Anatomy and physiology of chronic scrotal pain
Scrotal anatomy
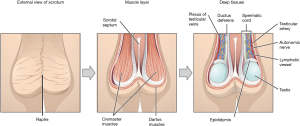
The scrotum is a pigmented external sac of skin and muscle that physically protects and facilitates temperature regulation of the testes to ensure optimal spermatogenesis. It is formed from fusion of the left and right labioscrotal folds, and has a septum that separates the two halves (Figure 1).
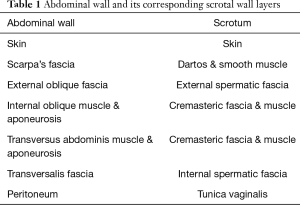
The layers of the scrotum are continuation of the abdominal wall layers (Table 1). From superficial to deep, scrotal layers include: skin, superficial “Dartos” fascia, external spermatic fascia, cremaster muscle, internal spermatic fascia, and tunica vaginalis. Dartos fascia is contiguous with Scarpa’s fascia in the abdomen and Colles’ fascia in the perineum.
Full table
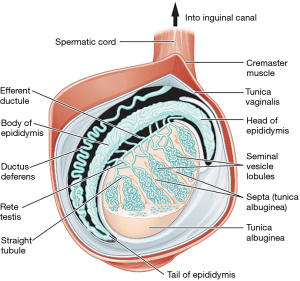
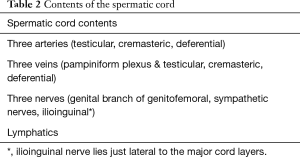
The testis, epididymis, and spermatic cord are housed within the scrotum. Other than being attached to the base of the scrotum by the gubernaculum to prevent torsion, the testes are free to move around. The right testicle in most cases rests at a higher level than the left. The epididymis has three parts—head, body and tail. Only the epididymal head is fixed to the upper part of the testis; relationship of the body and tail to the testis is often variable (Figure 2). Blood and nerve supply for the epididymis and testis are generally found on the posterior side (1). The spermatic cord is a connective tissue matrix that contains the vas deferens, three arteries, three veins, lymphatics, and two nerves. A third nerve, the ilioinguinal, lies just lateral to the cord (Table 2).
Full table
Arteries
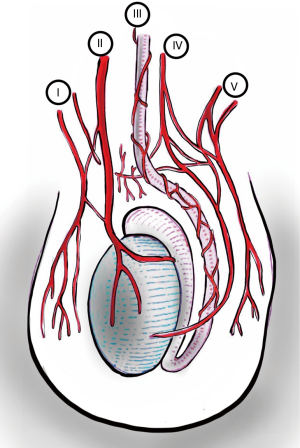
The scrotum is well supplied with blood from both the internal and external iliac arteries and has rich interconnected anastomoses. Anterior scrotum is supplied by the anterior scrotal artery, a branch of the deep external pudendal artery (from external iliac). Posterior scrotum is supplied by the posterior scrotal artery, a branch of the internal pudendal artery (from internal iliac).
Main source of blood to the testis is via the testicular artery (also known as the internal spermatic artery), which arises from the aorta. Artery of the vas deferens (deferential artery) branches from the internal iliac artery. Cremasteric artery comes from the external iliac artery via inferior epigastric artery.
Due to the rich inter-connected anastomoses amongst the arteries that supply blood to the scrotum and its contents (Figure 3), even the division of the spermatic cord will likely only cause testicular atrophy, and not gangrene (2).
Veins
The scrotum has both a superficial and deep venous network (Figure 4). The superficial network drains the scrotum, and these veins mostly follow the arteries, with the anterior scrotum draining into the great saphenous vein through the external pudendal branches, and the posterior scrotum draining into the internal iliac vein through the internal pudendal branches.
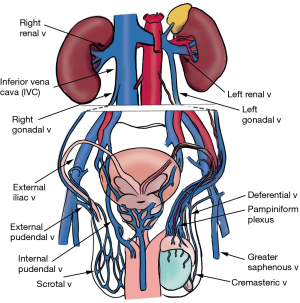
The deep network consists of an aggregate of 10–12 small veins that drain the testis and epididymis, called the pampiniform plexus, which coalesce to become the gonadal vein, emptying into the renal vein on the left or the inferior vena cava (IVC) on the right. Deferential vein empties into the pelvic plexus, and cremasteric vein drains into the inferior epigastric vein (3). These pathways are not absolute; there is a significant amount of inter-individual variation with venous drainage.
Lymphatics
Lymph from the skin, scrotal layers, and tunica vaginalis drains into the superficial then deep inguinal lymph nodes. Lymph from testes and epididymis drains into the retroperitoneum along a defined path, due to the migration route of the testes during development.
Innervation
The somatic supply to the testes and scrotum originates from the L1–L2 and S2–4 nerve roots through the iliohypogastric, ilioinguinal, genitofemoral, and pudendal nerves (Figure 5) (4). The iliohypogastric nerve provides sensory innervation to skin above the pubis. The ilioinguinal nerve innervates skin of the inner thigh, penile base, and upper scrotum. The genitofemoral nerve divides into genital and femoral branch after passing through the psoas muscle. The femoral branch provides sensory innervation to a small area of skin on the inside of the thigh and the genital branch travels with the spermatic cord to provide innervation to the cremaster muscle, as well as the tunica vaginalis (5).

Somatic innervation to the scrotum varies based on the specific scrotal region. The anterolateral surface is supplied by genital branch of the genitofemoral nerve. Anterior surface is supplied by the anterior scrotal nerves (branching from ilioinguinal nerve). Posterior surface is supplied by posterior scrotal nerves (from perineal nerve, branch of pudendal nerve), and the inferior surface is supplied by the long scrotal branches of posterior femoral cutaneous nerve (1).
The testes are embryologically derived from the same level as the kidneys. Therefore, they share a common level of autonomic innervation, which is 90% sympathetic originating from the T10-L1 segments, and the rest parasympathetic originating from the S2–4 segments. Three groups of autonomic nerves travel with the gonadal vessels and vas deferens to the epididymis and testis—superior spermatic nerves, middle spermatic nerves, and inferior spermatic nerves (Figure 5).
Superior spermatic nerves, composed of fibers from the renal and intermesenteric plexuses follow the testicular artery to the testis. This association between the intestinal (intermesenteric) and testicular nerves may explain the “kick in the stomach” feeling accompanying testicular injury. Middle spermatic nerves arise from the superior hypogastric plexus, pass to the mid-ureter and travel alongside the vas deferens to the internal ring, where they join the spermatic cord. The ureteral proximity may explain pain radiation to the scrotum of an obstructing ureteral stone. Inferior spermatic nerves originate from the pelvic plexus (inferior hypogastric plexus), and join the middle spermatic nerves at the prostate-vesical junction. Some afferent and efferent fibers decussate to the contralateral pelvic plexus, which may explain how lesions in one testis affect the function of the other testis (6).
Physiology of pain
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. There are three types of pain: (I) nociceptive—refers to direct stimulation of the nociceptors in response to noxious insult or tissue injury (examples include musculoskeletal pain, skin pain, or pain from distension of hollow organs such as a full bladder). This pain is usually described as sharp, aching or throbbing pain and is usually the “normal” response resulting from a painful stimulus; (II) neuropathic—caused by a lesion or disruption of the nervous system (such as diabetic neuropathy or spinal cord injury). It can be perceived as tingling, burning and hypersensitivity to pain; (III) inflammatory—caused by release of mediators released at the site of tissue inflammation (such as rheumatoid arthritis). Any pain persisting after three months is typically classified as chronic (7).
How a stimulus turns into pain is shown in Figure 6. The stimulus first activates nociceptors, which are free nerve endings found in both somatic and visceral tissues. Prostaglandins, bradykinin, and cholecystokinin are chemicals released during tissue damage that also activate nearby nociceptors (of note, non-steroidal anti-inflammatory drugs work by inhibiting the production of these chemicals). Nociceptors then convert, or “transduce” the pain into action potentials and transmit them via afferent fibers (either A-delta, or C) to the dorsal root ganglion in the spinal cord, where they synapse with the second order neurons. The signal then ascends via the spinal cord to the thalamus. From here, the signals are relayed to multiple areas of the brain including the somatosensory cortex, the insula, frontal lobes and limbic system (7).
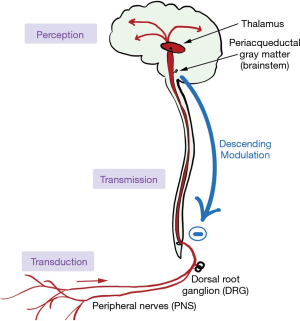
Prior to ascending to the thalamus, some signals branch to various brainstem nuclei. These nuclei, when activated by ascending impulses, release noradrenaline, serotonin, γ-amino butyric acid (GABA), and endogenous opioids that descend to the spinal cord and inhibit nociception. Certain antidepressants such as the tricyclics and selective norepinephrine reuptake inhibitors (SNRIs) enhance descending inhibition, providing a mechanism for their role in alleviating neuropathic pain.
The final stage of pain pathway involves integrating the ascending signals into the perception of pain by a conscious person. Multiple areas of the brain are involved; there is no one location where awareness of pain occurs.
Pathophysiology of chronic scrotal pain
The pathophysiology of chronic scrotal pain is complicated, multifactorial, and not well understood. Many patients recall their chronic pain starting after an injury to the scrotum or testes. The acute pain that results can cause nerve sensitization, leading to modulation of the nerve pathways, ultimately resulting in hypersensitivity and spontaneous firing. Altered or hyperactivated nerve sensation in and around the spermatic cord is considered a major factor in promoting chronic orchalgia. A potential mechanism for this hypersensitivity is Wallerian degeneration, characterized by auto destructive change in the axon after injury that normally promotes regrowth and healing. A heightened immune cell response initiated by neutrophils and macrophages causes inflammation surrounding the nerves which may then lead to neural hypersensitivity. Parekattil and colleagues found a high density of nerves in the spermatic cord with Wallerian degeneration in patients with chronic orchalgia, supporting this hypothesis (8). This hypersensitivity manifests itself as allodynia (perception of pain from a normally non-painful stimulus) or hyperalgesia (exaggerated response than what would be typically expected). Hyperalgesia and allodynia occur from sensitization in either the peripheral or central nervous systems. These changes are termed neural plasticity and can result in perception of pain even months after the injury has healed (9).
Any organ that shares the same nerve pathway with the scrotal contents (mostly L1, L2, and S2–4) may refer pain to this area. Back pain may radiate to the testicle due to sensory nerve root irritation (T10–L1). Inguinal hernias may stretch the genitofemoral and ilioinguinal nerves causing discomfort in the scrotum and testes. Pain arising in the ureter, hip, the presence of aortic aneurysm, intervertebral disc prolapse, or pudendal neuropathies can also cause chronic testicular pain. Pain which is generated by some change within the scrotum itself usually stimulates somatic as well as autonomic fibers and is therefore accurately localized to the scrotum (10).
Some authors have also suggested that chronic orchalgia might be part of a larger behavioral syndrome that begins with a painful episode that is then reinforced either internally or externally and provides secondary gain to the patient. Some of these reinforcements include emotional relief, attention from family and friends, time off work, obtaining pain medications, and socialization with the physician. Once the behavior is reinforced, it occurs in the absence of a noxious stimulus (11).
Conclusions
The scrotum, testes, epididymis and vas deferens have a rich inter-connected vascular supply. The iliohypogastric, ilioinguinal, genitofemoral, and pudendal nerves provide innervation and are involved in chronic scrotal pain. Irritation of these nerves by non-scrotal pathology results in referred pain to the scrotum. The pain pathway starts with nociceptor triggering, transduction via the peripheral nervous system, transmission to the central nervous system via the dorsal root ganglion through the thalamus and to various regions of the brain. Descending modulation through the brainstem serves to inhibit some of the nociceptive pain signals. The integration of multiple ascending and descending signals ultimately results in the perception of pain. Neural plasticity after injury may result in abnormal sensitization of nociceptors and ultimately chronic pain.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Skaldalakis JE, Colburn GL, Weidman TA, et al. Skaldalakis’ Surgical Anatomy. Athens: Paschalidis Medical Publication Ltd (P.M.P.), 2004.
- Neuhof H, Mencher WH. The viability of the testis following complete severance of the spermatic cord. Surg Gynecol Obstet 1960;8:672-85.
- Lechter A, Lopez G, Martinez C, et al. Anatomy of the gonadal veins: a reappraisal. Surgery 1991;109:735-9. [PubMed]
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [PubMed]
- Zorn BH, Watson LR, Steers WD. Nerves from pelvic plexus contribute to chronic orchidalgia. Lancet 1994;343:1161. [Crossref] [PubMed]
- Reynolds LW, Sills SM. Orchialgia. In: Waldman SD. editor. Pain Management. Philadelphia: Elsevier, 2011.
- Cross SA. Pathophysiology of pain. Mayo Clin Proc 1994;69:375-83. [Crossref] [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol 2006;2:95-106. [Crossref] [PubMed]
- Morgan RJ, Parry JR. Scrotal pain. Postgrad Med J 1987;63:521-3. [Crossref] [PubMed]
- Arnold LM, Choy E, Clauw DJ, et al. Fibromyalgia and Chronic Pain Syndromes: A White Paper Detailing Current Challenges in the Field. Clin J Pain 2016;32:737-46. [Crossref] [PubMed]