Comprehensive pelvic floor physical therapy program for men with idiopathic chronic pelvic pain syndrome: a prospective study
Introduction
Idiopathic male chronic pelvic pain syndrome (CPPS) is a heterogeneous constellation of symptoms that causes significant impairment and is often challenging to treat. CPPS accounts for up to 90% of men with pelvic pain in outpatient clinics. CPPS is characterized by symptoms lasting at least 3 months during the past 6 months, in the absence of a urinary tract bacterial infection (1) CPPS is distressing to patients and causes physical, emotional, and relationship distress leading to significant impairment in quality of life (QOL). Difficulty in accurate diagnosis and delivery of efficacious treatment leads to patient dissatisfaction and frustration. By the time patients are referred to a specialist in CPPS they have often tried and failed multiple interventions such as antibiotics, alpha blockers and anti-inflammatory medications.
The 2013 European Urologic Association guidelines on chronic pelvic pain recommend a multimodal approach to achieve the best outcomes (2). Medical therapy focuses on the identifiable and treatable causes of pain. Medications include antibiotics when infection is identified; alpha blockers, 5 alpha reductase inhibitors, anticholinergics when there is associated LUTS; and anti-inflammatory and opioid pain medications. Psychosocial management with cognitive behavioral therapy improves QOL indices (3). Surgical therapies should be used as last resort.
Few studies have incorporated pelvic floor physical therapy (PFPT) into treatment algorithm for CPPS. PFPT encompasses a wide array of interventions that physicians are often unfamiliar with. Previous literature shows improvement in symptoms and QOL when interventions such as myofascial release (4,5), therapeutic stretching and exercise (6), biofeedback (7,8), and neuromodulation (9,10) were administered individually. We evaluated the role of the combination of these interventions in a comprehensive PFPT program for men with idiopathic CPPS. The mostly widely utilized questionnaire to evaluate idiopathic CPPS is the National Institute of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) (11). Some studies use the modified NIH-CPSI for men also called as Genitourinary pain index (GUPI) (12). We used male GUPI to evaluate outcomes after comprehensive PFPT program.
Methods
We identified men over the age of 18 referred for pelvic floor physical therapy from October 2015 to October 2016 with a diagnosis of idiopathic CPPS. Men with clearly identifiable causes of pelvic pain, such as previous surgery, chronic infection, trauma, prostatitis and epididymitis were excluded. Men who had concomitant urinary incontinence or were post prostatectomy were also excluded.
Patients were evaluated by two physical therapists trained in PFPT prior to enrollment. After evaluation and enrollment into the PFPT program, patients received the same modalities of therapy. All patents received education about CPPS and the therapies included in the program. Treatment included: (I) manual therapy for myofascial trigger point release, including internal and external manipulation of the pelvic floor and abdominal musculature; (II) therapeutic exercises to promote range of motion, improve mobility/flexibility and strengthening weak muscles; (III) biofeedback to facilitate strengthening and relaxation of pelvic floor musculature; (IV) neuromodulation for pain relief. Patient therapy schedules were based on insurance limitations, patient availability and physical therapist recommendations.
Patients’ progress was measured using the GUPI. Surveys were administered at the beginning of the initial evaluation and at the beginning of the tenth visit. GUPI scores range from 0–45 with higher scores reflecting worse symptoms. A decrease of 7 points in the GUPI total score robustly predicted being a treatment responder (sensitivity 100%, specificity 76%). A reduction of 4 points in the GUPI total score predicted a clinically perceptible difference in global response (sensitivity 79%, specificity 90%) (12).
Descriptive statistics were used to track patient progress. Mean pre and post therapy scores were compared using the student t-test. Individual’s changes in scores from initial to tenth visit were analyzed separately. Data was grouped based on response; robust (7 or greater), moderate [4–6] no response (less than 4) and worsening symptoms (increasing GUPI score). Patient characteristics, including age, race, medical comorbidities, time from initial to tenth evaluation, and severity of symptoms at initial evaluation were assessed to identify predictors of response to therapy.
Results
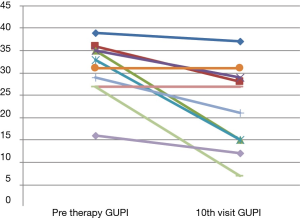
Fourteen patients referred for PFPT met criteria for inclusion. Out of 14, a total of 10 patients completed all 10 visits (Table 1). The median GUPI score at initial evaluation was 30.8 [16–39] and decreased to 22.2 [7–37] at the tenth visit. Five of the 10 patients (50%) in the study had a reduction of greater than 7 points and 2 (20%) had a change of greater than 4 but less than 7. Three patients (30%) did not have any meaningful change in GUPI (less than 4). No patients had an increase in GUPI (Figure 1). The remaining four are in the process of completing 10 sessions. Medical comorbidity, severity of symptoms, age, race, did not have any predictive value on response. Duration from initial evaluation to tenth visit i.e., the longer the interval of therapy appeared to be associated with better response.
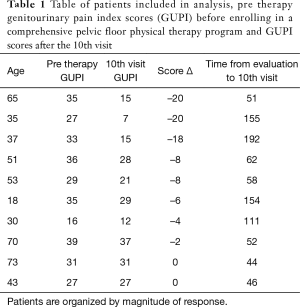
Full table
Discussion
A comprehensive PFPT program that includes myofascial release, therapeutic exercises, biofeedback and neuromodulation improves symptoms as measured on the GUPI in men with idiopathic CPPS. In our study 50% of men had a robust improvement in GUPI scores and 20% had moderate improvement. No participants had worsening of symptoms. This indicates that a comprehensive PFPT is a reasonable option for treatment of men with idiopathic CPPS and is unlikely to be harmful or worsen symptoms.
CPPS has often remained an enigma for physicians and patients alike due to the heterogeneity of symptoms. Monotherapy with medications or psychotherapy often proves to be ineffective and can lead to patient and practitioner frustration. For most practitioners, medical therapy directed at discrete symptoms is the first line option. Antibiotics are frequently prescribed even if evidence of infection is lacking. Widely available medications, such as alpha blockers, anticholinergics and anti-inflammatories can be helpful in some cases. A 2012 network metanalysis that included comparison of antibiotics, alpha blockers, anti-inflammatories, and the combination of antibiotics and alpha blockers to placebo (13). Alpha blockers with antibiotics had the greatest change in NIH-CPSI (‒13.6 compared to placebo), however they also had the lowest percentage of patients respond (RR 0.9). For single drug therapy alpha blockers had the greatest change in NIH-CPSI (‒10.8 compared to placebo) however anti-inflammatories had the largest number of responders (RR 1.8). Another study examining anti-inflammatory celecoxib, showed a significant decrease in NIH-CPSI scores compared to placebo (‒8.03 vs. ‒4.75)—however response was not durable and was limited to the duration of therapy (14). Data on the use of anticholinergics are less (15). Evaluation of the somewhat confusing data on medical intervention reiterates the difficulty in selecting the most appropriate and efficacious medical therapy.
Pelvic floor therapy requires special training to be effective. Previous literature has proven that therapists lacking training in PFPT have worse outcomes then those who have specialized PFPT training (16). Studies individually evaluating myofascial release, therapeutic exercises, biofeedback and electrical stimulation show improvements in NIH-CPSI scores.
Myofascial release is manual manipulation of internal and external trigger points with the goal or relieving muscle tension and related pain. Techniques utilized include direct pressure, contraction and release/hold-relax/contract-relax/reciprocal inhibition, and deep tissue mobilization, including stripping, strumming, skin rolling and effleurage (17). In one large study including 138 men with refractory CPPS, 72% reported marked or moderate improvement as measured by NIH-CPSI scores. More recently a study of 106 men with refractory CPPS showed improvement in pain by visual analogue scale with the use of a specially designed wand that can be used at home to release internal trigger points (7.5 to 4) (5).
Stretching and strengthening are the interventions most associated with physical therapy. Kegel exercises are the most well-known form of pelvic floor physical therapy that urologists are familiar with, however they are not the correct therapy for the majority of patients with CPPS. In fact, many patients have overactive or hypertonic musculature that requires relaxation as opposed to strengthening and Kegels can worsen symptoms in some men with CPPS (16). In a study of 97 men with CPPS comparing general stretching and aerobic exercise, both groups had significant decreased in CPSI scores by more than 7 points (81% vs. 75%) at 18 weeks (6). As a result, PFPT programs incorporate therapeutic stretching and exercise.
Biofeedback allows for the patients and therapists to visualize the actions of the pelvic floor muscles with the goal of re-educating the pelvic floor muscles. Multiple devices exist. Some use an internal probe such as a manometer to measure muscular activity and strength of contractions, others uses external EMG leads, while some use a combination. Biofeedback has been successfully used to treat pain symptoms in a variety of pathologies, including rectal pain, vaginal pain and levator pain. Additionally, biofeedback can improve symptoms of men with CPPS. In one study of 31 men with CPPS, mean age 43.9 years (range, 23–70) years, NIH-CPSI scores decreased from 23.6 (range, 11–34) at baseline to 11.4 (range, 1–25) after treatment (P<0.001) (18). Thus biofeedback is a useful tool in the treatment of CPPS.
Neuromodulation uses electricity to stimulate the nervous system for a desired result. In urologic practice, neuromodulation is most often used for overactive bladder and urge urinary incontinence. Urologists are most familiar with the sacral nerve InterstimTM device and percutaneous tibial nerve stimulation (PTNS). In patients with pain syndromes the goal is to alter the perception of pain. Neuromodulation has been utilized as a treatment of pelvic pain for decades (19). A systematic review individually evaluated different methods of neuromodulation for patients with CPPS, including pudendal nerve stimulation, sacral nerve stimulation, percutaneous tibial nerve electrode stimulation, and transperineal electromagnetic stimulation. Improvement in QOL were seen regardless of modality. Unfortunately, both men and women were included and therefore these results are not completely translatable to male CPPS (20). In a small study of 14 men who elected for neuromodulation, the mean total National Institutes of Health prostatitis symptom score significantly decreased from 29 to 14 after 5 weeks of therapy (21). Interesting, combination therapy of biofeedback with electrical stimulation has a synergistic effect on CPPS by alleviating pain and urinary symptoms and QOL (22).
To our knowledge, our study is the first to evaluate a comprehensive physical therapy program that incorporates combination of modalities. As shown, this approach leads to significant reduction on GUPI scores with 70% reporting robust or moderate response. More importantly, no patients had increasing GUPI scores (worsened symptoms) after 10 sessions. Strengths of this study are it prospective design, careful selection criteria, and homogeneity of therapy provided. All patients received similar therapies and included biofeedback, myofascial release and neural stimulation despite being administered by two providers. This study’s limitations are its small number of patients and variability in therapy schedule. Additionally, we do not have a comparison control group and therefore we cannot assess for the placebo effect. We were unable to identify any patient characteristics that were predictors of treatment success or failure likely due to small sample size. Unfortunately due to patient availability and insurance limitations, the frequency of therapy sessions was not standardized and varied from multiple sessions a week to once a week.
Conclusions
Male CPPS is difficult to treat and often requires a multimodal approach. Based on the results of our study, a comprehensive PFPT may be an effective treatment option for select patients. A larger study with a control group is needed to validate the routine use of PFPT in men with idiopathic CPPS and predict characteristics of men who would respond to therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Study was reviewed by the University of Miami IRB was exempted, Non-Human subject research. Data was anonymized. The intervention provided no additional risk to patients.
References
- Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA 1999;282:236-7. [Crossref] [PubMed]
- Engeler DS, Baranowski AP, Dinis-Oliveira P, et al. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol 2013;64:431-9. [Crossref] [PubMed]
- Tripp DA, Nickel JC, Katz L. A feasibility trial of a cognitive-behavioural symptom management program for chronic pelvic pain for men with refractory chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J 2011;5:328-32. [Crossref] [PubMed]
- Fitzgerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol 2013;189:S75-85. [Crossref] [PubMed]
- Anderson R, Wise D, Sawyer T, et al. Safety and effectiveness of an internal pelvic myofascial trigger point wand for urologic chronic pelvic pain syndrome. Clin J Pain 2011;27:764-8. [Crossref] [PubMed]
- Giubilei G, Mondaini N, Minervini A, et al. Physical activity of men with chronic prostatitis/chronic pelvic pain syndrome not satisfied with conventional treatments--could it represent a valid option? The physical activity and male pelvic pain trial: a double-blind, randomized study. J Urol 2007;177:159-65. [Crossref] [PubMed]
- Chiarioni G, Nardo A, Vantini I, et al. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology 2010;138:1321-9. [Crossref] [PubMed]
- Heah SM, Ho YH, Tan M, et al. Biofeedback is effective treatment for levator ani syndrome. Dis Colon Rectum 1997;40:187-9. [Crossref] [PubMed]
- Marinkovic SP, Gillen LM, Marinkovic CM. Minimum 6-year outcomes for interstitial cystitis treated with sacral neuromodulation. Int Urogynecol J 2011;22:407-12. [Crossref] [PubMed]
- Peters KM, Killinger KA, Boguslawski BM, et al. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 2010;29:1267-71. [Crossref] [PubMed]
- Schneider H, Ludwig M, Weidner W, et al. Experience with different questionnaires in the management of patients with CP/CPPS: GPSS, IPSS and NIH-CPSI. World J Urol 2003;21:116-8; discussion 115. [Crossref] [PubMed]
- Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology 2009;74:983-7, quiz 7 e1-3.
- Thakkinstian A, Attia J, Anothaisintawee T, et al. alpha-blockers, antibiotics and anti-inflammatories have a role in the management of chronic prostatitis/chronic pelvic pain syndrome. BJU Int 2012;110:1014-22. [Crossref] [PubMed]
- Zhao WP, Zhang ZG, Li XD, et al. Celecoxib reduces symptoms in men with difficult chronic pelvic pain syndrome (Category IIIA). Braz J Med Biol Res 2009;42:963-7. [Crossref] [PubMed]
- Kim DS, Kyung YS, Woo SH, et al. Efficacy of anticholinergics for chronic prostatitis/chronic pelvic pain syndrome in young and middle-aged patients: a single-blinded, prospective, multi-center study. Int Neurourol J 2011;15:172-5. [Crossref] [PubMed]
- Polackwich AS, Li J, Shoskes DA. Patients with Pelvic Floor Muscle Spasm Have a Superior Response to Pelvic Floor Physical Therapy at Specialized Centers. J Urol 2015;194:1002-6. [Crossref] [PubMed]
- Anderson RU, Wise D, Sawyer T, et al. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol 2005;174:155-60. [Crossref] [PubMed]
- Cornel EB, van Haarst EP, Schaarsberg RW, et al. The effect of biofeedback physical therapy in men with Chronic Pelvic Pain Syndrome Type III. Eur Urol 2005;47:607-11. [Crossref] [PubMed]
- Landau B, Levy RM. Neuromodulation techniques for medically refractory chronic pain. Annu Rev Med 1993;44:279-87. [Crossref] [PubMed]
- Yang CC. Neuromodulation in male chronic pelvic pain syndrome: rationale and practice. World J Urol 2013;31:767-72. [Crossref] [PubMed]
- John H, Ruedi C, Kotting S, et al. A new high frequency electrostimulation device to treat chronic prostatitis. J Urol 2003;170:1275-7. [Crossref] [PubMed]
- Yang ZS, Zu XB, Qi L, et al. Combination therapy of biofeedback with electrical stimulation for chronic prostatitis/chronic pelvic pain syndrome. Zhonghua Nan Ke Xue 2011;17:611-4. [PubMed]


