
Clinical outcomes of modified partial cystectomy in muscle-invasive bladder cancer: balancing tumor control and quality of life
Highlight box
Key findings
• This study introduces a modified partial cystectomy (MPC) combining the advantages of laparoscopic and open techniques for bladder preservation in muscle-invasive bladder cancer (MIBC).
• MPC demonstrates superior postoperative outcomes to laparoscopic partial cystectomy (LPC) and similar outcomes to radical cystectomy (RC), while preserving bladder function and quality of life.
What is known and what is new?
• Conventional approaches, such as LPC and open partial cystectomy, have limitations in tumor resection, prevention of tumor spread, and performance of ureteral reimplantation (UR) or bladder reconstruction.
• Our improvements include the following: (I) preoperative bladder intravesical chemotherapy, intraoperative gauze padding, and distilled water irrigation are employed to reduce tumor cell dissemination; (II) laparoscopic techniques are used for comprehensive bladder mobilization and systematic pelvic lymph node dissection (PLND), minimizing intraoperative damage and enhancing staging accuracy; (III) open surgical techniques are applied for UR and bladder reconstruction in tumors near the ureteral orifice, ensuring complete resection with margins >2 cm. These modifications, combined with postoperative adjuvant therapy, improve tumor control and overall therapeutic outcomes.
What is the implication, and what should change now?
• MPC offers tumor control comparable to RC with fewer complications, faster recovery, and improved quality of life.
• MPC can be implemented in patients with single, localized tumors (≤5 cm), no carcinoma in situ, and clear surgical margins.
• MPC can be integrated as an alternative approach into multimodal treatment strategies, including combinations with radiation therapy and chemotherapy.
• Future studies should further validate the clinical benefits and applicability of MPC in the treatment of bladder cancer.
Introduction
Muscle-invasive bladder cancer (MIBC) is a highly malignant urological tumor, ranking eleventh in global incidence and fourteenth in mortality (1). Radical cystectomy (RC), the current gold standard treatment, effectively controls tumors but severely impacts patients’ physiological functions and quality of life, driving a shift towards bladder-sparing strategies (2,3). Trimodality therapy (TMT), a mainstream bladder-sparing approach, which combines transurethral resection, chemotherapy, and radiotherapy, shows comparable efficacy to RC but faces challenges due to its complexity and dependence on multidisciplinary cooperation (4,5).
For a carefully selected group of patients, surgical bladder-sparing methods like transurethral resection of bladder tumor (TURBT) or partial cystectomy (PC) can be effective (6). PC, compared to TURBT, offers advantages such as complete bladder wall removal and pelvic lymph node dissection (PLND), enabling more comprehensive tumor resection and accurate staging (7,8). Laparoscopic partial cystectomy (LPC), a minimally-invasive bladder-sparing technique, provides a clear view and facilitates certain procedures but has limitations in precisely localizing and excising tumors in complex positions, leading to difficulties in ensuring clean margins and performing post-excision reconstruction.
In this study, we aimed to evaluate a modified version of LPC, named modified partial cystectomy (MPC), which integrates laparoscopic access with open surgical advantages. Our study aimed to conduct a retrospective analysis to evaluate the feasibility and short-term oncological and functional outcomes of MPC in patients with MIBC, compared to conventional LPC and RC, focusing on perioperative parameters, oncological control, and postoperative quality of life, with the hope of offering a new treatment option. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-243/rc).
Methods
This retrospective cohort study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Guangzhou Medical University (No. 2024-ZN034) and conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Informed consent was obtained from all patients. Patients who underwent RC or PC at the Affiliated Cancer Hospital of Guangzhou Medical University between January 2020 and January 2022 were identified. A complete TURBT, along with random biopsies, was performed preoperatively to exclude multifocal disease or an association with carcinoma in situ (CIS). PC was carried out either laparoscopic or with our modification of combined laparoscopic and open technique. Tumor location does not influence the decision for PC, while tumor size (≤5 cm) is a factor in patient selection, though not a strict criterion. Ureteral reimplantation (UR) is performed when needed.
Inclusion criteria and group allocation
Patients were considered eligible if they met the following criteria: (I) normal preoperative bladder function; (II) histopathologically confirmed pure urothelial carcinoma (UC), excluding UC with variant histology (adenocarcinoma, sarcomatoid, etc.); (III) primary or solitary tumor with clinical staging of T2 or T3; (IV) no evidence of lymph node metastasis on imaging; (V) no evidence of CIS; (VI) no other malignancies.
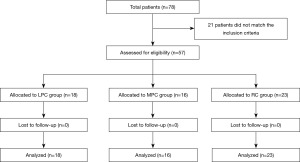
All surgeries were performed by the same lead surgeon with experienced assistants, ensuring consistency and standardization of the procedures. The study included 57 patients: 16 underwent MPC with PLND, 18 underwent LPC, and 23 underwent RC with PLND and urinary diversion (UD).
For RC patients, preoperative preparation included mechanical bowel preparation, while postoperative recovery followed an enhanced recovery after surgery (ERAS) protocol, with a standard nutritional regimen initiated upon starting oral feeding.
Study design, data collection and outcome measures
A retrospective cohort study of two groups of patients who underwent PC using one of the two techniques mentioned above was conducted. The PC groups were compared with a gold standard group of patients who had the same selection criteria for PC but underwent standard RC and PLND. We excluded patients with incomplete perioperative or follow-up (Figure 1).
We recorded preoperative data, including age, gender, estimated glomerular filtration rate (eGFR), hemoglobin levels, body mass index (BMI), chronic conditions (e.g., hypertension, diabetes), American Society of Anesthesiologists (ASA) score, prior abdominal surgery, tumor location and size, and neoadjuvant chemotherapy. Intraoperative data included operation time, complications, estimated blood loss, and UR.
The three groups were compared in terms of postoperative complications, functional, pathological and oncological outcomes. Postoperative complications were identified and categorized according to the Clavien-Dindo classification, and the timing of postoperative feeding, drainage and catheter removal was determined. We recorded pathological features including T- and N-staging, and surgical margin status. The International Prostate Symptom Score (IPSS) (9) and the Functional Assessment of Cancer Therapy-Bladder Bladder Symptom Scale (FACT-BL-BBS) (10) were used to assess functional outcomes. Recurrence and survival were used to compare the oncological outcome.
Preoperatively, surgeons should counsel patients on the potential need to convert from PC to RC based on intraoperative findings. It is crucial to emphasize that RC remains the gold standard treatment and that, even in highly selected patients, long-term oncological outcomes with PC may not be equivalent to those of RC. The following section will outline the key steps of the surgical technique.
LPC
The patients were operated on under general anesthesia. First, an 18-Fr Foley catheter was inserted to drain the bladder and the patient was placed in the Trendelenburg position. The procedure was started by creating a pneumoperitoneum and exposing the bladder. A 2-cm incision was made above the umbilicus, through which a 10-mm trocar was inserted along with the laparoscope. Next, at approximately two finger widths below the umbilicus, the external edge of the rectus abdominis muscle was punctured to insert 12- and 10-mm trocars. Finally, two 5-mm trocars were inserted at the level of the iliac crest near the midline, two finger widths from the umbilicus. Once the location of the tumor was confirmed, we selected a site distant from the tumor for the incision, taking care to ensure that it was suitable for optimal exposure of the bladder. Using an ultrasonic scalpel, a full resection was performed along the tumor margin, maintaining a safety margin of 1–2 cm. The tumor specimen was placed in the bag immediately after resection. The bladder wall was then closed in two layers. We use absorbable sutures to perform a two-layer water tight closure of the bladder. Pelvic drainage tube was left into the retropubic space.
MPC (Figure 2)
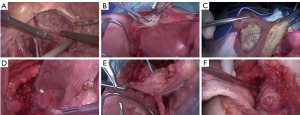
First, an 18-Fr Foley catheter was inserted into the bladder, then 100 mg of mitomycin dissolved in 100 mL of saline was injected. The catheter was then firmly clamped to ensure that no drug leaked out. Under general anesthesia, the patient was positioned and the trocar was inserted according to the standard procedure for LPC. During the procedure, intestinal adhesions were first loosened with the laparoscope to fully expose the surgical area in the pelvic region. A standard PLND was then performed up to the common iliac bifurcation. Once the PLND was complete, the peritoneum of the bladder tip and anterior wall was incised to fully expose the bladder and ureters. The operation was then performed via an open approach. Small abdominal incision was then performed to expose the bladder. The anterior bladder wall was pulled with tissue forceps to prevent the tumor seeding. An electric scalpel was then used to make a small incision in the bladder wall away from the tumor and the chemotherapy fluid was suctioned out using a suction device. The surrounding organs were shielded with gauze to prevent contamination by the bladder fluid or the tumor. The incision was then enlarged to fully expose the tumor margin, and a complete resection was performed with at least a 2-cm safety margin. In cases where tumor margins were difficult to locate, an Olympus flexible cystoscope was inserted through the urethra to provide intracavitary imaging and guide tumor localization using light source guidance on the imaging platform. An intraoperative frozen section was performed to ensure negative margins. After resection, the wound was irrigated with sterile distilled water. If the ureteral orifice was affected during tumor resection, the distal portion of the ureter was surgically clamped, and the tumor was resected together with the ureteral orifice. After resection, UR with stent placement was carried out. Finally, the bladder and abdominal walls were closed, leaving an 18-Fr Foley catheter and a pelvic drain.
Laparoscopic RC plus PLND plus ileal conduit
Laparoscopic cystectomy was performed according to the standard protocol. After bladder resection, an ileal segment formed a UD, and the ureters were implanted into the ileum and drained via the abdominal wall, necessitating a postoperative urine collection bag.
Neoadjuvant chemotherapy and postoperative treatment
Regarding the use of neoadjuvant chemotherapy, it was generally recommended for patients with T2–4a stage disease, but the decision to administer it was based on the individual patient’s preferences. For patients with postoperative pathology indicating pT3–4 and N+, we considered using the gemcitabine and cisplatin regimen for adjuvant chemotherapy.
Statistical Analysis
Statistical analysis was executed via SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data were expressed as the mean ± standard deviation and were compared via the t-test; nonnormally distributed data were expressed as the median and interquartile range and were analyzed through the Wilcoxon test. Categorical data are expressed as frequency (percentage) and were tested through the Chi-squared test, continuity correction, or the Fisher’s exact test. Survival curves were constructed using the Kaplan-Meier method, with group comparisons made via the log-rank test. The statistical significance level was set at P<0.05.
Results
Basic patient data
A total of 57 patients were enrolled: 16 in the MPC group, 18 in the LPC group, and 23 in the RC group. The majority (73.7%) were male, 68.4% had clinical T2 disease, and 22.8% received neoadjuvant chemotherapy. The median age was highest in the LPC group (72.5 years), compared to 66.5 years in MPC and 65 years in RC (P=0.007). Tumor locations differed significantly in RC (39.13% trigonal), while LPC and MPC tumors mainly occurred on the lateral wall. Tumor size distribution was as follows: for tumors <3 cm, 37.50% were in MPC, 72.22% in LPC, and 60.87% in RC; for tumors 3–5 cm, 62.50% were in MPC, 27.78% in LPC, and 34.78% in RC; for tumors >5 cm, 4.35% were in RC. Other baseline characteristics, including gender, BMI, and staging, did not differ among groups (Table 1).
Table 1
| Variables | LPC (n=18) | MPC (n=16) | RC (n=23) | P | P (LPC vs. MPC) |
|---|---|---|---|---|---|
| Gender | 0.98 | 0.91 | |||
| Male | 13 (72.22) | 12 (75.00) | 17 (73.91) | ||
| Female | 5 (27.78) | 4 (25.00) | 6 (26.09) | ||
| Age (years) | 72.5 [68.0, 76.5] | 66.5 [64.0, 71.3] | 65 [59.5, 71.0] | 0.007* | 0.02* |
| Preoperative eGFR (mL/min/1.73 m2) | 68.89 [65.6, 82.2] | 75.78 [72.59, 98.73] | 73.41 [69.13, 87.85] | 0.87 | 0.29 |
| Preoperative hemoglobin (g/dL) | 13.37 [12.2, 14.7] | 13.53 [12.3, 14.9] | 13.58 [11.6, 16] | 0.79 | 0.63 |
| Preoperative BMI (kg/m2) | 27.06 [24.6, 28.5] | 26.89 [24.6, 28.3] | 26.98 [25.1, 28.2] | 0.88 | 0.65 |
| Clinical stage | 0.98 | 0.93 | |||
| T2 | 12 (66.67) | 11 (68.75) | 16 (69.57) | ||
| ≥T3 | 6 (33.33) | 5 (31.25) | 7 (30.43) | ||
| Chronic disease | 9 (50.00) | 8 (50.00) | 11 (47.83) | 0.99 | >0.99 |
| ASA score | 0.21 | 0.80 | |||
| 2 | 8 (44.44) | 7 (43.75) | 4 (17.39) | ||
| 3 | 7 (38.89) | 8 (50.00) | 16 (69.57) | ||
| 4 | 3 (16.67) | 1 (6.25) | 3 (13.04) | ||
| Prior abdominal surgery | 0.60 | 0.55 | |||
| Yes | 3 (16.67) | 5 (31.25) | 6 (26.09) | ||
| No | 15 (83.33) | 11 (68.75) | 17 (73.91) | ||
| Tumor location | 0.042 | 0.13 | |||
| Trigone | 1 (5.56) | 3 (18.75) | 9 (39.13) | ||
| Lateral wall | 8 (44.44) | 10 (62.50) | 9 (39.13) | ||
| Anterior wall | 9 (50.00) | 3 (18.75) | 5 (21.74) | ||
| Tumor size (cm) | <0.001* | 0.09 | |||
| <3 | 13 (72.22) | 6 (37.50) | 14 (60.87) | ||
| 3–5 | 5 (27.78) | 10 (62.50) | 8 (34.78) | ||
| >5 | 0 | 0 | 1 (4.35) | ||
| Neoadjuvant chemotherapy | 0.87 | 0.88 | |||
| Yes | 4 (22.22) | 3 (18.75) | 6 (26.09) | ||
| No | 14 (77.78) | 13 (81.25) | 17 (73.91) |
Data are presented as n (%) or median [interquartile range]. *, statistically significant difference. ASA, American Society of Anesthesiology; BMI, body mass index; eGFR, estimated glomerular filtration rate; LPC, laparoscopic partial cystectomy; MPC, modified partial cystectomy; RC, radical cystectomy.
Perioperative and pathological outcome
RC patients had longer operation times, more blood loss, and extended recovery periods compared to the PC groups. Major complication rates were 21.74% in RC, versus 5.56% in LPC and 6.25% in MPC (P<0.001). Ureter reimplantation was required in 75.00% of MPC patients. A proportion of 16.67% of LPC patients had positive surgical margins, compared to none in MPC and RC (Table 2).
Table 2
| Variables | LPC (n=18) | MPC (n=16) | RC (n=23) | P | P (LPC vs. MPC) |
|---|---|---|---|---|---|
| Operation time (min) | 206.94±5.98 | 191.13±6.55 | 337.78±10.71 | <0.001* | 0.24 |
| Estimated blood loss (mL) | 175.0 [150.0, 200.0] | 150 [137.5, 200.0] | 500.0 [500.0, 600.0] | <0.001* | 0.51 |
| Time to postoperative feeding (days) | 2.0 [2.0, 3.0] | 2.0 [2.0, 3.0] | 6.0 [6.0, 7.0] | <0.001* | 0.53 |
| Time to drainage tube removal (days) | 6.0 [5.0, 6.0] | 6.5 [6.0, 7.0] | 15.0 [14.0, 15.5] | <0.001* | 0.08 |
| Time to catheter removal (days) | 14.0 [13.0, 15.0] | 15.0 [14.0, 15.0] | N/A | 0.22 | |
| Length of stay (days) | 7.0 [6.0, 8.0] | 7.5 [7.0, 8.0] | 16.0 [15.0, 17.0] | <0.001* | 0.24 |
| Clavien-Dindo complications | <0.001* | 0.70 | |||
| Grade 1 | 6 (33.33) | 7 (43.75) | 2 (8.70) | ||
| Grade 2 | 3 (16.67) | 2 (12.50) | 16 (69.57) | ||
| Grade 3 or higher | 1 (5.56) | 1 (6.25) | 5 (21.74) | ||
| Ureteric reimplantation | N/A | 12 (75.00) | 23 (100.00) | – | – |
| Postoperative adjuvant chemotherapy | 4 (22.22) | 5 (31.25) | 3 (13.04) | – | 0.67 |
| Postoperative T stage | 0.97 | 0.88 | |||
| T2 | 11 (61.11) | 10 (62.50) | 14 (60.87) | ||
| T3 | 6 (33.33) | 6 (37.50) | 8 (34.78) | ||
| T4 | 1 (5.56) | 0 | 1 (4.35) | ||
| Postoperative N stage | – | – | |||
| N0 | N/A | 14 (87.50) | 20 (86.96) | ||
| N1 | N/A | 2 (12.50) | 3 (13.04) | ||
| N2 or higher | N/A | 0 | 0 | ||
| Tumor margin | 0.03* | 0.42 | |||
| Positive | 3 (16.67) | 0 | 0 | ||
| Negative | 15 (83.33) | 16 (100.00) | 23 (100.00) |
Data are presented as mean ± standard deviation, median [interquartile range], or n (%). N/A: there are no data available for this item. *, statistically significant difference. LPC, laparoscopic partial cystectomy; MPC, modified partial cystectomy; RC, radical cystectomy.
Functional and oncological outcome
MPC and LPC had similar IPSS scores. Both had higher FACT-BL-BBS scores than RC (P<0.001). The median follow-up time was 18 months. After 36 months of follow-up, 34.78% of patients with RC and 33.33% patients with LPC experienced disease relapse, versus 6.25% patients with MPC (P=0.19). The 3-year recurrence-free survival was significantly higher in MPC than in LPC (P=0.049) and RC (P=0.034) (Figure 3), while 3-year overall survival (OS) was comparable among groups (Table 3).
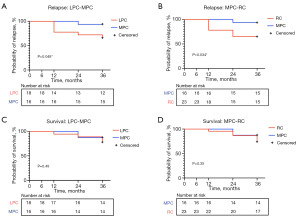
Table 3
| Variables | LPC (n=18) | MPC (n=16) | RC (n=23) | P | P (LPC vs. MPC) |
|---|---|---|---|---|---|
| IPSS | 7.0 [7.0, 9.0] | 7.5 [7.0, 9.3] | N/A | – | 0.70 |
| FACT-BL-BBS score | 28.5 [28.0, 30.0] | 28.5 [28.0, 30.0] | 24.0 [22.0, 25.5] | <0.001* | 0.67 |
| Recurrence | 6 (33.33) | 1 (6.25) | 8 (34.78) | 0.10 | 0.19 |
| Local recurrence | 5 (27.78) | 1 (6.25) | 5 (21.74) | – | – |
| Systemic recurrence | 1 (5.56) | 0 | 3 (13.04) | – | – |
| Alive (36 months) | 14 (77.78) | 14 (87.50) | 17 (73.91) | 0.59 | 0.65 |
| Dead (36 months) | 4 (22.22) | 2 (12.50) | 6 (26.09) | – | – |
Data are presented as median [interquartile range] or n (%). N/A: there are no data available for this item. *, statistically significant difference. FACT-BL-BBS, Functional Assessment of Cancer Therapy-Bladder Bladder Symptom Scale Bladder-Specific Signature; IPSS, International Prostate Symptom Score; LPC, laparoscopic partial cystectomy; MPC, modified partial cystectomy; RC, radical cystectomy.
Discussion
RC remains the gold standard for treating MIBC. However, bladder-preserving surgical techniques, such as PC, have gained increasing attention in recent years (11,12). Initially criticized for uncertain therapeutic outcomes, a study now suggests that MIBC patients undergoing PC may face a higher risk of recurrence, and those who experience recurrence are unlikely to achieve the same curative results as with RC, leading to poorer clinical outcomes (13). Consequently, research on bladder-preserving treatments has been limited. In the 1970s and 1980s, PC had a 5-year survival rate ranging from 25–48%, with local recurrence rates as high as 54–78%, resulting in a decline in its use among urologists (14,15). A study indicated that only 3–10% of MIBC patients meet the criteria for PC, and its application accounts for just 7–10% of all bladder cancer surgeries (16), highlighting the clinical challenges it faces. However, with more defined patient selection criteria for PC in recent years (e.g., a single lesion located on the anterior bladder wall, no concomitant CIS, and tumor margins greater than 2 cm), the 5-year survival rate has improved to 53.7–70%, and the local recurrence rate has decreased to 18.5–38%, making the therapeutic outcomes of PC comparable to those of RC (17-21).
A key advantage of PC is its ability to excise the entire bladder layer. Compared to TURBT, PC offers several benefits: it effectively removes tumors located in challenging areas, such as bladder diverticula or visual blind spots; ensures thorough excision of tumors invading the ureter or those near the ureteral orifice; and provides a clear surgical margin, facilitating UR. Additionally, PC involves PLND, which enhances the accuracy of pathological staging (22). However, PC has limitations, including the risk of tumor dissemination, less precise resection, and a higher recurrence rate, which have contributed to skepticism about its use.
Open PC is a classic surgical technique suitable for patients with localized tumors in favorable positions. However, for complex or deeply located tumors, the difficulty and risks of the procedure increase, as larger incisions result in more tissue damage and prolonged recovery times (23). With the growing use of laparoscopic techniques, the application of open PC has decreased. Nevertheless, open surgery provides superior visualization and a larger working space, ensuring more precise tumor excision. These advantages have been demonstrated in rare but successful cases. For example, Nhungo et al. reported favorable outcomes using open PC to treat muscle-invasive squamous cell carcinoma of the bladder, highlighting the oncological and functional safety of this approach in carefully selected patients (24).
Robotic surgery offers advantages, but its high technical demands and costs limit its widespread use. As a result, laparoscopic surgery remains the preferred approach in many regions. Laparoscopic techniques provide clear visualization and excel in bladder triangle dissection and pelvic lymph node removal. However, traditional LPC encounters challenges in accurately localizing tumors located on the lateral and posterior walls or near the ureteral orifice. This difficulty hinders complete tumor excision and achieving clear margins. Additionally, bladder reconstruction and UR following tumor removal become more complex. Although innovations such as green lasers and dye injections have enhanced tumor localization and excision precision (25,26), their clinical application is still limited due to issues such as the availability of markers, additional training requirements, and obstacles to widespread clinical implementation.
In this study, all surgeries were performed by the same surgical team, ensuring consistency and standardization of techniques while minimizing potential biases from operator variability. Moreover, we integrated laparoscopic techniques with the advantages of open surgery in MPC, effectively overcoming the limitations of traditional laparoscopic approaches. Laparoscopic techniques facilitated lymph node dissection and bladder mobilization, providing clear visualization and simplifying the procedure. Upon transitioning to open surgery, MPC offers several advantages in tumor localization, notably through the use of preoperative electrocautery markers and palpation, which ensure precise tumor identification. In cases where tumor margins are difficult to locate, an Olympus flexible cystoscope can be inserted through the urethra to provide intracavitary imaging, guiding tumor localization with light source guidance on the imaging platform. This method is simple, cost-effective, and improves precision while preventing tumor perforation, ensuring adequate margins, and minimizing bladder incision size. For certain patients, intraoperative frozen-section margin analysis may be necessary to confirm a safe margin (>2 cm). In cases involving the ureteral orifice or bladder trigone, resection of the affected area and UR may be required. Previous study has demonstrated that UR reduces recurrence and improves prognosis (27). We believe that these advantages extend the applicability of MPC, particularly for patients with a single lesion and tumor diameter less than 5 cm. However, for tumors located near the ureteral orifice, RC remains the preferred treatment.
Preoperative bladder perfusion chemotherapy reduces the risk of tumor dissemination (28) due to urine leakage or other factors during surgery. In this study, we performed mitomycin bladder perfusion with ureteral occlusion, followed by aspiration of bladder fluids after incising the bladder wall. After tumor resection, continuous irrigation with sterile distilled water was performed. Additionally, gauze pads were used to protect surrounding tissues, preventing contamination from the fluids and effectively reducing the risk of tumor spread.
Chemotherapy effectively reduces tumor size, eliminates residual tumor cells and metastatic lesions, downstages tumors, and decreases the risk of recurrence and metastasis, ultimately prolonging patient survival (29). Although novel low-toxicity systemic drugs, such as anti-PD-1, anti-CTLA-4, and FGFR3 inhibitors, may offer improved outcomes, these treatments were not explored in this study (30,31). Neoadjuvant chemotherapy is currently recommended for patients with T2–T4aN0M0 stage tumors. However, some patients, due to resistance to chemotherapy, opt for surgical treatment. For pT3–4 or N+ patients who have not received neoadjuvant chemotherapy, postoperative adjuvant chemotherapy may help extend disease-free survival (DFS) and OS (32). In our study, some low-risk patients did not receive chemotherapy, likely due to limited chemotherapy benefits, individualized treatment decisions, and patient compliance. While this study is retrospective and treatment protocols may lack standardization, we maintain that chemotherapy plays a pivotal role in improving prognosis.
PLND plays a pivotal role in the management of MIBC by assisting in postoperative pathological staging and reducing the risk of tumor recurrence. Lymph node metastasis is frequently present at the time of diagnosis in MIBC patients, with a metastatic risk exceeding 20%. According to Thalmann’s study, the 5-year survival rate for MIBC patients undergoing RC with PLND ranges from 58% to 68% (33), whereas the 5-year survival rate for those treated with PC varies between 67% and 90% (34,35). While the National Comprehensive Cancer Network (NCCN) guidelines recommend routine PLND for MIBC management, historical practices show inconsistency in its application, particularly among patients undergoing PC, especially with laparoscopic approaches aimed at minimizing surgical trauma (36). In our study, the LPC group did not undergo PLND, likely to minimize trauma and recovery time. In contrast, all MPC patients received PLND, resulting in promising outcomes. The recurrence rate in the MPC group was 6.25%, compared to 33.33% in the LPC group, with 36-month survival rates of 87.5% and 77.78%, respectively. These findings underscore the superior oncological outcomes and patient survival associated with MPC. Our study bridges this gap by incorporating standardized PLND into MPC, thereby improving staging accuracy and reducing recurrence risk.
This study has several inherent limitations. First, the retrospective design, small sample size, short follow-up period, and intra-group heterogeneity may affect the accuracy and interpretation of oncological outcomes. Significant variations in baseline characteristics (such as tumor size, treatment protocols, and surgical techniques) increased the complexity of inter-group comparisons, making it challenging to control for these factors and complicating the evaluation of treatment effectiveness. Additionally, while all surgeries were performed by the same lead surgeon, which helped standardize procedures, this limitation reduces the external validity and reproducibility of the study. Variability in surgeon skills and clinical settings could lead to outcome differences, limiting the generalizability of the findings. Therefore, future studies should focus on more homogeneous patient populations or prospective controlled trials to better assess the impact of treatment variables on oncological outcomes and further validate the clinical benefits and applicability of MPC in bladder cancer treatment.
Despite these limitations, which constrain the broader use of MPC, it offers significant advantages for carefully selected patients with a single tumor, no CIS, and clear tumor margins, particularly in terms of bladder preservation and functional outcomes. Integrating MPC into TMT and refining surgical techniques could enhance treatment results and quality of life for this patient group.
Conclusions
In our study, MPC demonstrated lower recurrence and complication rates compared to LPC, while offering comparable survival outcomes to RC. These results suggest that MPC may serve as a viable bladder-preserving alternative in carefully selected patients with MIBC. Therefore, further prospective studies with larger, homogeneous populations and longer follow-up are needed to evaluate the long-term efficacy and safety of MPC in MIBC management.
Acknowledgments
The authors thank all the volunteers and staff involved in this research.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-243/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-243/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-243/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-243/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Ethics Committee of the Affiliated Cancer Hospital of Guangzhou Medical University (No. 2024-ZN034). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. [Crossref] [PubMed]
- Adamczyk P, Pobłocki P, Kadlubowski M, et al. Complication Rate after Radical Cystectomy Depends on the Surgical Technique and Patient's Clinical Status. Urol Int 2022;106:163-70. [Crossref] [PubMed]
- Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol 2012;61:705-11. [Crossref] [PubMed]
- Inamoto T, Ibuki N, Komura K, et al. Can bladder preservation therapy come to the center stage? Int J Urol 2018;25:134-40. [Crossref] [PubMed]
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin 2020;70:404-23. [Crossref] [PubMed]
- Ebbing J, Heckmann RC, Collins JW, et al. Oncological outcomes, quality of life outcomes and complications of partial cystectomy for selected cases of muscle-invasive bladder cancer. Sci Rep 2018;8:8360. [Crossref] [PubMed]
- Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol 2004;172:878-81. [Crossref] [PubMed]
- Ölçücü MT, Aydın ME, Avcı S, et al. Comparison of a Visual Prostate Symptom Score and International Prostate Symptom Score: A Prospective Multicenter Study and Literature Review. Urology 2020;146:230-5. [Crossref] [PubMed]
- Degboe A, Ivanescu C, Rohay JM, et al. Validity and performance of the Functional Assessment of Cancer Therapy-Bladder (FACT-Bl) among advanced urothelial cancer patients. Support Care Cancer 2019;27:4189-98. [Crossref] [PubMed]
- Koga F, Kihara K, Yoshida S, et al. Selective bladder-sparing protocol consisting of induction low-dose chemoradiotherapy plus partial cystectomy with pelvic lymph node dissection against muscle-invasive bladder cancer: oncological outcomes of the initial 46 patients. BJU Int 2012;109:860-6. [Crossref] [PubMed]
- Konieczkowski DJ, Efstathiou JA, Mouw KW. Contemporary and Emerging Approaches to Bladder-Preserving Trimodality Therapy for Muscle-Invasive Bladder Cancer. Hematol Oncol Clin North Am 2021;35:567-84. [Crossref] [PubMed]
- Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol 2017;14:221-34. [Crossref] [PubMed]
- Fosså SD, Odegaard A, Kaalhus O. Partial cystectomy followed by postoperative irradiation in treatment of bladder carcinoma (P2/P3). Eur Urol 1981;7:150-6. [Crossref] [PubMed]
- Peress JA, Waterhouse K, Cole AT. Complications of partial cystectomy in patients with high grade bladder carcinoma. J Urol 1977;118:761. [Crossref] [PubMed]
- Girardi DM, Ghatalia P, Singh P, et al. Systemic therapy in bladder preservation. Urol Oncol 2023;41:39-47. [Crossref] [PubMed]
- Smaldone MC, Jacobs BL, Smaldone AM, et al. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology 2008;72:613-6. [Crossref] [PubMed]
- Golombos DM, O'Malley P, Lewicki P, et al. Robot-assisted partial cystectomy: perioperative outcomes and early oncological efficacy. BJU Int 2017;119:128-34. [Crossref] [PubMed]
- Leveridge MJ, Siemens DR, Izard JP, et al. Partial cystectomy for urothelial carcinoma of the bladder: Practice patterns and outcomes in the general population. Can Urol Assoc J 2017;11:412-8. [Crossref] [PubMed]
- Knoedler J, Frank I. Organ-sparing surgery in urology: partial cystectomy. Curr Opin Urol 2015;25:111-5. [Crossref] [PubMed]
- Pon Avudaiappan A, Prabhakar P, Lusnia C, et al. A comparative study of survival outcomes between partial and radical cystectomy in octogenarians with muscle-invasive bladder cancer. Transl Androl Urol 2024;13:1486-97. [Crossref] [PubMed]
- Sun S, Wang H, Zhang X, et al. Transurethral Resection of Bladder Tumor: Novel Techniques in a New Era. Bladder (San Franc) 2023;10:e21200009. [Crossref] [PubMed]
- Albisinni S, Veccia A, Aoun F, et al. A systematic review and meta-analysis comparing the outcomes of open and robotic assisted radical cystectomy. Minerva Urol Nefrol 2019;71:553-68. [Crossref] [PubMed]
- Nhungo CJ, Lori JM, Kashaija JM, et al. Favorable outcome of open partial cystectomy for muscle-invasive squamous cell carcinoma of the bladder: A case report and literature review. Clin Case Rep 2024;12:e9019. [Crossref] [PubMed]
- Fan J, Wu K, Zhang P, et al. Green-laser assisted laparoscopic partial cystectomy for selective muscle-invasive bladder cancer: technique and initial outcome. World J Urol 2019;37:2671-5. [Crossref] [PubMed]
- Kim BK, Song MH, Yang HJ, et al. Use of cystoscopic tattooing in laparoscopic partial cystectomy. Korean J Urol 2012;53:401-4. [Crossref] [PubMed]
- Ma B, Li H, Zhang C, et al. Lymphovascular invasion, ureteral reimplantation and prior history of urothelial carcinoma are associated with poor prognosis after partial cystectomy for muscle-invasive bladder cancer with negative pelvic lymph nodes. Eur J Surg Oncol 2013;39:1150-6. [Crossref] [PubMed]
- O'Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 2011;60:703-10. [Crossref] [PubMed]
- Sternberg CN, Pansadoro V, Calabrò F, et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer 2003;97:1644-52. [Crossref] [PubMed]
- Janisch F, Rink M, Shariat SF. The promise and challenges of neoadjuvant immunotherapy in the management of non-metastatic muscle-invasive bladder cancer. BJU Int 2020;125:753-5. [Crossref] [PubMed]
- Abel M, Burkenroad A, Sun A, et al. The Evolving Landscape of Antibody-Drug Conjugates for Urothelial Carcinoma. Clin Genitourin Cancer 2021;19:183-93. [Crossref] [PubMed]
- Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [Crossref] [PubMed]
- Thalmann G. Editorial comment on: characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur Urol 2010;57:309. [Crossref] [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Mistretta FA, Cyr SJ, Luzzago S, et al. Partial Cystectomy With Pelvic Lymph Node Dissection for Patients With Nonmetastatic Stage pT2-T3 Urothelial Carcinoma of Urinary Bladder: Temporal Trends and Survival Outcomes. Clin Genitourin Cancer 2020;18:129-137.e3. [Crossref] [PubMed]


