
Adjustable continence therapy for men (ProACTTM): systematic review and compendium of adverse events
Highlight box
Key findings
• The most common adverse events (AEs) associated with adjustable continence therapy for men (ProACTTM) are mechanical failure, device migration/malposition, and device erosion. Devices were explanted in 24% of patients and revised or reimplanted in 28% of cases.
What is known and what is new?
• Individual case series have summarized AEs related to ProACTTM.
• This systematic review summarizes the published literature on the device’s safety profile. We also summarize “real-world” AEs through analysis of the Manufacturer and User Facility Device Experience database.
What is the implication, and what should change now?
• Use of the ProACTTM device is associated with a high rate of AEs. This data should be used to educate risk/benefits discussions with patients considering utilizing the device.
Introduction
Stress urinary incontinence (SUI) is a growing problem facing our aging population (1). SUI results from surgery for prostate cancer or benign prostatic hypertrophy, the most common non-cutaneous cancer and benign urologic disease in men, respectively (2). SUI rates increase as men grow older, with prevalence in men aged 65+ years ranging from 10–30% (1).
Treatment options are limited for severe male SUI. Though male urethral slings and artificial urinary sphincters (AUS) are effective treatments for many men with incontinence, these devices require open perineal surgery and are not adjustable once they are surgically placed. The adjustable continence therapy for men (ProACTTM) has been developed as an alternative treatment for male SUI and is also an effective treatment option per American Urologic Association (AUA) guidelines (3). This device is an inflatable para-urethral balloon approved for consumer use in the United States by the Food and Drug Administration (FDA) in 2015 (Figure 1). The device compresses the urethra passively and offers a unique advantage compared to other SUI interventions by allowing inflation adjustment to optimize continence for each patient (4-6).
Pre-approval safety reports highlight side effects of ProACT device including urinary retention, pain, ongoing incontinence, device migration, and bladder perforation (7-9). After FDA approval, adverse events (AEs) can be submitted to the Manufacturer and User Facility Device Experience (MAUDE) database, through which voluntary and mandated reporters can submit anonymous reports of real-world device complications. Reviews of MAUDE reports regarding other urologic technologies have uncovered unforeseen, severe complications related to post-approval device use (10,11). The current study systematically reviews all published safety data related to ProACT in both literature and the MAUDE database. The work will further inform implanters and patients of the risks of device placement. We present this article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-587/rc).
Methods
Review of published safety data
Bibliometric search engines PubMed and Google Scholar were used to identify peer-reviewed articles related to ProACT implantation in humans using the combination of keywords “ProACT”, “balloon”, and “continence” on October 15, 2023. Search results dated from first-in-human studies published in 2005 through December 2023, the time of the search. Potential studies were screened by one author (A.M.F.). Conference abstracts, articles not in English, and studies with overlapping populations were excluded. If two publications included overlapping populations, the article with fewer patients was excluded.
We reviewed selected studies in detail by one author (A.M.F.) for safety data related to ProACT implantation. We extracted total number of patients treated, length of follow-up, balloons placed, complications sustained, and post-implantation procedures related to the ProACT placement. AEs documented included surgical site infection, device erosion into nearby structures (urethra, bladder, rectum, skin), ProACT migration away from its site of placement, pain, urinary retention, hematuria, and perforation of adjacent organs (bladder, urethra, colon) on placement of the device. Post-implantation procedures documented included device explantation, ProACT reimplantation, bladder catheterization, cystoscopy, suprapubic tube placement, and any other procedures mentioned. Severity of AEs were categorized using the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 (12).
MAUDE database review
MAUDE reports were identified on October 14, 2023 by searching the MAUDE database (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm) for AEs that related to the placement of ProACT. Injuries related to the Proact Transfer Sling, a patient mobility device, were excluded. ProACTTM MAUDE narratives spanned from November 2018 through December 2023. Narratives were reviewed individually by two authors (A.M.F. and either K.D.L., U.G., or J.B.) to reduce risk of bias. All AEs and post-implantation procedures described were catalogued in a similar fashion to the AEs reviewed in the published literature.
Statistical analysis
Descriptive statistics were arranged and computed using Microsoft Excel, version 16.75 to evaluate safety data, including results from published literature and the MAUDE database.
The study protocol is available upon request, and no amendments were made after initiation of the protocol.
Results
Published AEs
Thirty-one peer-reviewed publications were identified using our search criteria. Eleven studies were excluded: seven because they reported on overlapping populations, three because full text versions could not be found in English, and one because it studied simultaneous implant of ProACT with another device. Ultimately 20 peer-reviewed publications were analyzed (Figure 2, Table S1) (7-9,13-29).
One thousand six hundred and seven patients were treated with ProACTTM in published studies. Seven hundred and fifty-two AEs were identified, yielding an AE rate of 47% of patients. The most common AEs in published studies were mechanical failure (n=224, 30%), device migration/malposition (n=155, 21%), device erosion (n=120, 16%), surgical site infection (n=62, 8%), urethral perforation (n=48, 6%), bladder perforation (n=46, 6%), urinary retention (n=35, 5%), and scrotal hematoma (n=20, 3%). Eight hundred and twenty-five post-implantation procedures were described, consisting of device revision or reimplantation (n=442, 54%) and device explantation (n=383, 46%) (Tables 1,2). See Table S1 for AE rates published in each study.
Table 1
| AE | N [%] |
|---|---|
| Published literature | |
| Total | 752 [100] |
| Mechanical defect/failure | 224 [30] |
| Device malposition/migration | 155 [21] |
| Device erosion (any) | 120 [16] |
| Surgical site/device infection | 62 [8] |
| Urethral perforation | 48 [6] |
| Bladder perforation | 46 [6] |
| Urinary retention | 35 [5] |
| Other/unspecified | 29 [4] |
| Hematoma/bleeding | 20 [3] |
| Pain | 12 [2] |
| MAUDE database | |
| Total | 80 [100] |
| Surgical site/device infection | 15 [19] |
| Urinary retention | 9 [11] |
| Erosion to bladder | 9 [11] |
| Erosion to skin | 9 [11] |
| Erosion to urethra | 8 [10] |
| Other† | 7 [9] |
| Unspecified erosion | 6 [10] |
| Bladder perforation | 4 [7] |
| Unspecified perforation | 4 [7] |
| Device malposition/migration | 4 [7] |
| Hematoma/bleeding | 3 [5] |
| Rectal erosion | 2 [3] |
†, “Other” AEs identified in the MAUDE database included hematuria (n=2, 3%), pain (n=2, 3%), urinary tract infection (n=1, 1%), urinary urgency (n=1, 1%), and unspecified issue with the device (n=1, 1%). AE, adverse event; MAUDE, Manufacturer and User Facility Device Experience; ProACT, adjustable continence therapy.
Table 2
| Procedure | N [%] |
|---|---|
| Published literature | |
| Total | 825 [100] |
| Device reimplantation or revision | 442 [54] |
| Device explantation | 383 [46] |
| MAUDE database | |
| Total | 65 [100] |
| Device explantation | 37 [57] |
| Foley catheterization | 10 [15] |
| Device reimplantation | 9 [14] |
| Cystoscopy | 5 [8] |
| Suprapubic tube placement | 2 [3] |
| Bowel diversion | 1 [2] |
| Unspecified | 1 [2] |
MAUDE, Manufacturer and User Facility Device Experience; ProACT, adjustable continence therapy.
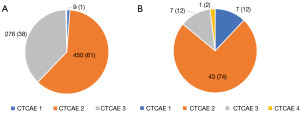
Fourteen AEs could not be graded according to the CTCAE system due to lack of clinical details. Of the remaining 738 AEs identified, 1.2% were CTCAE grade 1 (n=9), 61% grade 2 (n=450), and 37% grade 3 (n=276). There were 1 and 2 events that were grade 4 and 5, respectively, which were deemed unrelated to ProACT placement (Figure 3).
Post-approval AEs
Fifty-eight MAUDE reports were identified that related to the placement of ProACT. In the MAUDE database, 80 AEs were identified. The most common AEs identified were surgical site or device infection (n=15, 19%); urinary retention (n=9, 11%); device erosion to bladder (n=9, 11%); skin (n=9, 11%); urethra (n=8, 10%); or other location (n=6, 10%); bladder perforation on device placement (n=4, 7%); device malposition/migration (n=4, 7%); hematoma formation (n=3, 5%); rectal erosion (n=2, 3%); and unspecified/other AEs (n=7, 9%). “Perforation” differs from “erosion” in that perforation occurs at device placement, while erosion occurs afterward.
Sixty-five post-implantation procedures were described including device explantation (n=37, 57%), bladder catheterization (n=10, 15%), ProACTTM reimplantation (n=9, 14%), cystoscopy (n=5, 8%), suprapubic tube placement (n=2, 3%), and one unspecified procedure (n=1, 2%). MAUDE database complications also include one event of rectal perforation on device placement necessitating colostomy.
Of the AEs described in the MAUDE database, 12% were CTCAE grade 1, 74% grade 2, 12% grade 3, 2% grade 4, and 0% grade 5 (Figure 3).
Discussion
The current work summarizes the complications associated with placement of ProACT. We include injuries noted in both the literature and in the post-approval, “real world” setting. Complications are common and are predominantly categorized as CTCAE grade 1 or 2.
Across all ProACT implants reported in academic literature, the most common AEs in were mechanical failure (14% of implants), device malposition/migration (10%), and device erosion (7%). Device infection rate was 4% across all cases, and the bladder and urethra were each perforated in 3% of implants. These rates should inform risk/benefit discussions as patients and providers consider utilizing this incontinence device. Devices were explanted in 24% of patients and revised or reimplanted in 28% of cases. AUA guidelines indicate “mean all-cause (i.e., erosion, infection, balloon migration or balloon failure) explantation rate of 27% (range: 7% to 55%)” (3,4).
While the MAUDE database does not provide complication rates, reviewing these narratives provides important insights into the safety landscape for devices in the “real world”. MAUDE has historically been studied to highlight unforeseen safety concerns in FDA-approved devices (11). The AE reports from the MAUDE database are overall consistent with the pre-approval safety profile from published literature. Review of the database revealed one AE (report number 3003477176-2018-00026), in which both ProACT balloons were extruded from the patient’s rectum shortly after device placement. The rectum was perforated during percutaneous device placement, and the patient ultimately required a diverting colostomy. Though this may represent an isolated event, patients and providers should be aware of the possibility of this rare but real complication.
Review of the published literature provides insights into optimizing the safety of ProACTTM device placement. Though the transrectal ultrasound (TRUS) guided placement is not an approved delivery strategy per the manufacturer’s information for users, ProACT has been placed under fluoroscopic or TRUS guidance (30). In a case series by Finazzi Agrò et al., 16 bladder or urethral perforations all occurred with placement under fluoroscopic guidance (14). The MAUDE case of rectal perforation did not disclose whether this occurred after fluoroscopic or TRUS guidance, but the transrectal approach may be favorable to prevent such catastrophic injuries in the future. Additionally, in the case series of Crivellaro et al., all patients sustaining an AE had been previously undergone pelvic radiation therapy (17). Future studies should carefully consider the comparative safety of ProACT placement in irradiated vs. non-radiated patients and when using the fluoroscopic vs. TRUS-guided approach.
Evaluation of the safety profile of ProACT should consider the safety of other implantable urologic devices for incontinence. In the case of AUS, a large retrospective, multicenter study recently indicated a total complication rate of 28% at 32 months follow-up, with infection and erosion occurring in 4.2% and 6.7% of implants, respectively (31). Systematic review of complications from male urethral sling placement demonstrate overall complication rates of 2–83%, infection rates of 0–19.6% urinary retention rates of 0–44%, and erosion in 0–6% of cases (32). With ProACTTM, rates of serious AEs such as infection (4%), device failure (14%), and erosion (7%) were similar to AUS and urethral sling placement, though the rate of total AEs were higher with ProACT (47%) than AUS.
Limitations of the current study include those intrinsic to the MAUDE database. As stated by the MAUDE website, “MAUDE data is based on voluntary reporting, meaning it may not capture all AEs. The databank does not establish a cause-and-effect relationship between the device and the reported complications” (33). Post-approval complications may go under-reported as the MAUDE database requires submission from voluntary and mandated reporters, and complication rates cannot be ascertained from this source. Additionally, full clinical details of complications are not available in the published literature or the MAUDE database. This precludes granular analysis such as the relationship between placement approach or radiation status and ProACTTM safety. Additionally, certain publications were excluded for overlapping populations with larger included studies. Though publication exclusion poses the possibility of losing important data on ProACTTM safety, we minimized this by including the larger of studies with overlapping populations.
Conclusions
Comparison of the published and “real world” safety data reveals similar complications, with the exception of a very rare, severe rectal perforation identified in the post-approval MAUDE safety database. Providers should inform patients that the most common risks ProACT placement include device failure, balloon erosion, device infection, urinary tract perforation, and urinary retention. Patients should understand that the overall complication rate according to published literature is approximately 47%, with most AEs representing CTCAE 1–2 complications.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-587/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-587/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-587/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buckley BS, Lapitan MC. Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children--current evidence: findings of the Fourth International Consultation on Incontinence. Urology 2010;76:265-70. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Breyer BN, Kim SK, Kirkby E, et al. Updates to Incontinence After Prostate Treatment: AUA/GURS/SUFU Guideline (2024). J Urol 2024;212:531-8. [Crossref] [PubMed]
- Tricard T, Song QX, Munier P, et al. Adjustable continence therapy (proACT) for the treatment of male stress urinary incontinence post-prostatectomy: a systematic review and meta-analysis (2023 update). World J Urol 2023;41:1793-802. [Crossref] [PubMed]
- Larson T, Jhaveri H, Yeung LL. Adjustable continence therapy (ProACT) for the treatment of male stress urinary incontinence: A systematic review and meta-analysis. Neurourol Urodyn 2019;38:2051-9. [Crossref] [PubMed]
- den Hoedt S, Blok BFM. Adjustable continence therapy (ProACT/ACT(TM)) with periurethral balloons for treatment of stress urinary incontinence: a narrative review. Transl Androl Urol 2024;13:1744-61. [Crossref] [PubMed]
- Kjær L, Fode M, Nørgaard N, et al. Adjustable continence balloons: clinical results of a new minimally invasive treatment for male urinary incontinence. Scand J Urol Nephrol 2012;46:196-200. [Crossref] [PubMed]
- Hübner WA, Schlarp OM. Treatment of incontinence after prostatectomy using a new minimally invasive device: adjustable continence therapy. BJU Int 2005;96:587-94. [Crossref] [PubMed]
- Rouprêt M, Misraï V, Gosseine PN, et al. Management of stress urinary incontinence following prostate surgery with minimally invasive adjustable continence balloon implants: functional results from a single center prospective study. J Urol 2011;186:198-203. [Crossref] [PubMed]
- Porto JG, Arbelaez MCS, Blachman-Braun R, et al. Complications associated with minimally invasive surgical therapies (MIST) for surgical management of benign prostatic hyperplasia: a Manufacturer and User Facility Device Experience (MAUDE) database review. World J Urol 2023;41:1975-82. [Crossref] [PubMed]
- Fernandez AM, Jones CP, Patel HV, et al. Real-World Complications of the SpaceOAR Hydrogel Spacer: A Review of the Manufacturer and User Facility Device Experience Database. Urology 2024;183:157-62. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). U.S. Department of Health and Human Services; 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Ammirati E, Manassero A, Giammò A, et al. Management of male and female neurogenic stress urinary incontinence in spinal cord injured (SCI) patients using adjustable continence therapy. Urologia 2017;84:165-8. [Crossref] [PubMed]
- Finazzi Agrò E, Gregori A, Bianchi D, et al. Efficacy and safety of adjustable balloons (Proact™) to treat male stress urinary incontinence after prostate surgery: Medium and long-term follow-up data of a national multicentric retrospective study. Neurourol Urodyn 2019;38:1979-84. [Crossref] [PubMed]
- Nash S, Aboseif S, Gilling P, et al. Four-year follow-up on 68 patients with a new post-operatively adjustable long-term implant for post-prostatectomy stress incontinence: ProACT™. Neurourol Urodyn 2019;38:248-53. [Crossref] [PubMed]
- Bada M, Crocetto F, Barone B, et al. ProACT in the management of stress urinary incontinence after radical prostatectomy. What happens after 8 years of follow up? monocentric analysis in 42 patients. J Basic Clin Physiol Pharmacol 2023;34:49-54. [Crossref] [PubMed]
- Crivellaro S, Tosco L, Palazzetti A, et al. Geometrical stepper-guided navigation system for ProACT implant under transrectal ultrasound control: preliminary data. Urol Int 2012;89:473-9. [Crossref] [PubMed]
- Kocjancic E, Crivellaro S, Ranzoni S, et al. Adjustable Continence Therapy for the treatment of male stress urinary incontinence: a single-centre study. Scand J Urol Nephrol 2007;41:324-8. [Crossref] [PubMed]
- Lebret T, Cour F, Benchetrit J, et al. Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology 2008;71:256-60. [Crossref] [PubMed]
- Martens FM, Lampe MI, Heesakkers JP. ProACT for stress urinary incontinence after radical prostatectomy. Urol Int 2009;82:394-8. [Crossref] [PubMed]
- Munier P, Nicolas M, Tricard T, et al. What if artificial urinary sphincter is not possible? Feasibility and effectiveness of ProACT for patients with persistent stress urinary incontinence after radical prostatectomy treated by sling. Neurourol Urodyn 2020;39:1417-22. [Crossref] [PubMed]
- Nestler S, Thomas C, Neisius A, et al. Long-term results of ProACT primary and repeat implantation for treatment of stress urinary incontinence in men. World J Urol 2019;37:1173-9. [Crossref] [PubMed]
- Noordhoff TC, Scheepe JR, Blok BFM. Outcome and complications of adjustable continence therapy (ProACT™) after radical prostatectomy: 10 years' experience in 143 patients. Neurourol Urodyn 2018;37:1419-25. [Crossref] [PubMed]
- Ricard H, Léon G, Branchereau J, et al. Adjustable continence balloons in postprostatectomy incontinence: Outcomes and complications. Neurourol Urodyn 2022;41:1414-22. [Crossref] [PubMed]
- Trigo-Rocha F, Gomes CM, Pompeo AC, et al. Prospective study evaluating efficacy and safety of Adjustable Continence Therapy (ProACT) for post radical prostatectomy urinary incontinence. Urology 2006;67:965-9. [Crossref] [PubMed]
- Venturino L, Dalpiaz O, Pummer K, et al. Adjustable Continence Balloons in Men: Adjustments Do Not Translate Into Long-term Continence. Urology 2015;85:1448-52. [Crossref] [PubMed]
- Yiou R, Butow Z, Baron T, et al. Adjustable continence therapy (ProACT™) after male sling failure for patients with post-radical prostatectomy urinary incontinence: a prospective study with one-year follow-up. World J Urol 2015;33:1331-6. [Crossref] [PubMed]
- Baron MG, Delcourt C, Nouhaud FX, et al. Sequential treatment with ProACT™ device implantation after male sling failure for male urinary incontinence. Prog Urol 2017;27:1098-103. [Crossref] [PubMed]
- Ronzi Y, Le Normand L, Chartier-Kastler E, et al. Neurogenic stress urinary incontinence: is there a place for Adjustable Continence Therapy (ACT™ and ProACT™, Uromedica, Plymouth, MN, USA)? A retrospective multicenter study. Spinal Cord 2019;57:388-95. [Crossref] [PubMed]
- ProACT Adjustable Continence Therapy for Men: Physician Instructions for Use. Accessed February 9, 2025. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130018D.pdf
- Tutolo M, Cornu JN, Bauer RM, et al. Efficacy and safety of artificial urinary sphincter (AUS): Results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol Urodyn 2019;38:710-8. [Crossref] [PubMed]
- Meisterhofer K, Herzog S, Strini KA, et al. Male Slings for Postprostatectomy Incontinence: A Systematic Review and Meta-analysis. Eur Urol Focus 2020;6:575-92. [Crossref] [PubMed]
- Manufacturer and User Facility Device Experience (MAUDE) Database. Accessed February 11, 2025. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm



