
Association of sperm DNA fragmentation with higher miscarriage rates in non-male factor infertility reproductive cycles
Highlight box
Key findings
• Men whose spouses experienced recurrent spontaneous abortion (RSA) had a significantly higher sperm DNA fragmentation (SDF) index (DFI) as compared to controls.
• DFI was positively correlated with age and negatively associated with sperm concentration, total motility, normal morphology, and progressive motility.
• Occupational factors were associated with sperm motility, while lifestyle factors (smoking, alcohol, sleep duration) demonstrated no significant association.
What is known and what is new?
• SDF has been linked to impaired reproductive outcomes, but its specific role in RSA remains controversial.
• Our findings indicate that higher sperm DFI is associated with RSA in non-male factor infertility cycles, emphasizing the impact of paternal factors on pregnancy loss.
What is the implication, and what should change now?
• Sperm DNA integrity should be considered in RSA evaluations, even in cases without clear male infertility.
• Age-related sperm DNA damage suggests that early reproductive planning and fertility counseling for men may be beneficial.
• Improving workplace environments may help enhance semen quality and reduce the risk of RSA.
Introduction
Recurrent spontaneous abortion (RSA), defined as the occurrence of two or more lost pregnancies in succession with the same partner (including biochemical pregnancy), is considered one of the most common pregnancy complications. RSA affects up to 5% of women of childbearing age (1) and has become a globally significant issue. A combination of thyroid dysfunction, structural and anatomical irregularities, endocrine imbalances, immune-related disruptions, genetic predisposition, coagulation disorders, and infectious agents plays a key role in this multifactorial disease (2). RSA is a pathological pregnancy that can result in infertility, cardiovascular disease, and venous thromboembolism. It has been linked with psychological issues, including anxiety, depression, and posttraumatic stress disorder (3). Women with RSA may have an elevated risk of pregnancy-associated issues, such as preeclampsia, fetal growth restriction, and premature birth as compared to those with normal fertility (4). Effective therapeutic modalities for RSA are currently scarce, highlighting the clinical significance of identifying reliable strategies for early diagnosis and treatment to reduce its incidence.
Concerning the clinical diagnosis of male factors contributing to RSA, routine semen analysis only assesses the parameters of motility, morphology, and sperm concentration. However, these conventional indicators offer only incomplete predictions of semen quality (5). It is important that DNA integrity be maintained in sperm cells to ensure the accuracy of the genetic information transferred. Loss of integrity impairs this process and has been found to adversely influence the formation and division of fertilized eggs and embryonic development, resulting in infertility and habitual abortion (6,7).
Among the various parameters in the sperm chromatin structure assay (SCSA), the sperm DNA fragmentation (SDF) index (DFI) is regarded as the most reliable measure of DNA integrity and potential damage in the sperm. This approach demonstrates superior diagnostic value compared to traditional routine semen analysis (8,9). Moreover, several systematic reviews have established a significant association between elevated sperm DFI and increased risk of pregnancy loss in both spontaneous and assisted conception, suggesting that higher rates of SDF may contribute to miscarriage (10). A meta-analysis further confirmed that couples experiencing RSA exhibit a higher level of SDF than do fertile couples, suggesting that sperm DFI may be an indicator for RSA (11). However, other studies have reported that SDF levels do not significantly differ between couples experiencing RSA and those with normal fertility (12,13). Thus, there is no consensus regarding the value of DNA integrity assessments in RSA diagnosis and outcome prediction. Further research is required to determine whether sperm DNA integrity correlates with RSA.
This study used SCSA to analyze sperm DFI in 438 RSA cases at a single center to assess the potential of DFI in predicting pregnancy outcome in RSA. Furthermore, the significance of other factors with SDF, including lifestyle variables (smoking and drinking), occupation, and routine semen parameters, was examined to assess the value of SDF in evaluating male fertility in RSA and its influencing factors. We present this article in accordance with the STARD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-322/rc).
Methods
Participants and data
RSA was defined as pregnancy loss before 28 weeks of gestation that occurred at least twice with the same spouse and could include biochemical pregnancies. This study enrolled 438 males (aged 24–50 years) whose spouses had received diagnoses of RSA between January 2022 and October 2023 in the Outpatient Department of Reproductive Medicine at the Second People’s Hospital of Wuhu (the experimental group). The inclusion criteria were (I) healthy married adult males; (II) couples with a regular, healthy sex life; and (III) spouses diagnosed with RSA. The exclusion criterion was males using medications that could affect semen test results within the previous 6 months. In addition, 183 males (aged 24–50 years) whose spouses had normal fertility in the previous year without RSA were enrolled as the controls. This study collected data on lifestyle factors from all participants, including age, occupation, smoking habits, alcohol consumption, sleep duration, abstinence time, and occupational status. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Institutional Review Board of the Second People’s Hospital of Wuhu (No. SZ2021002) approved the study, and all patients provided informed consent.
Collection and evaluation of semen specimen
The methods for semen collection and analysis were carried out following the World Health Organization’s (WHO) 2010 recommendations, with minor adjustments. In our Andrology Laboratory, patients were instructed to masturbate and collect semen into a preweighed, sterile, wide-mouthed cup that was previously tested for compatibility with the human sperm collection in a container after 3–5 days of abstinence. The patient’s name and time of semen collection were documented using a marker. After color was assessed, the sperm volume was determined by weighing the specimen in the sperm cup with an electronic scale, while the measurement of the pH was conducted on an extensive precision test strip.
The sperm cup was incubated in a thermostatic water bath at 37 ℃, and a wet specimen was prepared after 20 min to determine whether the semen was completely liquefied. Sperm not completely liquefied after 60 min were recorded as abnormally liquefied. In this case, liquefaction was promoted by mechanical mixing or digestion with bromelain. The viscosity of the specimen was measured, and abnormalities were recorded after complete liquefaction.
Moreover, a preliminary microscopic examination was conducted to evaluate the aggregation or agglutination of the sperm. After being mixed, the semen specimens were subjected to auxiliary analysis using an SQA-V automated semen quality analyzer (Medical Electronic Systems, CA, USA) to assess various parameters, including semen concentration and motility. Following transient centrifugation of the semen, the sperm was resuspended and smeared onto a glass slide for optical microscopy (1,000×) to evaluate sperm morphology after Diff-Quik fixation staining. The reference values for semen quality analysis were established with strict adherence to the WHO 2010 guidelines as follows: semen volume ≥1.5 mL, pH 7.2–8.0, sperm concentration ≥15×106/mL, total sperm number ≥39×106, total motility ≥40%, progressive motility ≥32%, and normal form ≥4%. A semen specimen was defined as normal if it satisfied all of the above criteria and abnormal if it did not satisfy any of the criteria.
Measurement of DFI
The DFI was evaluated using SCSA, and semen (10 µL; final concentration 2×106 cells/mL) was added to 90 µL of Tris-NaCl-EDTA buffer (Abcam, Cambridge, UK). Subsequently, 200 µL of a pH 1.2 buffer (NaCl, Triton X-100) was added and mixed gently. Following a precise timing of 30 s, 600 µL of 0.0006% (v/v) acridine orange in phosphate buffer was introduced. The prepared samples were examined within a time frame of 30 min. A flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA, USA) was used to analyze the samples. Two independent measurements were performed for each sample, with at least 10,000 cell particles being analyzed in each instance. Following acid treatment and staining with acridine orange, the sperm samples demonstrated red fluorescence due to single-stranded DNA fragments with irregular chromatin structures. Conversely, a green fluorescence was emitted when intact double-stranded DNA came into contact with acridine orange. The proportion of red-fluorescing sperm relative to the overall sperm count was used to determine the sperm DFI.
Statistical analysis
Data were analyzed with SPSS 26.0 (IBM Corp., Armonk, NY, USA). Normally distributed variables are shown as the mean ± standard deviation. Independent samples t-tests were conducted when data satisfied the assumption of variance homogeneity, whereas nonparametric rank-sum tests were used for nonnormally distributed variables. The Spearman rank correlation coefficient and linear regression analyses were performed to assess relationships among various factors. Moreover, receiver operating characteristic (ROC) curves were used to examine sensitivity, specificity, and the most suitable cutoff value for predicting risk factors associated with abnormal sperm DFI. Statistical significance was defined as a P value <0.05.
Results
Comparison of routine semen parameters and sperm DFI between the two groups
The experimental and control groups were comparable in terms of normal forms, abstinence duration, volume of semen, concentration of sperm, and progressive and total sperm motility. The DFI values were significantly higher in the experimental group than in the control group (Table 1; P=0.002), indicating a significant association between DNA integrity and the RSA.
Table 1
| Item | Experimental group (n=444) | Control group (n=183) | Z | P |
|---|---|---|---|---|
| DFI (%) | 18.20 (14.42, 23.63) | 15.92 (11.26, 23.1) | −3.147 | 0.002 |
| Normal form (%) | 3.37 (2.40, 5.77) | 4.15 (2.41, 5.51) | −0.50 | 0.96 |
| Abstinence time (days) | 3.00 (3.00, 4.00) | 3.00 (3.00, 4.00) | −0.97 | 0.92 |
| Semen volume (mL) | 3.40 (2.40, 4.36) | 3.30 (2.30, 4.23) | −0.912 | 0.36 |
| Sperm concentration (×106/mL) | 65.80 (37.20, 99.30) | 70.60 (39.53, 99.30) | −0.551 | 0.58 |
| Progressive motility (%) | 36.00 (31.00, 44.00) | 36.00 (30.00, 45.00) | −0.06 | 0.95 |
| Total motility (%) | 53.00 (45.25, 60.00) | 53.00 (45.00, 59.00) | −1.015 | 0.31 |
Data are presented as median (P25, P75). DFI, DNA fragmentation index.
Correlation analysis of age and sperm quality
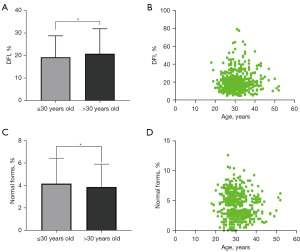
The participants were classified into two subgroups based on age (≤30 and >30 years). Sperm DFI in the subgroup ≤30 years old is lower than that in the subgroup >30 years old (Figure 1A; P=0.01). A positive relationship between DFI and age was indicated in the Spearman correlation analysis (Figure 1B; P=0.03), with older males demonstrating a higher sperm DFI. However, no significant differences were observed in sperm concentrations, total sperm numbers, sperm motility, or progressive motility between the two subgroups. The ≤30-year subgroup demonstrated a significantly higher rate of normal form than did the >30-year group (Figure 1C; P=0.02). Moreover, a negative association was observed between age and normal form (Figure 1D; P=0.01). Since some of these clinical variables were potential confounding factors, age was considered the independent variable in the linear regression analysis. The findings revealed a significant association of age with sperm DFI and normal form (P<0.05), indicating that age might influence sperm quality.
Analysis of correlation and difference between lifestyle and sperm quality factors
In the Spearman correlation analysis, there were negative correlations between sperm DFI and sperm concentration (P=0.04), total motility (P<0.001), normal form (P<0.001), and progressive motility (P<0.001). However, sperm DFI did not correlate with semen volume (P=0.45) or total sperm count (P=0.07) (Table 2 and Figure 2). These data suggest that sperm DFI correlates to varying degrees with routine semen parameters.
Table 2
| Sperm parameter | Sample (n) | R | P |
|---|---|---|---|
| Semen volume (mL) | 627 | 0.030 | 0.45 |
| Sperm concentration (×106/mL) | 627 | −0.083 | 0.04 |
| Total motility (%) | 627 | −0.240 | <0.001 |
| Total sperm number (×106/mL) | 627 | −0.071 | 0.07 |
| Normal form (%) | 627 | −0.350 | <0.001 |
| Progressive motility (%) | 627 | −0.281 | <0.001 |
DFI, DNA fragmentation index.

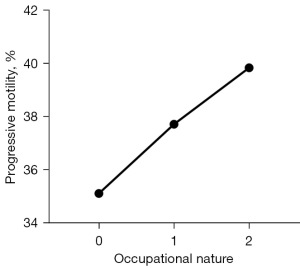
The analysis revealed no significant differences in sperm concentration, total sperm number, semen volume, progressive motility, normal morphology, or total motility between the subgroups categorized by sleep duration, alcohol consumption, and smoking (P>0.05). Participants were classified into three distinct labor types (mental labor, manual labor, and military work or law enforcement). No significant differences were identified in semen volume, sperm concentration, total motility, total sperm number, or normal sperm form between these groups (P>0.05). However, significant variations in progressive motility were found between participants with different occupation types. Furthermore, occupation was positively linked with progressive motility (r=0.100, P<0.05). Workers in sedentary occupations had significantly lower progressive motility than did those in other occupations (Figure 3; P<0.05).
ROC curve determinations of the predictive value of risk factors for sperm DFI
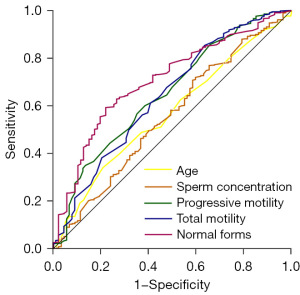
To assess the predictive factors for sperm DFI, multivariate logistic regression was applied, with DFI ≤30 being the dependent variable and age, sperm concentration, progressive motility, total motility, and normal form being the predictors. The findings demonstrated that these variables were significantly associated with a DFI diagnosis (P<0.05). However, the diagnostic accuracy was relatively low, with cutoff values for age, sperm concentration, progressive motility, total motility, and normal form being 19.5 years, 42.9×106/mL, 33.5%, 44.5%, and 3.31%, respectively; the corresponding sensitivities were 0.977, 0.708, 0.601, 0.853, and 0.593, respectively, while the specificities were 0.002, 0.414, 0.614, 0.363, and 0.779, respectively. The analysis of the ROC curve indicated that the area under the curve (AUC) values ranged from 0.568 to 0.706, demonstrating the good predictive value of age (P=0.03), sperm concentration (P=0.01), progressive motility (P<0.001), total motility (P<0.001), and normal form (P<0.001) for sperm DFI (Figure 4).
Discussion
In-depth research on the etiology and intervention targets of RSA suggests a potential link between male sperm quality and RSA incidence. A comprehensive meta-analysis revealed that the partners of patients with RSA had markedly reduced sperm concentration, vitality, and normal form in comparison to those with normal fertility (7). Another study reported significantly higher progressive motility and sperm abnormalities associated with RSA relative to normal controls (14). However, Eisenberg et al. identified no obvious relationship between RSA and semen parameters in their observational study (15). In our study, a comparison of an RSA group and a healthy control group revealed no significant variations in normal sperm morphology, semen volume, concentration, or progressive or total motility (P>0.05). This implies that routine semen parameters are inadequate for accurately assessing male fertility in the examination of the male pathogenic factors for RSA. Therefore, further research is needed to examine the roles and mechanisms of male factors in embryonic development. This would allow for the identification of more specific sperm detection indicators to clarify the complex relationship between sperm quality and RSA.
It is widely recognized that 50% of the genetic material in progeny originates from the father, emphasizing the importance of sperm DNA, the carrier of human genetic material, in facilitating embryonic development and pregnancy outcomes by ensuring complete transmission of genetic information to the offspring (16). Although protamine encloses most sperm DNA, a portion is located at the periphery and is susceptible to oxidative damage. Notably, a considerable proportion of SDF irreparable by the oocyte can result in anomalous embryonic growth and spontaneous abortion following the activation of the paternal genome. In clinical practice, the sperm DFI serves as a key measure for evaluating sperm DNA quality. According to Bhattacharya, DNA integrity in sperm is not significantly associated with sperm parameters (17).
In contrast, other researchers have reported there to be a negative correlation between SDF and routine semen parameters such as sperm morphology, concentration, and progressive motility (18). In line with this, our study found the same negative correlations between sperm DFI and sperm concentration, total motility, the normal form, and progressive motility. This suggests that a higher probability of SDF is associated with inferior-quality sperm parameters. A study has confirmed there to be a strong correlation between sperm DFI and RSA, indicating higher rates of RSA in the spouses of males with a higher DFI than in those with a lower DFI (19). Aitken proposed high DFI as a potential risk factor for RSA (20). In our study, the DFI values were markedly higher in the RSA group relative to the control group. The sperm DFI can thus be used as an indicator of RSA risk.
Lower semen quality and fertility have been observed in older males. In our study, we found that as age increased, there was a decrease in the proportion of morphologically normal sperm, while the DFI value increased. Previous research suggests that aging in males contributes to a lower proportion of normal sperm, with a sharp reduction observed after 35 years of age (21). This may be explained by the diminishing antioxidant capacity of sperm, overproduction of reactive oxygen species (ROS), and age-related rise in oxidative stress resulting in a decrease in the abundance of normal form (22). A few studies have reported there to be a higher likelihood of DNA damage in older men and that advancing age has an adverse effect on sperm chromatin integrity (23,24).
An increase in age may induce excessive ROS production and decrease antioxidant capacity, leading to oxidative DNA injury (25,26). Markers of oxidative stress, such as malondialdehyde (MDA), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and total antioxidant capacity (TAC), have been closely associated with impaired sperm function and reduced DNA integrity (27). It has been reported that high levels of markers for oxidative stress in men are associated with poor semen quality and high DFI (28). These results hint that oxidative stress biomarkers may contribute to explain the biological mechanism of this association between poor sperm quality and RSA. Moreover, older age may alter the permeability of the mitochondrial membrane in the sperm, reducing the mitochondrial membrane potential, promoting DNA fragmentation, and eventually producing a higher DFI (29). Normal sperm form and DNA integrity are essential for sperm development and have profound implications for fertilization, embryonic development, implantation, and pregnancy outcomes. Overall, age is a critical factor for assessing the risk factors associated with fertility issues and unfavorable pregnancy outcomes in males.
Among various lifestyle and environmental factors, occupational exposure has emerged as a significant contributor to male fertility. Exposure to higher temperatures, toxins, and workplace pollutants can reduce sperm concentration, total motility, and abundance of normal form (30). This study demonstrated that sedentary employees experience a decline in progressive motility. It has been previously hypothesized that the physiological temperature of human testes is crucial for normal sperm production and that sedentary work obstructs blood flow in the groin area, resulting in increased testicular temperature. Heat stress hinders sperm production and decreases inhibin B levels, thereby reducing sperm quality (31,32). In contrast, appropriate exercise to reduce sedentary behavior can enhance antioxidant capacity to mitigate the effect of oxidative stress on sperm quality (33). Therefore, a long-term sedentary occupation represents a potential risk factor for decreased progressive motility, thereby increasing the risk of RSA.
In this study, ROC curve analysis confirmed that male age, sperm concentration, progressive motility, total motility, and normal sperm form were independently associated with sperm DFI. Although statistically significant associations were observed, the AUC values for sperm DFI in this study ranged from 0.568 to 0.706, indicating limited utility as a standalone predictive test. These findings suggest that sperm DFI alone may not serve as a highly reliable independent predictor for RSA (34,35). Instead, it should be considered in combination with other clinical parameters—such as age, sperm morphology, and oxidative stress markers—to improve risk stratification and predictive accuracy. Further large-scale, prospective studies are warranted to validate the incremental predictive value of DFI within an integrated risk assessment model for RSA.
This study still has certain limitations. Since this study mainly focused on the fathers and did not consider the maternal variables, including age, hormonal status, uterine abnormalities, or immune mechanisms. This may limit the full adjustment of all possible confounding factors contributing to RSA. Future studies should collect comprehensive data from both parents and use multivariate models to sort out the relative impacts of fathers and mothers on RSA. Additionally, this was a single-center study conducted in southern Anhui, China, which may limit the generalizability of the findings to broader populations with different ethnic, environmental, or lifestyle characteristics. While oxidative stress is recognized as a key factor contributing to sperm DNA damage, direct measurements of oxidative stress biomarkers were not performed, which limits mechanistic insights.
Conclusions
The established correlation between sperm chromatin structural integrity and RSA suggests that a reduced SDF rate may decrease RSA incidence. In addition, age may affect sperm quality, with normal morphology and compromised DNA integrity intensifying as men age. Given the potential adverse influence of older paternal age on fertility, preventive and treatment strategies for RSA should account for age. Furthermore, occupational exposure was also found to be associated with sperm quality. Therefore, the identified risk factors should be integrated into clinical practice to prevent, diagnose, and treat RSA.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-322/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-322/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-322/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-322/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study protocol was reviewed and approved by the Institutional Review Board of the Second People’s Hospital of Wuhu (No. SZ2021002). Informed consent was provided by all participants when they were enrolled.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deng T, Liao X, Zhu S. Recent Advances in Treatment of Recurrent Spontaneous Abortion. Obstet Gynecol Surv 2022;77:355-66. [Crossref] [PubMed]
- Zhang Y, Feng M, Gao Y, et al. Depression outcome in women with recurrent spontaneous abortion: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2024;300:54-62. [Crossref] [PubMed]
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021;397:1658-67. [Crossref] [PubMed]
- Fee N, McEvoy A, Cullen S, et al. Pregnancy outcomes following recurrent miscarriage. Ir J Med Sci 2023;192:2255-8. [Crossref] [PubMed]
- Pourmasumi S, Sabeti P, Rahiminia T, et al. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int J Reprod Biomed 2017;15:331-44.
- Yao G, Dou X, Chen X, et al. Association between sperm DNA fragmentation index and recurrent pregnancy loss: results from 1485 participants undergoing fertility evaluation. Front Endocrinol (Lausanne) 2024;15:1493186. [Crossref] [PubMed]
- Li J, Luo L, Diao J, et al. Male sperm quality and risk of recurrent spontaneous abortion in Chinese couples: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24828. [Crossref] [PubMed]
- Liu K, Mao X, Pan F, et al. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci Rep 2023;13:2717. [Crossref] [PubMed]
- Qiu Y, Yang H, Li C, et al. Progress in Research on Sperm DNA Fragmentation. Med Sci Monit 2020;26:e918746. [Crossref] [PubMed]
- Malić Vončina S, Stenqvist A, Bungum M, et al. Sperm DNA fragmentation index and cumulative live birth rate in a cohort of 2,713 couples undergoing assisted reproduction treatment. Fertil Steril 2021;116:1483-90. [Crossref] [PubMed]
- Tan J, Taskin O, Albert A, et al. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online 2019;38:951-60. [Crossref] [PubMed]
- Coughlan C, Clarke H, Cutting R, et al. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl 2015;17:681-5. [Crossref] [PubMed]
- Best JC, Kohn T, Patel P, et al. Elevated sperm DNA fragmentation does not predict recurrent implantation failure. Andrologia 2021;53:e14094. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Eisenberg ML, Sapra KJ, Kim SD, et al. Semen quality and pregnancy loss in a contemporary cohort of couples recruited before conception: data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril 2017;108:613-9. [Crossref] [PubMed]
- Raheem OA, Ana M, Urol M, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2017;6:S322-3. [Crossref] [PubMed]
- Bhattacharya SM. Association of various sperm parameters with unexplained repeated early pregnancy loss--which is most important? Int Urol Nephrol 2008;40:391-5. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril 2010;94:1748-52. [Crossref] [PubMed]
- Andrabi SW, Ara A, Saharan A, et al. Sperm DNA Fragmentation: causes, evaluation and management in male infertility. JBRA Assist Reprod 2024;28:306-19. [Crossref] [PubMed]
- Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol 2017;6:S761-4. [Crossref] [PubMed]
- Demirkol MK, Barut O, Dogan NT, et al. At What Age Threshold does the Decline in Semen Parameters Begin? J Coll Physicians Surg Pak 2021;31:4-7. [Crossref] [PubMed]
- Gunes S, Hekim GN, Arslan MA, et al. Effects of aging on the male reproductive system. J Assist Reprod Genet 2016;33:441-54. [Crossref] [PubMed]
- Lahimer M, Montjean D, Cabry R, et al. Paternal Age Matters: Association with Sperm Criteria's- Spermatozoa DNA Integrity and Methylation Profile. J Clin Med 2023;12:4928. [Crossref] [PubMed]
- Mohammed LM, Maged YM. Does advanced paternal age affect global DNA methylation of human spermatozoa and intracytoplasmic sperm injection outcome? J Turk Ger Gynecol Assoc 2023;24:18-27. [Crossref] [PubMed]
- Xie D, Lu C, Zhu Y, et al. Analysis on the association between sperm DNA fragmentation index and conventional semen parameters, blood microelements and seminal plasma ROS in male patients with infertility. Exp Ther Med 2018;15:5173-6. [Crossref] [PubMed]
- Lira FT. From pathophysiology to practice: addressing oxidative stress and sperm DNA fragmentation in Varicocele-affected subfertile men. Int Braz J Urol 2024;50:530-60. [Crossref] [PubMed]
- Soria-Meneses PJ, Jurado-Campos A, Gómez-Rubio V, et al. Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA Animals (Basel) 2022;12:3286. [Crossref] [PubMed]
- Collodel G, Moretti E, Micheli L, et al. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology 2015;3:280-6. [Crossref] [PubMed]
- Koppers AJ, De Iuliis GN, Finnie JM, et al. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008;93:3199-207. [Crossref] [PubMed]
- Zhang X, Fan Z, Wang Q, et al. Association between ambient temperature and semen quality among sperm donation volunteers in South China. Environ Int 2023;173:107809. [Crossref] [PubMed]
- Rao M, Xia W, Yang J, et al. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology 2016;4:1054-63. [Crossref] [PubMed]
- Jensen TK, Andersson AM, Hjollund NH, et al. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab 1997;82:4059-63. [Crossref] [PubMed]
- Zańko A, Siewko K, Krętowski AJ, et al. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. Int J Environ Res Public Health 2022;20:732. [Crossref] [PubMed]
- Jiang H, Xia X, Luo Y, et al. Sperm DNA fragmentation index: limited effectiveness on predicting embryo quality in assisted reproduction technology treatments. Reprod Biol Endocrinol 2025;23:14. [Crossref] [PubMed]
- Zhu C, Zhang S, Chen F, et al. Correlations between elevated basal sperm DNA fragmentation and the clinical outcomes in women undergoing IUI. Front Endocrinol (Lausanne) 2022;13:987812. [Crossref] [PubMed]
(English Language Editor: J. Gray)


