Pelvic radiotherapy and sexual function in women
Introduction
Pelvic radiotherapy remains an important part of the treatment or constitutes the primary treatment of many cancers e.g., advanced cervical cancer, rectal cancer, and anal cancer. There is a continuous development of new radiation methods to save normal tissue without compromising cure rate and survival (1-4). Earlier studies have revealed that patients undergoing radiotherapy for their pelvic cancer suffer from a considerable amount of late effects influencing negatively on their quality of life (QOL) and sexual functioning (5-8). In female cancer patients vaginal morbidity seems to be critical for the resumption and maintenance of a healthy sexual life. The introduction of modern radiotherapy modalities has generated a hope that late effects following treatment of pelvic cancers may decrease in the future.
Pelvic radiation may be delivered as external beam and/or brachytherapy. Lately, technical progress has been made to determine the dose intensity pattern that will best conform to the tumor shape; e.g., intensity modulated radiation therapy (IMRT) and 3-D/4-D computed tomography (CT) or magnetic resonance images (MRI) that is used in conjunction with computerized dose calculations. The main purpose is to allow a higher radiation dose to be focused within the tumour while minimizing the dose to surrounding normal critical structures. The 4D target concept for image-guided adaptive brachytherapy (IGAB) purposes to take into account that following external radiotherapy the residual tumor volume at the time of brachytherapy is smaller than the primary tumor. Therefore the volume of the residual tumor can be considered as the high risk target while the primary tumor volume can be considered as an intermediate risk target and may not need as high a radiation dose (3). Hence normal tissue may be spared. The addition of brachytherapy allows a high localized target doses with tissue sparing low non-target doses. For cervical cancer, brachytherapy is delivered using an applicator inserted close to or in the tumor target. To optimize the dose lateral in the pelvis, applicators have been developed that allows insertion of angled needles into the pelvis in which the radiation source can travel and dwell in different positions according to the target calculations. The limiting factor for the total dose delivery of radiation is normal tissue tolerance. Hence, in managing pelvic cancer with radiotherapy, a delicate balance between cure and tissue tolerance have to be dealt with. In general, the occurrence of complications is dose and fractionation dependent and there may be a long latency periods for many adverse late effects to emerge, probably due to scattered irradiation remaining in the organs around the tumour.
The accumulated radiation dose to the pelvic organs is important for acute bowel, bladder, and genital toxicity. Radiation effects are progressive and may become symptomatic after a latent period but there may be a continuous progression from the acute oedema, mucosal and sub-mucosal inflammation and persistent ulceration and necrosis to fibrosis (9-11). The rapid cell-turnover of the vaginal and vulva epithelium make them very sensitive to the effects of radiation. Following pelvic radiation, acute radiation effects include vaginal erythema, moist desquamation, and a confluent mucositis. The mucosa may demonstrate severe congestion and submucosal hemorrhage (hyperemia). These effects usually resolve within 2 to 3 months after radiotherapy. In some patients, however, there is a progressive vascular compromise and tissue hypoxia may result in epithelial sloughing, ulcer formation and necrosis. On the longer term, vaginal wall thinning, adhesions, atrophia and fibrosis may occur often followed by decreased vaginal elasticity, narrowing, shortening and ultimately total vaginal stenosis (8,12-16). With a median period of 1 to 2 years late effects may arise when the sub-mucosa undergoes varying degrees of fibrotic change, organ capacity reduces and teleangectasia may develop. Further, ischaemia from radiation-induced endarteritis obliterans may give rise to a fragile neovasculature that tends to bleed. The end result may include vaginal and vaginal entrance stenosis and fragility. Similar effects are observed in the bladder and rectum resulting in late effects as urgency, hemorrhagic cystitis, tenesmi and fecal incontinence (5,9,11,17,18).
A healthy sexual response is described having four phases; desire, excitement, orgasm and resolution whereas female sexual dysfunction (FSD) includes desire, arousal, orgasmic and sexual pain disorders (19). Current knowledge of the complexities related to female hypoactive sexual desire, arousal and pain sexual disorders has prompted recommendation of a classification system based on physical as well as psychological pathophysiology, and a personal distress criterion for most diagnostic categories (19,20). This includes a re-definition of sexual desire to include the concept of receptivity. Sexual arousal disorders are separated into genital and subjective subtypes while the definition of dyspareunia reflects the possibility of having pain that precludes sexual intercourse. The anticipation and fear of pain characteristic of vaginismus is noted while the assumed muscular spasm is omitted given the lack of evidence (19,20).
Late effects of radiotherapy and QOL aspects are most validly measured by the patient and patient reported outcomes (PROs) are today widely accepted as outcome measures. Very few measures of FSD have included the above described new conceptual considerations regarding FSD. Further, significant vaginal, vulval, and perineal changes may arise after radiotherapy and cause considerable pain during all phases of sexual interaction in the first months following radiotherapy. Later, chronic changes may arise which may render sexual intercourse impossible. These radiation induced changes are usually not included in questionnaires assessing FSD; e.g., the Female Sexual Function Index (FSFI) (21) and the Derogatis Sexual Functioning Inventory (DFSI) (22). However, questionnaires and late effects scales have emerged that explicitly address mucosal changes and their impact on sexuality following radiotherapy; e.g., the Sexual function Vaginal changes Questionnaire (SVQ) (23), the European Organisation for Research and Treatment of Cancer (EORTC) cervical cancer module CX-24 (24), the EORTC Endometrial Cancer Module EN-24 (25), The Common Terminology Criteria for Adverse Events (CTCAE) (26), and the Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic scale (LENT SOMA) (27).
The goal of the present review was to summarize the latest knowledge on pelvic radiotherapy and sexual function in women focusing on studies (I) from the period 2010 to 2014; (II) that used PROs as outcome measure; (III) that were randomized controlled trials (RCTs) where FSD at least constituted a secondary outcome; and (IV) that reported from modern radiotherapy modalities.
Methods
A literature search was performed, first with the general terms: pelvic radiotherapy* AND female sexuality* OR female sexual function* OR FSD*. More specific search criteria were then used: rectal/anal/bladder/cervical/endometrial/vulva cancer AND female sexuality* OR female sexual function* OR FSD*. A further aspect primarily related to vaginal or perineal brachytherapy was evaluated. The literature search therefore also included the terms: Brachytherapy*, vaginal*, toxicity*, late effects* AND pelvic radiotherapy*. A similar search has been done before (7) and the present search was therefore focused on the past 5 years and with a special interest in identifying RCTs that evaluated PROs related to FSD. Further selection criteria were: (I) sexual functioning assessed as PRO or as part of a qualitative study; (II) the instrument for assessment of sexual function described; (III) pelvic radiotherapy and/or brachytherapy given; (IV) ≥15 patients participated in quantitative studies.
Results
Two randomized controlled studies were identified that reported PROs dealing with issues on sexuality following radiotherapy for rectal or endometrial cancer (1,28,29). Both studies will be described in details below in the respective disease-specific sections. Studies included in the present narrative review are summarized in Table 1. These studies represent a selected part of studies retrieved as described above. A few studies are included that do not fulfill the selection criteria of ≥15 patients included (30,31). These two studies are summarized due to their longitudinal design and the existing sparse knowledge on sexual function after radiotherapy for bladder and vulva cancer, respectively.
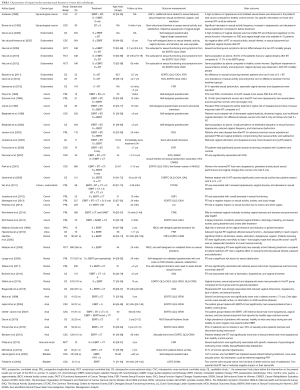
Full table
Most studies were retrospective cross-sectional in design while a few older studies were prospective longitudinal (8,16,32,33). Summarizing the general findings in observational studies published before 2010, it can be concluded that pelvic radiotherapy is associated with multiple organic changes and psychological issues that have the potential to negatively impact on female sexuality (12,13,34-47). Women reported a feeling of lack of femininity, sexual attractiveness and confidence besides being distressed by vaginal bleeding, vaginal pain, vaginal dryness, vaginal shortening, and decreased elasticity resulting in fear of having sex and less sexual enjoyment (8,12,14-16,36,40).
During the past 5 years several studies have emerged that focuse on a presumed less toxic effect of IGAB and IMRT but few studies included PROs and the literature published after 2010 did not reveal any new knowledge or evidence of less toxicity related to pelvic radiotherapy. Results from both periods (before and after 2010) will be addressed in each disease-specific section below with a special focus on results from the few RCTs and from studies evaluating modern radiotherapy methods.
Gynecological cancer
Endometrial cancer
Few studies have dealt with sexual functioning/FSD in endometrial cancer patients. The past 15 years of research within the field of using PROs to assess late effects in this patient group has assessed more general aspect of QOL, gastrointestinal, and urological late effects (39,48-50) and only a few older studies (51) have included data on sexual functioning. However, a few newer studies assessed sexual functioning in more details (1,47,52) although sexual functioning was not the primary outcome in any of these studies. The earlier studies used non-validated questionnaires or items (47,51) while the randomized controlled study of Nout et al. used scales/items related to other cancer diagnoses (prostate or ovarian cancer) (1,29,53).
Nunns et al. assessed vaginal morbidity and sexual functioning in a subset of patients (75 patients out of 252) who had post-operative brachytherapy (N=32) or external beam radiation and brachytherapy (N=43) (51). The overall incidence of vaginal stenosis was 54.7% of which most (75%) were confined to the upper third part of the vagina. In addition, vaginal vault scarring was found in 63%, vaginal adhesions in 53%, teleangiectasia in 60%, and mucosal atrophy in 61% with no difference between those who had additional external beam radiation besides brachytherapy. Only 20 out of 75 women were sexually active prior to treatment. Of these, 13 women (65%) reported reduced sexual interest and activity post-treatment and 12 women (60%) reported dyspareunia (51).
In the PORTEC study (1,29,53), women, median age 70 years, operated for stage 1 endometrial cancer and with intermediate or high-risk of lymph node metastases, were randomly assigned to receive either external beam radiotherapy (EBRT) (N=214) or vaginal brachytherapy (VBT) (N=213). The primary end-point was vaginal vault recurrence while QOL aspects were secondary outcomes. The sample size was large enough to show significant differences in both recurrence and QOL-related endpoints. QOL was evaluated prior to radiotherapy and 2 and 4 weeks and 6, 12, 18, 24, 36, 48, and 60 months after radiotherapy by self-assessment of questionnaires or single items developed and internationally validated by the European Organization of Research and Treatment of Cancer (EORTC) group. No difference was observed in overall survival between the two radiation groups. Sexual interest and activity increased during the first six months post-radiation and no difference was observed between the two radiation groups in sexual interest, activity, enjoyment, or vaginal dryness. However, compared to an age-matched control group of women from the general population significant impairment was observed both regarding sexual interest, activity, enjoyment and vaginal dryness in both groups (53).
Some smaller, recently published, cross-sectional studies did not find any difference in sexual functioning between women undergoing hysterectomy and postoperative VBT compared to women who had hysterectomy only (54,55) or compared to healthy postmenopausal women (56). All three studies used validated questionnaires but conclusions may very well have been biased by a very small number of patients actually receiving VBT (54,55). In the study of Damast et al. women who had hysterectomy and VBT scored significantly worse on all sexual dysfunction domains of the Female Sexual Dysfunction Index (FSFI) (21); desire, arousal, vaginal dryness, orgasm, dyspareunia and sexual satisfaction than the index population of healthy women between age 18-74, however, not significantly worse than a comparison group of postmenopausal women (56). However, using the cut-off point for FSD in the FSFI questionnaire showed that 81% of the women receiving VBT reported sexual dysfunction.
It is concluded that applying postoperative VBT to women with endometrial cancer carries a comparatively high risk of vaginal changes and sexual impairment at the same level as that found after EBRT. However, VBT carries a significantly lower risk of impairment on all other QOL domains (53).
Cervical cancer
Advanced cervical cancer is treated with EBRT, brachytherapy and concomitant chemotherapy. Patients, identified with histologically high risk factors for recurrence after surgery for early stage disease, e.g., lymph node metastases, are given adjuvant EBRT and chemotherapy. A couple of longitudinal studies have been published assessing sexual functioning following primary or postoperative radiotherapy for cervical cancer (8,16,32) and found persistent sexual dysfunction with limited or no indication of improvement over time. In the longitudinal study of Jensen et al. scores were compared with those of age-matched control women. Throughout the study period of two years, significant severe sexual dysfunction and vaginal morbidity were reported by the patients compared to controls: e.g., 42% reported that the size of vagina was bothersome during sexual intercourse because it felt too small [relative risk (RR) =4.8], and 45% were never or only occasionally able to complete sexual intercourse (RR =2.4) (8). About one third of the patients reported persistent lubrication problems (RR =5.3) and of these 50% reported severe distress (8). Several cross-sectional studies have confirmed these results in comparatively large samples (12,13,40,46,57-59). Hence, results from the literature before 2010 including both longitudinal and cross-sectional studies in patients receiving either adjuvant or primary radiotherapy showed that the effects of radiotherapy is progressive and persistent over time especially related to the vaginal mucosal morbidity with no or little improvement to be expected. Further, significant psychological impairment; e.g., feeling of guilt, self-blame, fear of recurrence, and anxiety were related to the diagnosis and treatment of cervical cancer (32,40,45).
Up to the period before 2010, studies on QOL issues after radiotherapy reported on late effects after traditional EBRT and brachytherapy. Searching the literature after 2010 revealed that a few studies have emerged reporting on the use of MRI based IGAB and IMRT (3,4,60). However, most studies still reported on traditional radiotherapy (61) or did not provide sufficient information on radiotherapy modality applied to assess potential improvement related to modern radiotherapy modalities (62-66).
The literature after 2010 still revealed significant sexual impairment following pelvic radiotherapy for advanced cervical cancer, especially related to vaginal changes; tightness, shortness, dryness, dyspareunia, vaginal bleeding (62,64-67). Several authors have explored more psychological aspects of sexual functioning in cervical cancer patients. Abbott-Anderson et al. identified several concerns about both physical, psychological, and social aspects of sexuality e.g., alterations in body image, maintaining previous sexual roles, emotional distancing from the partner, and perceived change in partner’s level of sexual interest etc(62). Similar worries were found in the study of Mantegna et al., 15.6% of patients with advanced cervical cancer reported anxiety and body-image changes two years after treatment with significant improvement short after treatment but without long-term recovery (65). Despite a significant improvement in sexual activity during the first three months following treatment, patients treated with chemo-radiation reported significantly lower levels of sexual activity compared to patients treated with surgery only for early stage disease (65). Juraskova et al. evaluated patients with cervical or endometrial cancer, of whom one third had adjuvant radiotherapy and stressed the importance of assessing women’s perceived quality of sexual life, not just the frequency, since overall sexual function was predicted more strongly by the perceived quality than the quantity of sexual interactions and even a small change in perceived quality had a large impact on overall sexual function (63).
In the study of Lindegaard et al. the focus was to describe the radiotherapy modality, to compare survival with historical data, and to evaluate late effects as assessed by the health care professional following IGAB (3). Hence, no PROs were included but overall, an improvement in survival was observed besides significant less urological, gastrointestinal, and vaginal morbidity as assessed by the doctor at gynaecological examination (3). The same author participated in a multicenter study prospectively evaluating the effects of definite chemo-radiation and IGAB which had the main purpose of comprehensively assess early and late vaginal morbidity (60). The assessment of vaginal morbidity was also in this study evaluated by objective assessments by the doctor at gynaecological examination: vaginal mucositis, vaginal bleeding, vaginal stenosis (shortening and/or tightening), vaginal dryness, and vaginal fistula and assessed regarding being fluctuating or persisting. The CTCAE, version 3 (26) was used to grade vaginal morbidity and by nature, as mentioned by the authors, potentially biased by subjective interpretation by the doctor assessing the morbidity and knowing what treatment modality that was given (60,68). Further, as the author claims, this grading system does not work for e.g., assessing “vaginal dryness” since e.g., no life-threatening (grade 4) vaginal dryness exists. It is concluded that the prevalence of severe or life-threatening vaginal morbidity is limited after definite radiation chemotherapy including IGAB. However, mild to moderate vaginal morbidity was very prevalent: the actuarial probability that a patient would have any vaginal morbidity grade ≥1 within 2 years after treatment was 89% (95% CI: 85.2-92.6) while it was 29% (95% CI: 23.6-33.4) for having ≥ grade 2. Vaginal stenosis was most frequent with an actuarial probability for grade ≥1 of 75% and 22% for grade ≥2 (60). Vaginal dryness was reported by nearly half of the patients with an actuarial probability for grade ≥1 at 2 years post-treatment of 62% and for grade ≥grade 2 of 8%. One third of the patients reported bleeding (mild) with an actuarial probability of 40% (95% CI: 35.3-45.5) (60). PROs were included in a recent in press study by the same author in a mono-institutional prospective study of 50 patients receiving IGAB for advanced cervical cancer and assessed before, during, 1 week and 3 months after radiotherapy (4). However, despite reporting from the EORTC QOL Questionnaire Cervix-24 (24), very sparse information is given on vaginal morbidity and sexual dysfunction. These results are awaited in a later paper (personal correspondence).
It is concluded that patients with advanced cervical cancer still represent a group at high risk of experiencing persistent sexual dysfunction both related to vaginal morbidity and to more psychologically related elements of femininity and body-image. Results on PROs including sexual dysfunction and vaginal morbidity after introducing IGAB and/or IMRT are awaited (4,69).
Vulvar cancer
Radiation therapy can be used in different settings for the management of patients with vulvar cancer. Adjuvant therapy may be given to the vulva region in case of close or positive resection margins and to the groins and the external iliac region in case of positive inguinal and/or pelvic nodes to prevent loco-regional recurrence and to improve survival in early stage vulvar cancer. Definitive chemo-radiation is used only if the vulvar cancer is considered too advanced to be surgically resected or if complete resection will endanger function of the urethra and/or anal sphincter.
Studies evaluating sexual function after vulvar cancer are scarce and based on small sample sizes and further characterized by heterogeneity on disease stage, treatment modality, and used methodology to assess sexual function. Most studies relied on a retrospective or cross-sectional design, control groups were seldom included, and most often dealt with extensive surgical procedures that were used decades ago (70). Although substantial surgical modifications towards less radicality have been made during the past decades in the treatment of vulvar cancer, considerable sexual dysfunction due to scarring and narrowing of the vaginal entrance, vaginal dryness and pain besides considerable orgasmic problems has been reported up to 2010 (35,71-74).
The literature after 2010 is also mainly concerned with sexual dysfunction related to the surgical procedure (75-78). Some studies have included small sub-groups of patients who received adjuvant or primary chemoradiation (31,73,75,79,80). Due to small sample sizes, very vague conclusions can be drawn regarding the impact of radiotherapy on the women’s sexual functioning. However, scarring, fibrosis, reduced sensation and elasticity of the vaginal entrance and vagina has been reported (81). In the longitudinal study of Weimer Schultz et al. a very high risk for stenosis of the vaginal entrance and a severe reduction in the perception of genital sensations during sexual arousal and orgasm was found with no recovery over time after definite surgery and radiotherapy for vulva cancer (31). In the exploratory cross-sectional study on long-term sexual function in survivors of vulvar cancer, adjuvant inguinal radiotherapy negatively affected sexual function by decreased ability to achieve orgasm (75). In a recent Cochrane review on the role of radiotherapy in the management of vulvar cancer, no QOL data was reported (82).
Further research is needed to better characterize survivorship issues and risk factors for impaired sexual function in vulvar cancer patients. Furthermore, there is a need for a valid instrument to address specific issues of relevance to the QOL including sexual functioning in vulvar cancer patients. The EORTC Quality of Life Group is developing a new questionnaire for QOL assessment of vulvar cancer patients which will complement the generic QOL questionnaire (QLQ-C30) and which among other issues assesses sexually related issues (personal communication).
Gastro-intestinal cancers
Rectal cancer
Pre-operative radiotherapy (PRT) has become an important part of the multimodality treatment of locally advanced rectal cancer showing a positive effect on local recurrence (83). The effect of chemo-radiation has been evaluated in a recent Cochrane review showing a significant positive effect on the local recurrence rate while no effect was found on overall survival (84). None of the Cochrane reviews included PROs but the addition of PRT has been shown to increase the prevalence of sexual dysfunction in several earlier and recent studies (5-8,28,43,85-91) In the study of Marijnen et al. female rectal cancer patients were randomly assigned to PRT + surgery [total mesorectal excision (TME)] or surgery only and followed for 24 months after treatment with PROs on QOL and sexual functioning (28). The Rotterdam Symptom Checklist was used for QOL assessment and supplemented with new items on voiding, defecation, and sexual problems. These additional items/scales were not validated prior to the study. Patients undergoing PRT were significantly less sexually active following treatment compared to before treatment. Compared to patients who had surgery only, PRT had a negative effect on sexual functioning (sexual interest, pleasure, and satisfaction) while, surprisingly, a similar level of vaginal dryness and dyspareunia was found across randomisation (Marijnen 2005). In the study of Lange et al. patients were randomly assigned to receive PRT + TME or TME only for localized rectal cancer (33). Although PROs were used the authors did not provide any information on which questionnaires that were used or whether the questionnaires used were validated. QOL, including several items on sexual functioning, was assessed prospectively up to 24 months post-surgery and the main aim of the study was to identify risk factors for sexual dysfunction. In total, 267 female patients participated of whom 51.7% were sexually active before treatment, dropping to 32.5% three months after treatment and with a further decrease to 18.4% being sexually active at 2 years post-treatment. Sixty-two per cent of the sexually active women reported either newly developed sexually dysfunctions or aggravation of pre-existing sexual dysfunctions after treatment and PRT was the only significant risk factor (33).
The cross-sectional study of Bruheim et al. deserves attention due to the use of a validated questionnaire to assess sexual functioning in a comparatively large population of female patients treated for rectal cancer (87). The aim of the study was to assess the impact of PRT (53%) or post-operative radiotherapy (47%) on sexual functioning. Patients were assessed at a median of 4.5 years post treatment and compared to patients who did not receive pre- or postoperative radiotherapy. In logistic regression analyses radiotherapy was associated with an increased risk of vaginal dryness, dyspareunia and vaginal shortness, even when adjusting for age and the presence of a stoma (87).
Searching the literature after 2010 reveals very few relevant papers on our subject. However, two large cross-sectional studies have been published lately (85,91). In the study of Bregendahl et al. the impact of PRT and surgical modality on FSD and urinary function was addressed (85). In total, 261 women, who were sexually active before treatment, were included and a validated questionnaire was used (23). In line with the literature before 2010 they found a significant association between PRT and reduced vaginal dimensions, dyspareunia, lack of desire, and sexual inactivity. Of those patients who were sexually active post-treatment 72% had vaginal dryness and more than half of the patients had dyspareunia which had worsened in 35% and 60% respectively, compared to pre-cancer levels. The most important reasons for not being sexually active were lack of desire, vaginal dryness, and dyspareunia (85).
In the long-term follow-up study of Wiltink et al. patients had been randomly assigned to PRT followed by TME versus TME only (91). Patients, alive 14 years after randomization, were well balanced between the two groups and asked to complete a questionnaire on sexual functioning among other issues. In total, 478 patients (82%) responded to the questionnaire; of these 197 women. Validated questionnaires were used (92). Significant impairment regarding vaginal dryness, sexual enjoyment and dyspareunia was found in the irradiated group compared to the surgery-only group and compared to the general population (91).
From the literature published before and after 2010 it is concluded that radiotherapy, independent of modality, has a severe negative impact on the sexual functioning in women treated for rectal cancer. Studies evaluating late effects and sexuality after modern radiotherapy are awaited. In a recent study, Kunneman et al. discuss prerequisites for shared decision making based on the individual patients’ weighing of benefits and harms caused by PRT in the treatment of rectal cancer (93). The issue of no overall survival benefit of PRT was seldom addressed whereas increased local control was mentioned in all consultations. Sexual dysfunction is considered as one of two most important side-effects of PRT and was only addressed in 65% of the consultations with female rectal cancer patients with no association between discussing sexual dysfunction and the patient’s level of education or marital status. The authors conclude that there is considerable variation in information provision and that this indicates lack of clarity on which benefits and harms that should be discussed before deciding on whether or not to give PRT (93). They advocates for standard information provision which should include information on potential sexual dysfunction.
Anal cancer
The introduction of primary radical radiotherapy and concomitant chemotherapy for anal carcinoma has shown increased local control rate and enabled sphincter conservation (94-96). Different treatment strategies are possible; external beam radiation with an external boosts or use of perineal implants and interstitial radiation. Further, studies have emerged evaluating the use of vaginal dilator during radiotherapy to spare the genitals (97). During the past 10 years studies have emerged evaluating more modern radiotherapy technologies as IMRT but few patients were included and mainly basic toxicity data were available from these studies (98,99).
Searching the literature before 2010 does not reveal any prospective studies evaluating the impact of chemo-radiation on sexual dysfunction in female anal cancer patients. Three smaller studies (34,44,100) retrospectively evaluated sexuality using validated single items from the EORTC colorectal disease specific questionnaire (92). Different radiation modalities were used in the three studies; external boost and interstitial brachytherapy and in the study of Oehler-Jänne et al. the two boosts were compared. However, comparison and judgement of the impact of chemo-radiation for anal cancer on female sexual functioning in these studies were not possible due to very small patient groups, very low sexual activity, and low compliance. However, even when taking a very conservative interpretation approach all three studies reported severe deterioration in several domains of sexual functioning e.g., dyspareunia, vaginal dryness, and lack of sexual enjoyment compared to healthy controls as included in the study of Jephcott et al. (44). When comparing the two different boost modalities in the study of Oehler-Jänne et al. no difference was observed between the two radiation methods (100). However, scores of dyspareunia, vaginal dryness, and sexual enjoyment were severely negatively affected compared to those of healthy age-matched women (100).
The literature search after 2010 revealed a few newer studies on PROs following definite chemo-radiation for anal cancer (101-105). No randomized or prospective studies were identified. The largest study included 101 female anal cancer patients and assessed long-term adverse effects following chemo-radiotherapy using PROs and comparing results with those of age-matched healthy control women (103). Sexual interests and dyspareunia were assessed using validated items from the EORTC colorectal questionnaire module. Significant and severe impairment was reported in all domains of QOL besides significant lack of sexual interest and considerable dyspareunia (103). In the study of Das et al. 26 women were included at a median of five years post radiotherapy (105). Conventional EBRT was used and sexual dysfunction was assessed using a validated questionnaire. Data was compared with historical results from women who e.g., underwent surgery for benign uterine myomas or patients with serious medical conditions. The scale scores on sexual functioning confirmed earlier findings of severe impairment in several domains of sexual functioning including difficulties in becoming aroused, inability to relax and enjoy sex and inability to reach orgasm (105). Comparable sparse information can be extracted from the study of Provencher et al. where 32 female anal patients were recruited retrospectively and assessed as long-term survivors (101). Sixty-five percent of the patients had no interest in sex and of those who were sexually active 50% had pain or discomfort during sexual intercourse. For the study of Philip et al. patients treated for rectal or anal carcinoma were recruited for an intervention study on sexual dysfunction (102). Hence, selection bias was integrated in this study and results are not reliable concerning prevalence or impact of sexual dysfunction. They found that sexual dysfunction was significantly and consistently associated with specific measures of psychological well-being most notably sexual/relationship satisfaction. The authors therefore suggest focusing on sexual/relationship satisfaction in the development and implementation of interventions for these patients (102). One study evaluated local control, survival, and toxicity related to use of IMRT in anal cancer (104). Conclusions about FSD were made but relied on notes in the patients’ file and were therefore severely biased (104).
There is limited data available on FSD following chemo-radiation for anal cancer. It has not been possible to identify any prospective data neither before nor after 2010. FSD was most often assessed using single items along with other QOL issues and was therefore not the primary outcome. From a clinical perspective severe mucosal and skin morbidity is often present following radiotherapy for anal cancer and may very well cause considerable sexual and vaginal morbidity impacting negatively on the QOL. Prospective studies are urgently needed to more validly assess sexual dysfunction outcomes in anal cancer patients. As in other pelvic cancers, the addition of chemotherapy to pelvic radiotherapy has proved effective in improving the local control rate. However, no positive effect on overall survival has been identified.
Urological cancer
Bladder cancer
Radical radiotherapy may be used as a bladder-conserving curative treatment of muscle-invasive bladder cancer. A recent review evaluated the safety and efficacy of a trimodality treatment (TMT) consisting of transurethral resection of the tumor followed by chemotherapy and radiotherapy published during the past two decades (106) and found excellent 5 years overall survival rate even in advanced stage tumors. In some countries this treatment strategy is reserved to cases where the patient cannot be offered a cystectomy and radical pelvic lymphadenectomy due to severe comorbidity. However, lately Gofrit et al. published a large retrospective case-control study showing that despite a significantly higher comorbidity index, patients treated with chemo-radiotherapy had similar overall and disease-free survival rates with a lower toxicity than those patients treated with radical surgery (107). Hence, the optimal therapeutic strategy for bladder cancer still seems controversial and the literature search both before and after 2010 revealed very limited data about radiation induced patient assessed late effects.
In the literature before 2010 four studies were identified that assessed QOL following radical radiotherapy for bladder cancer (30,108-111). However, due to a very limited number of female bladder cancer patients included, no information on their sexual functioning could be extracted. One study was identified in the period after 2010 that assessed QOL after TMT for bladder cancer (112). The study was prospective and included 51 patients of whom only six were female. Sexual function was assessed with one item evaluating, male and females together, whether the patient was sexually active or not. Hence, it is concluded that the present literature on radical radiotherapy for bladder cancer does not provide any valid information on its influence on female sexual function.
Discussion
Despite considerable effort to develop improved radiation methods, the latest literature confirms that patients who receive pelvic radiotherapy still suffer from severe late effects; sexual dysfunction is one of these (5,54,85,91,113-117).
The present updated narrative review indicates that pelvic radiotherapy, independent on modality, has a persistent negative effect on the sexual functioning of female cancer patients; compared to data from age-matched control women and data from patients treated by surgery only (62,113,117,118). There are areas for which there is not data available in the literature, e.g., bladder cancer, but several general studies indicate that severe late effects related to both small and large intestine, the bladder, the vagina, and the vulva are prevalent following pelvic radiotherapy (62,113,117,118). These studies also indicate that FSD is multi-factorial and therefore by nature influenced by e.g., (fear of) fecal incontinence, bladder urgency or incontinence, having a stoma, being depressed or experiencing poor body-image or lack of femininity (102,119). Further, sexuality in gynecological cancer patients has been suggested to be more influenced by the quality of sexual function than the quantity (63). Lately, studies have indicated several partner related issues; for the elderly patients comorbidity and erectile dysfunction may cause cessation of sexual activity but for the younger patient thoughts about the partner’s view on body and mind changes following a cancer diagnosis and radical radiotherapy may give rise to sexual insecurity and a feeling of not feeling sexually attractive (102).
It could be argued that almost all reported results reflect late effects related to radiotherapy as delivered by conventional or “old-fashioned” radiation methods and not taking the advantage of the 4D target concept for IGAB and newer methods of EBRT as IMRT into account. These methods are presumed to cause less morbidity than conventional radiation methods and results of subjective measures of e.g., vaginal morbidity, sexual and social functioning by patients are awaited in future studies. However, at present and presumably many years from now large cohorts of women are long-term survivors after conventional pelvic radiation and their late effects should be addressed prospectively and much more systematically than they are now.
In general, the literature search showed that during the past 4-5 years there has been a growing focus on PROs to evaluate late effects. This is a new trend in the oncological setting where the main approach for assessing late effects has been objective measures as evaluated by the doctor or other health care professionals and using rating scales. When searching the literature after 2010 it appears that several studies have emerged evaluating late effects by patient reported outcome measures thus acknowledging that the patient is the best to assess the impact of disease and treatment on her body and mind. However, there is still paucity on large scale studies on e.g., vaginal mucosal effects of radiotherapy and its impact on sexuality of cervical, rectal, bladder, and anal cancer patients; in particular, it is expected that the introduction of image guided adaptive brachytherapy will have less negative impact on vaginal morbidity.
Only few and small series of intervention studies for sexual problems after pelvic radiotherapy have been conducted. These have been evaluated in a recent Cochrane review (120). They document an effect of vaginal oestrogen, benzydamine, vaginal dilators, and hyperbaric oxygen therapy on acute and late vaginal radiation effect. However, only small series have been evaluated and the evidence is low. A protective effect of modern brachytherapy is mentioned but not sufficiently evaluable (120). Psychosocial intervention as e.g., communicating with the patient and her partner about sexual problems following radiotherapy may reduce fear and worries about sexuality. This may be the first and most important step towards sexual rehabilitation after cancer treatment. However, although most oncologists declare feeling comfortable with addressing issues on sexuality, sexual problems are very rarely discussed in the clinical setting (41,45,120-124). Hence, there still seems to be an apparent reticence to discuss the sexual consequences of cancer treatment with women. As mention by White et al. in a recent study of whether discussion of sexual matters is part of a routine follow up consultation following pelvic radiotherapy, this may partly be explained by the lack of biomedical intervention possibilities to treat FSD compared to those available for the management of erectile dysfunction in men (123). However, as mentioned by several authors and confirmed in several qualitative studies many problems may partly resolve just by the acknowledgement of their problem being treatment induced, being common among survivors, deserves attention, and may improve over time (45,123). It is our hope that the increasing focus on survivorship management will lead to a higher degree of openness and understanding that sexuality is an important part of cancer patients’ QOL and that patients who have received pelvic radiotherapy constitutes a vulnerable group.
Conclusions
Pelvic radiotherapy when delivered as conventional EBRT and/or brachytherapy has a persistent negative impact on female sexual functioning. The most prevalent problems are vaginal dryness, dyspareunia, varying degrees of vaginal shortness/stenosis, lack of sexual desire, and orgasmic problems. Radiotherapy induced vaginal morbidity may further preclude the ability to complete sexual intercourse and negatively influence enjoyment with having sex. The time has come for openness about sexual matters within the healthcare system and for intervention studies to defeat sexual impairment in long-term cancer survivors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010;375:816-23. [PubMed]
- Nout RA, Creutzberg CL. Point: Vaginal brachytherapy should be a standard adjuvant treatment for intermediate-risk endometrial cancer. Brachytherapy 2011;10:1-3. [PubMed]
- Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510-9. [PubMed]
- Kirchheiner K, Nout RA, Czajka-Pepl A, et al. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery - A mono-institutional prospective study. Gynecol Oncol 2015;136:415-23. [PubMed]
- Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790-9. [PubMed]
- Dunberger G, Lind H, Steineck G, et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int J Gynecol Cancer 2010;20:449-60. [PubMed]
- Incrocci L, Jensen PT. Pelvic radiotherapy and sexual function in men and women. J Sex Med 2013;10 Suppl 1:53-64. [PubMed]
- Jensen PT, Groenvold M, Klee M, et al. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2003;56:937-49. [PubMed]
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007-17. [PubMed]
- Grigsby PW, Russell A, Bruner D, et al. Late injury of cancer therapy on the female reproductive tract. Int J Radiat Oncol Biol Phys 1995;31:1281-99. [PubMed]
- Majewski W, Tarnawski R. Acute and late toxicity in radical radiotherapy for bladder cancer. Clin Oncol (R Coll Radiol) 2009;21:598-609. [PubMed]
- Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 1999;340:1383-9. [PubMed]
- Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Patient-rating of distressful symptoms after treatment for early cervical cancer. Acta Obstet Gynecol Scand 2002;81:443-50. [PubMed]
- Denton AS, Maher EJ. Interventions for the physical aspects of sexual dysfunction in women following pelvic radiotherapy. Cochrane Database Syst Rev 2003.CD003750. [PubMed]
- Jensen PT, Roed H, Engelholm SA, et al. Pulsed dose rate (PDR) brachytherapy as salvage treatment of locally advanced or recurrent gynecologic cancer. Int J Radiat Oncol Biol Phys 1998;42:1041-7. [PubMed]
- Schover LR, Fife M, Gershenson DM. Sexual dysfunction and treatment for early stage cervical cancer. Cancer 1989;63:204-12. [PubMed]
- Marks LB, Carroll PR, Dugan TC, et al. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1257-80. [PubMed]
- Parkin DE, Davis JA, Symonds RP. Long-term bladder symptomatology following radiotherapy for cervical carcinoma. Radiother Oncol 1987;9:195-9. [PubMed]
- Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol 2000;163:888-93. [PubMed]
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther 2001;27:395-403. [PubMed]
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208. [PubMed]
- Derogatis LR, Melisaratos N. The DSFI: a multidimensional measure of sexual functioning. J Sex Marital Ther 1979;5:244-81. [PubMed]
- Jensen PT, Klee MC, Thranov I, et al. Validation of a questionnaire for self-assessment of sexual function and vaginal changes after gynaecological cancer. Psychooncology 2004;13:577-92. [PubMed]
- Greimel ER, Kuljanic VK, Waldenstrom AC, et al. The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer 2006;107:1812-22. [PubMed]
- Greimel E, Nordin A, Lanceley A, et al. Psychometric validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Endometrial Cancer Module (EORTC QLQ-EN24). Eur J Cancer 2011;47:183-90. [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [PubMed]
- Davidson SE, Burns M, Routledge J, et al. Short report: a morbidity scoring system for Clinical Oncology practice: questionnaires produced from the LENT SOMA scoring system. Clin Oncol (R Coll Radiol) 2002;14:68-9. [PubMed]
- Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 2005;23:1847-58. [PubMed]
- Nout RA, van de Poll-Franse LV, Lybeert ML, et al. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J Clin Oncol 2011;29:1692-700. [PubMed]
- Fokdal L, Hoyer M, Meldgaard P, et al. Long-term bladder, colorectal, and sexual functions after radical radiotherapy for urinary bladder cancer. Radiother Oncol 2004;72:139-45. [PubMed]
- Weijmar Schultz WC, van de Wiel HB, Bouma J, et al. Psychosexual functioning after the treatment of cancer of the vulva. A longitudinal study. Cancer 1990;66:402-7. [PubMed]
- Flay LD, Matthews JH. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int J Radiat Oncol Biol Phys 1995;31:399-404. [PubMed]
- Lange MM, Marijnen CA, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 2009;45:1578-88. [PubMed]
- Allal AS, Sprangers MA, Laurencet F, et al. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer 1999;80:1588-94. [PubMed]
- Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Sexual functioning outcomes. J Consult Clin Psychol 1989;57:683-91. [PubMed]
- Bergmark K, Lundqvist EA, Steineck G. Swedish Study of Women treated for Cervical Cancer; Gynecologic cancer often affects Sexuality. Lakartidningen 2000;97:5347-55. [PubMed]
- Bruner DW, Lanciano R, Keegan M, et al. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int J Radiat Oncol Biol Phys 1993;27:825-30. [PubMed]
- Bye A, Ose T, Kaasa S. Quality of life during pelvic radiotherapy. Acta Obstet Gynecol Scand 1995;74:147-52. [PubMed]
- Bye A, Trope C, Loge JH, et al. Health-related quality of life and occurrence of intestinal side effects after pelvic radiotherapy--evaluation of long-term effects of diagnosis and treatment. Acta Oncol 2000;39:173-80. [PubMed]
- Cull A, Cowie VJ, Farquharson DI, et al. Early stage cervical cancer: psychosocial and sexual outcomes of treatment. Br J Cancer 1993;68:1216-20. [PubMed]
- Ekwall E, Ternestedt BM, Sorbe B. Important aspects of health care for women with gynecologic cancer. Oncol Nurs Forum 2003;30:313-9. [PubMed]
- Greimel ER, Freidl W. Functioning in daily living and psychological well-being of female cancer patients. J Psychosom Obstet Gynaecol 2000;21:25-30. [PubMed]
- Hendren SK, O'Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 2005;242:212-23. [PubMed]
- Jephcott CR, Paltiel C, Hay J. Quality of life after non-surgical treatment of anal carcinoma: a case control study of long-term survivors. Clin Oncol (R Coll Radiol) 2004;16:530-5. [PubMed]
- Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267-79. [PubMed]
- Park SY, Bae DS, Nam J, et al. Quality of life and sexual problems in disease-free survivors of cervical cancer compared with the general population. Cancer 2007;110:2716-25. [PubMed]
- van de Poll-Franse LV, Mols F, Essink-Bot ML, et al. Impact of external beam adjuvant radiotherapy on health-related quality of life for long-term survivors of endometrial adenocarcinoma: a population-based study. Int J Radiat Oncol Biol Phys 2007;69:125-32. [PubMed]
- Bradley S, Rose S, Lutgendorf S, et al. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecol Oncol 2006;100:479-86. [PubMed]
- Jereczek-Fossa BA, Jassem J, Badzio A. Relationship between acute and late normal tissue injury after postoperative radiotherapy in endometrial cancer. Int J Radiat Oncol Biol Phys 2002;52:476-82. [PubMed]
- Klee M, Machin D. Health-related quality of life of patients with endometrial cancer who are disease-free following external irradiation. Acta Oncol 2001;40:816-24. [PubMed]
- Nunns D, Williamson K, Swaney L, et al. The morbidity of surgery and adjuvant radiotherapy in the management of endometrial carcinoma. Int J Gynecol Cancer 2000;10:233-8. [PubMed]
- Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol 2009;27:3547-56. [PubMed]
- Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. L: Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 2012;48:1638-48. [PubMed]
- Quick AM, Seamon LG, Abdel-Rasoul M, et al. Sexual function after intracavitary vaginal brachytherapy for early-stage endometrial carcinoma. Int J Gynecol Cancer 2012;22:703-8. [PubMed]
- Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169-73. [PubMed]
- Damast S, Alektiar KM, Goldfarb S, et al. E: Sexual functioning among endometrial cancer patients treated with adjuvant high-dose-rate intra-vaginal radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e187-e193. [PubMed]
- Frumovitz M, Sun CC, Schover LR, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol 2005;23:7428-36. [PubMed]
- Greimel ER, Winter R, Kapp KS, et al. Quality of life and sexual functioning after cervical cancer treatment: a long-term follow-up study. Psychooncology 2009;18:476-82. [PubMed]
- Donovan KA, Taliaferro LA, Alvarez EM, et al. Sexual health in women treated for cervical cancer: characteristics and correlates. Gynecol Oncol 2007;104:428-34. [PubMed]
- Kirchheiner K, Nout RA, Tanderup K, et al. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study. Int J Radiat Oncol Biol Phys 2014;89:88-95. [PubMed]
- Harding Y, Ooyama T, Nakamoto T, et al. Radiotherapy- or radical surgery-induced female sexual morbidity in stages IB and II cervical cancer. Int J Gynecol Cancer 2014;24:800-5. [PubMed]
- Abbott-Anderson K, Kwekkeboom KL. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol Oncol 2012;124:477-89. [PubMed]
- Juraskova I, Bonner C, Bell ML, et al. Quantity vs. quality: an exploration of the predictors of posttreatment sexual adjustment for women affected by early stage cervical and endometrial cancer. J Sex Med 2012;9:2952-60. [PubMed]
- Lammerink EA, de Bock GH, Pras E, et al. Sexual functioning of cervical cancer survivors: a review with a female perspective. Maturitas 2012;72:296-304. [PubMed]
- Mantegna G, Petrillo M, Fuoco G, et al. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. BMC Cancer 2013;13:127. [PubMed]
- Pieterse QD, Kenter GG, Maas CP, et al. Self-reported sexual, bowel and bladder function in cervical cancer patients following different treatment modalities: longitudinal prospective cohort study. Int J Gynecol Cancer 2013;23:1717-25. [PubMed]
- Vaz AF, Conde DM, Costa-Paiva L, et al. Quality of life and adverse events after radiotherapy in gynecologic cancer survivors: a cohort study. Arch Gynecol Obstet 2011;284:1523-31. [PubMed]
- Kirchheiner K, Nout R, Lindegaard J, et al. Do clinicians and patients agree regarding symptoms? A comparison after definitive radiochemotherapy in 223 uterine cervical cancer patients. Strahlenther Onkol 2012;188:933-9. [PubMed]
- Chopra S, Engineer R, Mahantshetty U, et al. Protocol for a phase III randomised trial of image-guided intensity modulated radiotherapy (IG-IMRT) and conventional radiotherapy for late small bowel toxicity reduction after postoperative adjuvant radiation in Ca cervix. BMJ Open. BMJ Open 2012;2. pii: e001896.
- Aerts L, Enzlin P, Verhaeghe J, et al. Sexual and psychological functioning in women after pelvic surgery for gynaecological cancer. Eur J Gynaecol Oncol 2009;30:652-6. [PubMed]
- Andreasson B, Moth I, Jensen SB, et al. Sexual function and somatopsychic reactions in vulvectomy-operated women and their partners. Acta Obstet Gynecol Scand 1986;65:7-10. [PubMed]
- Green MS, Naumann RW, Elliot M, et al. Sexual dysfunction following vulvectomy. Gynecol Oncol 2000;77:73-7. [PubMed]
- Janda M, Obermair A, Cella D, et al. Vulvar cancer patients' quality of life: a qualitative assessment. Int J Gynecol Cancer 2004;14:875-81. [PubMed]
- Moth I, Andreasson B, Jensen SB, et al. Sexual function and somatopsychic reactions after vulvectomy. A preliminary report. Dan Med Bull 1983;30 Suppl 2:27-30. [PubMed]
- Hazewinkel MH, Laan ET, Sprangers MA, et al. Long-term sexual function in survivors of vulvar cancer: a cross-sectional study. Gynecol Oncol 2012;126:87-92. [PubMed]
- Aerts L, Enzlin P, Verhaeghe J, et al. Psychologic, relational, and sexual functioning in women after surgical treatment of vulvar malignancy: a prospective controlled study. Int J Gynecol Cancer 2014;24:372-80. [PubMed]
- de Melo Ferreira AP, de Figueiredo EM, Lima RA, et al. Quality of life in women with vulvar cancer submitted to surgical treatment: a comparative study. Eur J Obstet Gynecol Reprod Biol 2012;165:91-5. [PubMed]
- Günther V, Malchow B, Schubert M, et al. Impact of radical operative treatment on the quality of life in women with vulvar cancer--a retrospective study. Eur J Surg Oncol 2014;40:875-82. [PubMed]
- Oonk MH, van Os MA, de Bock GH, et al. A comparison of quality of life between vulvar cancer patients after sentinel lymph node procedure only and inguinofemoral lymphadenectomy. Gynecol Oncol 2009;113:301-5. [PubMed]
- Tamburini M, Filiberti A, Ventafridda V, et al. Quality of Life and Psychological State after Radical vulvectomy. Journal of Psychosomatic Obstetrics & Gynecology 1986;5:263-9.
- Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:683-701. [PubMed]
- Shylasree TS, Bryant A, Howells RE. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst Rev 2011.CD003752. [PubMed]
- Wong RK, Tandan V, De Silva S, et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev 2007.CD002102. [PubMed]
- McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 2012;12:CD008368. [PubMed]
- Bregendahl S, Emmertsen KJ, Lindegaard JC, et al. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2015;17:26-37. [PubMed]
- Bruheim K, Guren MG, Skovlund E, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:1005-11. [PubMed]
- Bruheim K, Tveit KM, Skovlund E, et al. Sexual function in females after radiotherapy for rectal cancer. Acta Oncol 2010;49:826-32. [PubMed]
- Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol 2011;8:51-7. [PubMed]
- Tekkis PP, Cornish JA, Remzi FH, et al. Measuring sexual and urinary outcomes in women after rectal cancer excision. Dis Colon Rectum 2009;52:46-54. [PubMed]
- Traa MJ, De VJ, Roukema JA, et al. Sexual (dys)function and the quality of sexual life in patients with colorectal cancer: a systematic review. Ann Oncol 2012;23:19-27. [PubMed]
- Wiltink LM, Chen TY, Nout RA, et al. Health-related quality of life 14 years after preoperative short-term radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomised trial. Eur J Cancer 2014;50:2390-8. [PubMed]
- Sprangers MA. te VA, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238-47. [PubMed]
- Kunneman M, Marijnen CA, Rozema T, et al. Decision consultations on preoperative radiotherapy for rectal cancer: large variation in benefits and harms that are addressed. Br J Cancer 2015;112:39-43. [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [PubMed]
- Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000;342:792-800. [PubMed]
- Young SC, Solomon MJ, Hruby G, et al. Review of 120 anal cancer patients. Colorectal Dis 2009;11:909-14. [PubMed]
- Briere TM, Crane CH, Beddar S, et al. Reproducibility and genital sparing with a vaginal dilator used for female anal cancer patients. Radiother Oncol 2012;104:161-6. [PubMed]
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354-61. [PubMed]
- Bazan JG, Hara W, Hsu A, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011;117:3342-51. [PubMed]
- Oehler-Jänne C, Seifert B, Lütolf UM, et al. Clinical outcome after treatment with a brachytherapy boost versus external beam boost for anal carcinoma. Brachytherapy 2007;6:218-26. [PubMed]
- Provencher S, Oehler C, Lavertu S, et al. Quality of life and tumor control after short split-course chemoradiation for anal canal carcinoma. Radiat Oncol 2010;5:41. [PubMed]
- Philip EJ, Nelson C, Temple L, et al. Psychological correlates of sexual dysfunction in female rectal and anal cancer survivors: analysis of baseline intervention data. J Sex Med 2013;10:2539-48. [PubMed]
- Bentzen AG, Balteskard L, Wanderas EH, et al. Impaired health-related quality of life after chemoradiotherapy for anal cancer: late effects in a national cohort of 128 survivors. Acta Oncol 2013;52:736-44. [PubMed]
- Mitchell MP, Abboud M, Eng C, et al. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol 2014;37:461-6. [PubMed]
- Das P, Cantor SB, Parker CL, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer 2010;116:822-9. [PubMed]
- Arcangeli G, Arcangeli S, Strigari L. A systematic review and meta-analysis of clinical trials of bladder-sparing trimodality treatment for muscle-invasive bladder cancer (MIBC). Crit Rev Oncol Hematol 2015;94:105-15. [PubMed]
- Gofrit ON, Nof R, Meirovitz A, et al. Radical cystectomy vs. chemoradiation in T2-4aN0M0 bladder cancer: a case-control study. Urol Oncol 2015;33:19.e1-5.
- Caffo O, Fellin G, Graffer U, et al. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer 1996;78:1089-97. [PubMed]
- Henningsohn L, Wijkstrom H, Dickman PW, et al. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol 2002;62:215-25. [PubMed]
- Henningsohn L, Wijkstrom H, Pedersen J, et al. G: Time after surgery, symptoms and well-being in survivors of urinary bladder cancer. BJU Int 2003;91:325-30. [PubMed]
- Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol 2003;170:1772-6. [PubMed]
- Lagrange JL, Bascoul-Mollevi C, Geoffrois L, et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: results of a multicenter prospective study (GETUG 97-015). Int J Radiat Oncol Biol Phys 2011;79:172-8. [PubMed]
- Lind H, Waldenstrom AC, Dunberger G, et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: a population-based cohort study. Br J Cancer 2011;105:737-45. [PubMed]
- Adams E, Boulton MG, Horne A, et al. The effects of pelvic radiotherapy on cancer survivors: symptom profile, psychological morbidity and quality of life. Clin Oncol (R Coll Radiol) 2014;26:10-7. [PubMed]
- Cakar B, Karaca B, Uslu R. Sexual dysfunction in cancer patients: a review. J BUON 2013;18:818-23. [PubMed]
- Dunberger G, Lind H, Steineck G, et al. Self-reported symptoms of faecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur J Cancer 2010;46:606-15. [PubMed]
- Vomvas D, Iconomou G, Soubasi E, et al. Assessment of sexual function in patients with cancer undergoing radiotherapy--a single centre prospective study. Anticancer Res 2012;32:657-64. [PubMed]
- Barraclough LH, Routledge JA, Farnell DJ, et al. Prospective analysis of patient-reported late toxicity following pelvic radiotherapy for gynaecological cancer. Radiother Oncol 2012;103:327-32. [PubMed]
- Milbury K, Cohen L, Jenkins R, et al. The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer 2013;21:793-802. [PubMed]
- Flynn P, Kew F, Kisely SR. Interventions for psychosexual dysfunction in women treated for gynaecological malignancy. Cochrane Database Syst Rev 2009.CD004708. [PubMed]
- Stead ML. Sexual function after treatment for gynecological malignancy. Curr Opin Oncol 2004;16:492-5. [PubMed]
- Wang J, Sun X, Cai R, et al. Attitudes and behavior of radiation oncologists toward sexual issues of cervical cancer patients who receive radiation therapy: a survey in China. Int J Gynecol Cancer 2013;23:393-8. [PubMed]
- White ID, Allan H, Faithfull S. Assessment of treatment-induced female sexual morbidity in oncology: is this a part of routine medical follow-up after radical pelvic radiotherapy? Br J Cancer 2011;105:903-10. [PubMed]
- Wiggins DL, Wood R, Granai CO, et al. Sex, intimacy, and the gynecologic oncologists: survey results of the New England Association of Gynecologic Oncologists (NEAGO). J Psychosoc Oncol 2007;25:61-70. [PubMed]


