heterogeneity among the outcomes of the included studies using Cochrane’s Q test. In instances of significant heterogeneity (P value of <0.05 in the Cochrane Q test), we attempted to investigate and explain the source of heterogeneity through sensitivity analysis. Publication bias was assessed using funnel plots (Figure S3).
Results
Study selection and characteristics
The search strategy is detailed in Figure 1. Based on our inclusion criteria, seven comparative studies (one RCT and six retrospective cohort studies) (14-20), comprising a total of 1,746 patients, were included in this study. Although the study by Huang et al. (16) included patients with proximal ureteral stones, these stones were pushed back into the kidney and treated as kidney stones. Therefore, we included this study in the kidney stone subgroup. The characteristics of the seven studies are summarized in Tables 1,2.
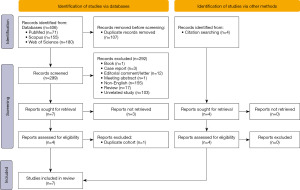
Table 1
| Author/year | Study design | Ureteroscope | Access sheath | Definition of stone free | Follow-up | Number of patients | Age† (years) | Sex (male/female) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUAS | Non-SUAS | SUAS | Non-SUAS | SUAS | Non-SUAS | SUAS | Non-SUAS | |||||||
| Wang et al. 2024 | RCS | 7.5 Fr flexible | 12/14 Fr SUAS (ClearPetraTM, Well Lead Medical Co.) | 12/14 Fr C-UAS (COOK, USA) | <4 mm | POD1: KUB; POM1: KUB or CT | 71 | 74 | 45.87±14.9 | 46.3±13.99 | 47/24 | 51/23 | ||
| Huang et al. 2023 | RCS | 8.7 Fr disposable flexible ureteroscope | 11/13 Fr or 12/14 Fr flexible SUAS | 11/13 Fr or 12/14 Fr C-UAS (Copper, Medical Technology Co., Ltd.) | <3 mm | POD1: CT; POM1: CT | 114; after matching: 103 | 257; after matching: 103 | 54.5±11.0; after matching: 54.5±11.0 | 56.3±11.6; after matching: 54.7±10.7 | 79/35; after matching: 71/32 | 169/88; after matching: 68/34 | ||
| Zhang et al. 2023 | RCS | 8/9.8 Fr semirigid ureteroscope and flexible ureteroscope | 12/14 Fr SUAS (Zhangjiagang Huamei Medical Equipment, China) | 12/14 Fr C-UAS (Shenzhen Kang Yi Bo Technology Development, China) | <2 mm | POD1: KUB or CT; POM1: KUB or CT | 102 | 112 | 47.69±9.18 | 46.75±11.87 | 55/47 | 71/41 | ||
| Qian et al. 2022 | RCS | 7.5 Fr flexible ureteroscope | 12/14 Fr SUAS (COOK, USA) | 12/14 Fr C-UAS (COOK, USA) | <4 mm | POD1: KUB or USG; POM1: KUB or CT | 81; after matching: 81 | 363; after matching: 81 | 51 (IQR, 42–57). After matching: 51 (IQR, 42–57) | 49 (IQR, 40–58). After matching: 50 (IQR, 43–57) | 52/29. After matching: 52/29 | 225/138. After matching: 56/25 | ||
| Zhu et al. 2019 | RCS | 9.8 Fr semirigid ureteroscope and 7.5 Fr flexible ureteroscope | 12/14 Fr SUAS (KYB, China) | 12/14 Fr C-UAS (KYB, China) | <2 mm | POD1: KUB; POM1: KUB or CT | 165 | 165 | 53.9±13.4 | 51.7±15.8 | 109/56 | 109/56 | ||
| Zhai et al. 2023 | RCS | 8/9.8 Fr semi-rigid ureteroscope | 12–14 Fr SUAS (Shenzhen Kang Yi Bo Technology Development Co. Ltd.) | No access sheath | 4 mm | NA | 60 | 60 | 45.7±6.5 | 46.2±6.9 | 34/26 | 32/28 | ||
| Du et al. 2019 | RCT | 7/8.4 Fr ureteroscope (Stroz) | 12/14 Fr SUAS (Jiangxi Inventor Technology Co., Ltd.) | No access sheath | ≤4 mm | POM1: KUB | 62 | 60 | 47.36±13.16 | 46.95±15.72 | 37/25 | 36/24 | ||
†, data are presented as mean ± standard deviation unless otherwise specified. CT, computed tomography; IQR, interquartile range; KUB, kidney, ureter, and bladder; NA, not applicable; POD, postoperative day; POM, postoperative month; RCT, randomized controlled trial; RCS, retrospective cohort study; SUAS, suction ureteral access sheath.
Table 2
| Author/year | Laterality (L/R) | Size or volume | Location | Number of stones | Hydronephrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUAS | Non-SUAS | SUAS | Non-SUAS | SUAS | Non-SUAS | SUAS | Non-SUAS | SUAS | Non-SUAS | |||||
| Wang et al. 2024 | NA | NA | 14.65±4.18 mm | 14.48±4.03 mm | Non-lower calyx: 43; lower calyx: 28 | Non-lower calyx: 45; lower calyx: 29 | Single: 39; multiple: 32 | Single: 42; multiple: 32 | 11 | 9 | ||||
| Huang et al. 2023 | NA | NA | 1.7±0.6 cm; after matching: 1.7±0.6 cm | 1.6±0.5 cm; after matching: 1.7±0.5 cm | NA | NA | NA | NA | 34; after matching: 31 | 74; after matching: 71 | ||||
| Zhang et al. 2023 | 46/56 | 58/54 | 18.47±4.67 mm | 18.20±4.46 mm | Pelvis: 41; upper calyx: 10; middle calyx: 9; lower calyx: 12; multiple calyxes: 30 | Pelvis: 43; upper calyx: 9; middle calyx: 11; lower calyx: 15; multiple calyxes: 34 | NA | NA | No: 27; mild: 47; moderate: 22; gross: 6 | No: 24; mild: 57; moderate: 29; gross: 2 | ||||
| Qian et al. 2022 | NA | NA | 19 (IQR, 17–23) mm; after matching: 19 (IQR, 17–23) mm | 19 (IQR, 16–22) mm; after matching: 20 (IQR, 17–23) mm | Upper/middle pole: 47; lower pole: 34; after matching; upper/middle pole: 47; lower pole: 34 | Upper/middle pole: 211; lower pole: 152; after matching; upper/middle pole: 56; lower pole: 25 | Single: 35; multiple: 46; after matching; single: 35; multiple: 46 | Single: 202; multiple: 161; after matching; single: 43; multiple: 38 | 71; after matching: 71 | 332; after matching: 76 | ||||
| Zhu et al. 2019 | NA | NA | 18.2±5.2 mm | 17.4±4.7 mm | Pelvis: 29; upper calyx: 14; middle calyx: 27; lower calyx: 40; multiple: 55 | Pelvis: 23; upper calyx: 18; middle calyx: 35; lower calyx: 42; multiple: 47 | NA | NA | NA | NA | ||||
| Zhai et al. 2023 | 23/37 | 26/34 | 136.5±26.6 mm2 | 131.8±25.1 mm2 | Proximal: 12; mid: 18; distal: 30 | Proximal: 13; mid: 17; distal: 30 | NA | NA | No: 10; 1–3 cm: 28; >3 cm: 22 | No: 12; 1–3 cm: 26; >3 cm: 22 | ||||
| Du et al. 2019 | NA | NA | 21.88±4.93 mm | 21.37±3.61 mm | L4 level | L4 level | NA | NA | NA | NA | ||||
IQR, interquartile range, L/R, left/right; NA, not applicable, SUAS, suction ureteral access sheath.
Assessment of risk of bias and quality of study
The risk of bias judgments of each domain for the included studies is summarized in Figures S1,S2. According to RoB2 for RCTs, the only RCT was considered to have some concerns. According to the ROBINS-I tool for non-randomized studies, all six studies were assessed as having a moderate risk of bias.
Standard pairwise meta-analysis of SUAS use
SFR
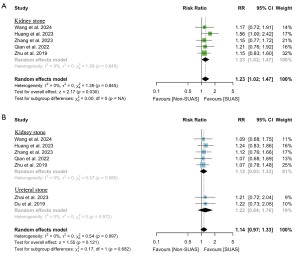
Data on immediate and follow-up SFR were available from five studies (14-18) (n=1,504) and seven studies (14-20) (n=1,746), respectively. As shown in Figure 2, the use of SUASs was associated with a higher SFR compared to no use of SUAS (RR: 1.23; 95% CI: 1.02 to 1.47; P=0.03), while no significant difference was observed in SFR at one month follow-up (RR: 1.14; 95% CI: 0.97 to 1.33; P=0.12). Cochran’s Q test indicated no significant heterogeneity among the included studies.
Operation time
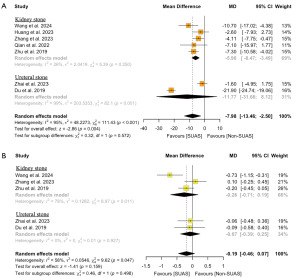
Seven studies (14-20) (n=1,746) were included in this analysis. The use of SUASs significantly reduced the operation time compared to no use of SUAS (MD: −7.98 minutes; 95% CI: −13.46 to −2.50; P=0.004) (Figure 3). Cochran’s Q test revealed significant heterogeneity among the included studies. Subgroup and sensitivity analyses indicated that the study by Du et al. (19) was the source of this heterogeneity (Figures S4,S5).
Hospital stay
Five studies (14,15,18-20) (n=931) reported the duration of hospital stay. No significant difference was found in the duration of hospital stay between URS/RIRS with and without SUASs (MD: −0.19 days; 95% CI: −0.46 to 0.07; P=0.16) (Figure 3). There was significant heterogeneity in this analysis. Subgroup and sensitivity analyses revealed that the study by Wang et al. (14) contributed to this heterogeneity (Figures S4,S5).
Complications
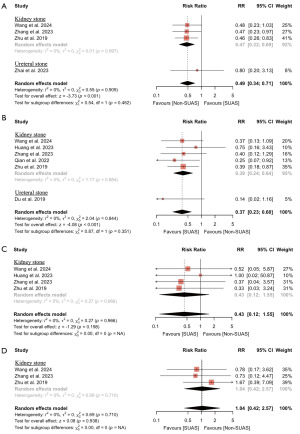
The risk of overall complications, fever, septic shock, and hematuria was reported by four (14,15,18,20) (n=809), six (14-19) (n=1,626), four (14-16,18) (n=1,060), and three studies (14,15,18) (n=689), respectively. As shown in Figure 4, the use of SUASs was associated with a significant decrease in the risk of overall complications (RR: 0.49; 95% CI: 0.34 to 0.71; P<0.001) and fever (RR: 0.37; 95% CI: 0.23 to 0.60; P<0.001). No significant differences between SUAS use and non-use were found regarding the risk of septic shock or hematuria. There were no significant heterogeneities among the included studies.
Discussion
In this meta-analysis, we systematically reviewed and synthesized the available evidence on the impact of SUASs in URS/RIRS on clinical outcomes in patients with urinary stones. Our findings demonstrate that SUASs contribute to a higher immediate SFR, shorter operative time, and fewer complications compared to not using SUASs, highlighting the utility of SUASs in URS/RIRS for urinary stones.
First, our analyses revealed that the use of SUASs significantly improved the immediate SFR by 23% compared to the non-SUAS group. This finding can be explained by the direct impact of suctional function on tiny fragments of broken stones. Chen et al. reported that in RIRS with SUAS, stones smaller than 1 mm can be directly aspirated through the gap between the ureteroscope and the SUASs (21). Additionally, fragments ranging from 2 to 4 mm can be effectively extracted using negative pressure and irrigation fluid when the ureteroscope is withdrawn (22). Owing to these features, the use of SUASs led to a significant improvement in immediate SFR. Conversely, no significant difference was found in follow-up SFR. One possible explanation for this is the spontaneous passage of residual fragments. The studies included in our analysis demonstrated that approximately 40–70% of cases experienced spontaneous stone passage (14-18). It should be noted that postoperative residual fragments are more likely to result in stone-related events compared to patients who are stone-free (23). Therefore, even though the improvement was observed only in immediate SFR, it can still contribute to a reduction in the patient’s subsequent potential stone-related events burden.
Second, our analyses showed a significant decrease in operation time when using SUAS with approximately 8-minute reduction. SUASs, which can maintain continuous irrigation, allow for the aspiration of remnant during the procedure, thereby ensuring a clearer surgical field and maintaining a better operative view (18). Additionally, the suctional function efficiently retrieves small fragments, allowing only the larger remaining fragments to be collected with a basket if necessary. Therefore, integrating a better surgical view and reducing the workload of retrieving the stone fragments can contribute to time savings (16). Whether eight minutes are clinically relevant remains to be reflected on. In a high-volume clinical setting with multiple URS/RIRS, eight minutes can be highly cost-effective.
Finally, our analysis revealed that the use of SUASs significantly reduced the risk of overall complications and fever compared to the non-SUAS group by 51% and 63%, respectively. Previous reports showed that intra-renal pressure (IRP) during URS/RIRS may be associated with subsequent adverse events. Additionally, IRP has been identified as a risk factor for postoperative infectious complications after the procedure (24,25). Croghan et al. (26) assessed the impact of IRP during URS/RIRS using 120 cases. They found that patients who developed postoperative sepsis had significantly higher IRP, with a mean of 71.16 mmHg, compared to 38.62 mmHg in those who did not develop sepsis. While a ureteroscope that can measure IRP has been released, maintaining appropriate low IRP is another important aspect of successful URS/RIRS (27). The use of SUASs allows for the maintenance of IRP levels via suctional function, potentially reducing the risk of fever. Additionally, a prospective multicenter, single-arm study by Gauhar et al. (28), which included the largest cohorts of SUAS-assisted RIRS cases to date (n=394), reported no cases of sepsis. Contrary to our expectations, our analysis revealed no significant difference in the risk of septic shock. One possible explanation for the lack of significant difference is the small number of events, which resulted in low statistical power. Although we also conducted an analysis using Peto’s method, which is recommended for the meta-analysis of rare events (29), no significant difference was also found [odds ratio (OR): 0.42; 95% CI: 0.13–1.37; P=0.1] (Figure S6). Upon examining each study in detail, three out of the four analyzed reports showed that the incidence of septic shock was significantly lower with the use of SUASs, while in the remaining report, there were no cases of septic shock in either group. The limited number of studies included in our meta-analysis may also have led to the lack of significant findings. Additionally, no significant differences were found in the risk of ureteral perforation and stricture (Figure S7), which are also rare but crucial complications. Further studies with a sufficient number of events are needed to clarify the risk of these rare complications.
Limitations
The present study has several limitations that should be considered. First, this meta-analysis included studies with varied patient populations and stone characteristics. Furthermore, even within the category of SUASs, there are differences among the products. Notably, the SUAS used in the studies by Huang et al. (16) and Zhang et al. (15) feature a flexible tip, allowing for more efficient suction of fragmentations near the stones. Additionally, variability in operating room environments and individual surgeons’ experience or preferences may also have influenced the surgical outcomes. Second, the definition of stone free and the imaging modalities used to evaluate residual fragments varied across the studies. Additionally, the imaging methods used for SFR assessment varied, even within the immediate and 1-month follow-up SFR assessment, potentially introducing bias. Third, in the comparisons involving URS, access sheaths were not used in the non-SUAS group. This difference prevented the pure impact of SUASs from being examined. Fourth, while we evaluated hospital stay, it is a subjective endpoint that depends on institutional practices and regional healthcare systems. Therefore, this outcome should be interpreted with caution. Fifth, the included studies did not perform adequate subgroup analyses to determine which specific patient populations would benefit most from the use of SUASs. Finally, there is only one RCT concerning SUASs; therefore, we included retrospective cohort studies in our analyses. However, most of the included retrospective cohort studies did not employ adjustments such as propensity score matching or inverse probability of treatment weighting, thus failing to minimize bias. These limitations highlight the need for large, well-designed RCTs to provide more robust evidence.
Conclusions
Our analysis revealed that the use of SUASs improved immediate SFR with favorable perioperative outcomes compared to the standard approach without SUASs. These findings suggest that URS/RIRS using SUASs could be a preferred method in clinical practice for patients requiring these procedures. Large, well-designed RCTs are needed to provide stronger evidence regarding clinically significant outcomes such as septic shock and surgeon satisfaction/perception.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the AMSTAR2 and the PRISMA reporting checklists. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-138/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-138/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-138/coif). S.F.S. has received honoraria from Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, and Takeda; has served in a consulting or advisory role for Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, and Takeda; and has participated in speakers bureau for Astellas, AstraZeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, and Takeda. T.K. is a paid consultant/advisor and has received honoraria for lectures and presentations from Astellas, Bayer, Janssen and Sanofi. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kachkoul R, Touimi GB, El Mouhri G, et al. Urolithiasis: History, epidemiology, aetiologic factors and management. Malays J Pathol 2023;45:333-52.
- Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol 2014;192:316-24. [Crossref] [PubMed]
- Türk C, Petřík A, Sarica K, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol 2016;69:475-82. [Crossref] [PubMed]
- Gauhar V, Chew BH, Traxer O, et al. Indications, preferences, global practice patterns and outcomes in retrograde intrarenal surgery (RIRS) for renal stones in adults: results from a multicenter database of 6669 patients of the global FLEXible ureteroscopy Outcomes Registry (FLEXOR). World J Urol 2023;41:567-74. [Crossref] [PubMed]
- Wang Q, Guo J, Hu H, et al. Rigid ureteroscopic lithotripsy versus percutaneous nephrolithotomy for large proximal ureteral stones: A meta-analysis. PLoS One 2017;12:e0171478. [Crossref] [PubMed]
- Haupt G, Pannek J, Herde T, et al. The Lithovac: new suction device for the Swiss Lithoclast. J Endourol 1995;9:375-7. [Crossref] [PubMed]
- Li P, Huang Z, Sun X, et al. Comparison of Vacuum Suction Sheath and Non-Vacuum Suction Sheath in Minimally Invasive Percutaneous Nephrolithotomy: A Meta-Analysis. J Invest Surg 2022;35:1145-52. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008.
- Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147-53. [Crossref] [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. [Crossref] [PubMed]
- Wang L, Zhou Z, Gao P, et al. Comparison of traditional and suctioning ureteral access sheath during retrograde intrarenal surgery in the treatment of renal calculi. Langenbecks Arch Surg 2024;409:81. [Crossref] [PubMed]
- Zhang Z, Xie T, Li F, et al. Comparison of traditional and novel tip-flexible suctioning ureteral access sheath combined with flexible ureteroscope to treat unilateral renal calculi. World J Urol 2023;41:3619-27. [Crossref] [PubMed]
- Huang J, Yang Y, Xie H, et al. Vacuum-assisted dedusting lithotripsy in the treatment of kidney and proximal ureteral stones less than 3 cm in size. World J Urol 2023;41:3097-103. [Crossref] [PubMed]
- Qian X, Liu C, Hong S, et al. Application of Suctioning Ureteral Access Sheath during Flexible Ureteroscopy for Renal Stones Decreases the Risk of Postoperative Systemic Inflammatory Response Syndrome. Int J Clin Pract 2022;2022:9354714. [Crossref] [PubMed]
- Zhu Z, Cui Y, Zeng F, et al. Comparison of suctioning and traditional ureteral access sheath during flexible ureteroscopy in the treatment of renal stones. World J Urol 2019;37:921-9. [Crossref] [PubMed]
- Du C, Song L, Wu X, et al. A study on the clinical application of a patented perfusion and suctioning platform and ureteral access sheath in the treatment of large ureteral stones below L4 level. Int Urol Nephrol 2019;51:207-13. [Crossref] [PubMed]
- Zhai Q, Zhang J, Wei Q, et al. Clinical application of novel integrated suctioning semi-rigid ureteroscopic lithotripsy. Minim Invasive Ther Allied Technol 2023;32:314-22. [Crossref] [PubMed]
- Chen Y, Zheng L, Lin L, et al. A novel flexible vacuum-assisted ureteric access sheath in retrograde intrarenal surgery. BJU Int 2022;130:586-8. [Crossref] [PubMed]
- Huang J, Xie D, Xiong R, et al. The Application of Suctioning Flexible Ureteroscopy With Intelligent Pressure Control in Treating Upper Urinary Tract Calculi on Patients With a Solitary Kidney. Urology 2018;111:44-7. [Crossref] [PubMed]
- Hein S, Miernik A, Wilhelm K, et al. Endoscopically Determined Stone Clearance Predicts Disease Recurrence Within 5 Years After Retrograde Intrarenal Surgery. J Endourol 2016;30:644-9. [Crossref] [PubMed]
- Tokas T, Herrmann TRW, Skolarikos A, et al. Pressure matters: intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J Urol 2019;37:125-31. [Crossref] [PubMed]
- Xu Y, Min Z, Wan SP, et al. Complications of retrograde intrarenal surgery classified by the modified Clavien grading system. Urolithiasis 2018;46:197-202. [Crossref] [PubMed]
- Croghan SM, Cunnane EM, O'Meara S, et al. In vivo ureteroscopic intrarenal pressures and clinical outcomes: a multi-institutional analysis of 120 consecutive patients. BJU Int 2023;132:531-40. [Crossref] [PubMed]
- Bhojani N, Koo KC, Bensaadi K, et al. Retrospective first-in-human use of the LithoVue™ Elite ureteroscope to measure intrarenal pressure. BJU Int 2023;132:678-85. [Crossref] [PubMed]
- Gauhar V, Traxer O, Castellani D, et al. Could Use of a Flexible and Navigable Suction Ureteral Access Sheath Be a Potential Game-changer in Retrograde Intrarenal Surgery? Outcomes at 30 Days from a Large, Prospective, Multicenter, Real-world Study by the European Association of Urology Urolithiasis Section. Eur Urol Focus 2024;10:975-82. [Crossref] [PubMed]
- Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health 2018;21:72-6. [Crossref] [PubMed]


