
Propensity-matched pair analysis of safety and efficacy between laparoscopic and open radical nephrectomy for the treatment of large renal masses (>10 cm): a retrospective cohort study
Introduction
Since laparoscopic radical nephrectomy (LRN) was first reported by Clayman et al. in 1991, the procedure has been widely accepted as the recommended surgical plan for T1 and selected T2 renal cell carcinoma (RCC) cases (1-4). LRN for localized RCC has shown clear benefits in terms of superior safety and prognosis, with reports of decreased blood loss, shorter hospital days, less pain, and fast recovery (5-7). The oncological equivalence of LRN to open radical nephrectomy (ORN) in T1 and T2a RCC has been confirmed by several studies (3,5,8,9). ORN is a feasible procedure for treating patients with RCC >10 cm in size, and few studies have focused on safety and efficacy of LRN. And the main concern regarding LRN treating large renal tumors is whether the small operating space caused by a large tumor complicates dissection and consequently causes more complications such as bleeding and peripheral organ injury. It remains to be determined whether minimally invasive surgery for large renal tumors can achieve excellent tumor control and may affect tumor-associated prognosis (3). Moreover, there might be potential confounders such as age, tumor size that would affect procedure selection and surgical outcomes between two groups. Therefore, in the current study, we conducted a propensity matched pair analysis to evaluate the safety and efficacy of LRN and ORN. In addition, we compared postoperative recovery and survival status in the 2 groups. We present our article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-449/rc).
Methods
Patients and selection criteria
In this cohort study, medical records were retrospectively reviewed for all patients with large (>10 cm) RCC (T2bN0M0) (10) in Changhai Hospital between October 2010 to October 2018. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Changhai Hospital (No. CHEC2021-191). Written informed consent was obtained from each patient for publication.
The inclusion criteria were listed as follows: (I) RCC patients with clinical stage T2bN0M0; (II) patients who underwent LRN or ORN. Clinical stage was determined by imaging with either CT or MRI, and tumor size was defined by the maximum diameter. For both LRN and ORN, the exclusion criteria were as follows: (I) benign renal masses; (II) tumor thrombi involving renal vein or inferior vena cava; (III) enlarged retroperitoneal lymph nodes; (IV) extensive involvement of adjacent structures; (V) metastatic disease; and with (VI) indeterminate pathological findings.
Measurements and outcomes
Patients’ demographic (gender, age, tumor laterality and size, and BMI), operative (estimated blood loss, operative time, open surgery conversion rate, perioperative serum creatinine levels, complications and hospital stay), pathological (histological type, pathological stage, tumor grade, and margin status), and postoperative (tumor recurrence) information were collected. Complications classifications were defined by the modified Clavien-Dindo system (11). Kaplan-Meier curve was used to analyze the overall survival (OS), cancer-specific survival (CSS), and progression-free survival (PFS). Patients received telephone or outpatient follow-up until the final follow-up or death. The follow-up contents included survival time, disease progression, and cause of death.
Surgical methods
All procedures were performed by surgeons who had experience in both laparoscopic and open surgery. Whether LRN or ORN to be conducted was determined according to patients’ comorbidities (clinical manifestations), previous surgical history, and surgeons’ or patients’ preferences. Lymph node dissection was not routinely performed but renal hilum lymphadenectomy was performed if intraoperative inspection revealed suspicious nodal enlargement. Open conversion was considered in those unable to curative resection or with uncontrollable bleeding.
Statistical analysis
Propensity score was estimated by binary logistic regression, considering gender, age, BMI, and tumor maximal diameter to adjust for potential baseline confounders (12). Continuous variables comparisons were conducted by student’s t-test or Mann-Whitney test, and categorical variables by Pearson’s chi-square or Fisher’s exact test. The Kaplan-Meier curve was used to analyze the survival data, and differences between groups were conducted by the log-rank test. A two-side P value less than 0.05 were considered statistically significant. The analyses were performed by the SPSS software package version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
For this series of 117 renal tumor patients (61 LRN and 56 ORN) with tumor size over 10 cm, 25 cases (neurilemmoma in 1 case, tumor thrombi involving the renal vein or inferior vena cava in 15 cases, lymphatic metastasis in 6 cases, lung metastasis in 5 cases, liver metastasis in 3 cases, bone metastasis in 2 cases, adrenal metastasis in 1 case, and involvement of adjacent structures in 2 cases) were excluded from the study. PSM identified 37 LRN and 37 ORN patients from 92 patients who met the inclusion criteria for this analysis. The demographic features and tumor characteristics of the patients are presented in Table 1. No significant differences were found in baseline features such as age, gender, BMI, ASA (American Society of Anesthesiologists) score, tumor laterality, and tumor size. All the 74 patients underwent surgical resection of tumors successfully. The average operating time in LRN was slightly longer than that in ORN, with no statistical significance (204.32±11.17 vs. 192.78±8.50 min, P=0.414). Estimated blood loss in the LRN group was considerably less than that in the ORN group (336.5±63.6 vs. 546.0±74.5 mL, P=0.036). Lymphadenectomy was performed in 7 patients (18.9%) in the LRN group and 13 patients (35.1%) in the ORN group. The rate of adrenalectomy was lower in the LRN group compared with that in the ORN group [43.2% (16/37) vs. 81.1% (30/37), P=0.002]. All lymph nodes and adrenalectomy specimens were free of tumor involvement on histopathological evaluation. No significant differences were observed in terms of the preoperative creatinine level, estimated glomerular filtration rate (eGFR), and hemoglobin. The postoperative eGFR decreased, and the declining trend was similar in the 2 groups, as shown in Table 1.
Table 1
| Characteristics | LRN (n=37) | ORN (n=37) | P value |
|---|---|---|---|
| Patient characteristics | |||
| Gender, n (%) | 0.815 | ||
| Male | 20 (54.1) | 22 (59.5) | |
| Female | 17 (45.9) | 15 (40.5) | |
| Age at surgery, years, mean ± SD | 51.7±2.4 | 50.8±2.3 | 0.773 |
| BMI (kg/m2), mean ± SD | 23.71±0.49 | 23.58±0.51 | 0.855 |
| ASA score, n (%) | 0.665 | ||
| 1 | 5 (13.5) | 3 (8.1) | |
| 2 | 29 (78.4) | 32 (86.5) | |
| 3 | 3 (8.1) | 2 (5.4) | |
| Creatinine, mean ± SD | 111.27±36.47 | 97.97±17.37 | 0.743 |
| eGFR (preoperative) (mL/min/1.73 m2) | 94.99±4.46 | 90.91±4.49 | 0.521 |
| eGFR (postoperative) (mL/min/1.73 m2) | 70.58±3.39 | 67.71±4.37 | 0.519 |
| LDH (U/L), mean ± SD | 179.9±9.2 | 225.6±20.3 | 0.044‡ |
| ALP (U/L), mean ± SD | 83.7±8.8 | 73.8±5.1 | 0.334 |
| Tumor characteristics | |||
| Laterality, n (%) | |||
| Left | 16 (43.2) | Matched | – |
| Right | 21 (56.8) | ||
| Maximal diameter, cm, mean ± SD | 11.37±0.30 | 11.76±0.33 | 0.375 |
| Operative characteristics | |||
| Laparoscopic conversion, n (%) | 3 (8.1) | None | – |
| Estimated blood loss, mL, mean ± SD | 336.49±63.58 | 545.95±74.52 | 0.036‡ |
| Operation time, minutes, mean ± SD | 204.32±11.17 | 192.78±8.50 | 0.414 |
| Adrenalectomy, n (%) | 16 (43.2) | 30 (81.1) | 0.002† |
| Lymphadenectomy, n (%) | 7 (18.9) | 13 (35.1) | 0.190 |
| Transperitoneal, n (%) | 29 (78.4) | 22 (59.5) | 0.131 |
| Retroperitoneal, n (%) | 8 (21.6) | 15 (40.5) | |
| Length of postoperative stay, days, median (IQR) | 6.0 (5.0–9.0) | 9.0 (6.0–11.5) | 0.015‡ |
| Removal time of the drainage tube, days, median (IQR) | 4.0 (3.0–5.0) | 5.0 (4.0–6.0) | 0.000‡ |
| Pathological subtype of RCC, n (%) | 0.467 | ||
| Clear cell | 21 (56.8) | 26 (70.3) | |
| Papillary | 3 (8.1) | 2 (5.4) | |
| Chromophobe | 8 (21.6) | 4 (10.8) | |
| TFE3 | 2 (5.4) | 1 (2.7) | |
| Sarcomatoid | 0 | 2 (5.4) | |
| Other types | 3 (8.1) | 2 (5.4) | |
| Fuhrman grade, n (%) | 0.510 | ||
| 1–2 | 13 | 13 | |
| 3–4 | 5 | 9 |
Significant (Student’s t test; P<0.05); †, Significant (Pearson’s chi-square test; P<0.05); ‡, Significant (Wilcoxon rank sum test; P<0.05). LRN, laparoscopic radical nephrectomy; ORN, open radical nephrectomy; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; IQR, interquartile range; RCC, renal cell carcinoma.
Complications
Complications are summarized in Table 2. The Clavien-Dindo complications included prolonged postoperative pain duration (3 LRN vs. 4 ORN) and drainage tube removal time (2 LRN vs. 5 ORN) requiring prolonged hospitalization. Grade II complications mainly included perioperative anemia requiring blood transfusion in 6 patients (16.2%) in the LRN group and 13 patients (35.1%) in the ORN group. There were 3 LRN patients with massive hemorrhage requiring intraoperative transfusion and eventual conversion to open surgery, among whom one had renal artery bleeding and 2 patients had renal vein injury. The rate of vascular injury was 8.1% (3/37) in the LRN group, which was higher than the rate of vascular injury in the ORN group. There was 1 patient who underwent rigid cystoscopy due to hematuria after LRN. Grade IV complications occurred in 1 LRN and 3 ORN cases. In the LRN group, 1 patient was treated in the intensive care unit (ICU) for respiratory insufficiency. In the ORN group, 3 patients required ICU-level care, including 2 patients with renal insufficiency who required hemodialysis and 1 patient who experienced atrial fibrillation and required hemodynamic monitoring. The postoperative stay was shorter in LRN [6.0 (5.0–9.0) vs. 9.0 (6.0–11.5) d, P=0.015], and the removal time of the drainage tube after surgery was also significantly shorter in LRN [4.0 (3.0–5.0) vs. 5.0 (4.0–6.0) d, P<0.001].
Table 2
| Grades and complications | LRN (n=37) | ORN (n=37) | P value |
|---|---|---|---|
| Grade I, n (%) | 6 (16.2) | 4 (10.8) | |
| Prolonged pain | 2 (5.4) | 2 (5.4) | |
| Fever | 1 (2.7) | 0 (0) | |
| Persistent drain output | 1 (2.7) | 1 (2.7) | |
| Transient ileus | 2 (5.4) | 1 (2.7) | |
| Grade II, n (%) | 7 (18.9) | 14 (37.8) | |
| Need for blood transfusion | 6 (16.2) | 13 (35.1) | |
| Pneumonia | 1 (2.7) | 1 (2.7) | |
| Grade III, n (%) | 1 (2.7) | 0 (0) | |
| Hematuria requiring rigid cystoscopy | 1 (2.7) | 0 (0) | |
| Grade IV, n (%) | 1 (2.7) | 3 (8.1) | |
| Renal insufficiency requiring hemodialysis | 0 (0) | 2 (5.4) | |
| Respiratory insufficiency requiring ICU care | 1 (2.7) | 0 (0) | |
| Atrial fibrillation requiring ICU care | 0 (0) | 1 (2.7) | |
| Overall | 15 (40.5) | 21 (56.7) | 0.279 |
LRN, laparoscopic radical nephrectomy; ORN, open radical nephrectomy; ICU, intensive care unit.
Pathology and follow-up data
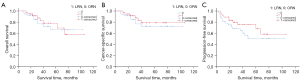
The pathological characteristics were comparable in the 2 groups, as shown in Table 3. In this study, all the 74 patients were eligible for follow-up analysis. The 2 groups were both followed up for a similar period (43.0±3.78 vs. 45.7±4.2 months, P=0.633). The five-year OS, CSS, and PFS rates following LRN were 69.4%, 78.8%, and 68.2%, respectively, which were higher than those following ORN, with no statistical significance (Figure 1, Table 3). No local or port site recurrence was found during the follow-up period. Metastasis or disease recurrence occurred in 10 patients (27.0%) in the LRN group and 16 patients (43.2%) in the ORN group. The distant metastasis pattern is shown in Table 3, and the most common site of metastasis was the lung. The median interval to metastasis was 26.1±7.0 months in the LRN group and 19.4±4.0 months in the ORN group. There were 6 out of 10 patients in the LRN group and 10 out of 16 patients in the ORN group with metastases who died of the disease. In addition, one LRP patient (myocardial infarction) and 2 patients in the ORN group (stroke and myocardial infarction) died of non-cancer related causes.
Table 3
| Follow-up and oncological outcomes | LRN (n=37) | ORN (n=37) | P value |
|---|---|---|---|
| Length of follow-up of censored patients, months, mean ± SD | 43.0±3.78 | 45.7±4.2 | 0.633 |
| Metastasis, n (%) | 10 (27.0) | 16 (43.2) | 0.182 |
| Metastasis sites, n (%) | 0.333 | ||
| Lung | 6 (16.2) | 10 (27.0) | |
| Contralateral kidney | 1 (2.7) | 0 | |
| Liver | 1 (2.7) | 2 (5.4) | |
| Brain | 0 (2.7) | 1(2.7) | |
| Skeleton | 2 (5.4) | 3 (8.1) | |
| Interval to metastasis, months, mean ± SD | 26.1±7.0 | 19.4±4.0 | 0.379 |
| % 5-year survival | |||
| Overall | 69.4 | 66.4 | 0.802 |
| Cancer-specific | 78.8 | 72.5 | 0.507 |
| Progression-free | 68.2 | 50.6 | 0.222 |
LRN, laparoscopic radical nephrectomy; ORN, open radical nephrectomy.
Discussion
Few studies have focused on the surgical safety and oncological outcomes for the treatment of renal tumors >10 cm, and these studies have been somewhat limited by biases caused by indeterminate results and small sample sizes as well as lower surgical success rates (13,14). The current study presented a comparison of 37 matched pairs of patients to evaluate the effectiveness and reliability of LRN for extremely large renal carcinomas. This study is novel in several respects. Firstly, 37 matched pairs of patients were carefully selected from 92 patients with clinical T2b disease through PSM to compare 2 surgical techniques. Due to the strict criteria employed in pair matching for tumor size, age, and BMI, group gaps in baseline data were reduced, resulting in comparable characteristics among patients and tumors. Additionally, other non-matched items, including tumor laterality, ASA score, and preoperative hemoglobin and eGFR, showed no significantly difference between the LRN and ORN groups. Secondly, each patient was treated and followed up with a standard protocol, including experienced consulting urological surgeons and pathologists from a single standardized diagnosis and treatment center. The follow-up was carried out with ultrasonography and CT scans every 6 months after surgery. Furthermore, various objective clinical data were collected during the perioperative period, which were used to evaluate surgical safety and patient status from other aspects.
Due to the cumulative risk of tumor rupture or spillage and extending the incision length to remove a larger specimen, larger renal masses negate the benefits of laparoscopic surgery. Earlier studies have suggested that LRN should be limited exclusively to patients with renal masses of less than 5 cm (6,15). As urologists have become more experienced and new techniques including laparoscopic specimen retrieval bags have been used for laparoscopic surgery, LRN has been a well-established alternative modality to ORN for T1 and some T2 renal tumors (16,17). However, the expanded criterion for the appropriate tumor size has posed more than a few practical difficulties for laparoscopic procedures. An increase in the diameter of the tumor creates a smaller working space and makes it more difficult to approach the renal hilum, especially in larger masses. Additionally, the rate of massive hemorrhage and blood transfusion for laparoscopic surgery for RCC >10 cm has been found to be markedly higher than that for RCC <10 cm, resulting from the abundance of nourish vessels and high probability of oncological violation (17). In this research, there were 4 patients in the LRN group with EBL greater than 1,000 mL, primarily due to arteriovenous bleeding related to traction of the vena cava and vascular avulsion injury. Additionally, a large mass located in the upper pole of the kidney significantly narrowed the operative field exposure. Once the vasculature behind the vena cava was torn, hemostasis and patching under laparoscopy became difficult, which is also a common reason for conversion to open surgery (18). However, ORN did not show a decreased rate of massive bleeding (>1,000 mL) for RCC >10 cm (10.8% for LRN vs. 21.6% for ORN), indicating an advantage of LRN on less EBL for larger tumors. Generally, LRN is superior to ORN in terms of perioperative outcomes and the overall complication rate.
This successive series of 37 LRN cases with an average tumor size of 11.37 cm confirmed comparable surgical feasibility with ORN, which was consistent with the corresponding values for previous laparoscopic operations. Hemal et al. described their experience with 41 patients treated with LRN, and the average tumor size, OT, and open conversion rate was comparable with this study (13). Steinberg et al. and Grimaud et al. reported comparable perioperative outcomes for tumors >10 cm (19,20). In all, the common results showed that LRN had a longer operation time and less blood loss than ORN. Additionally, our research identified some different outcomes following the procedures. The length of postoperative stay of patients was significantly shorter in previous studies (17), indicating a worse outcome in the current study. In addition, the complication rate was higher in our study (21), especially for the rate of blood loss and several complications needing ICU monitoring, and the main causes may include larger tumor volume and increased technical difficulty. In this series of patients, 3 LRN cases were converted to open surgery due to renal arterial hemorrhage (n=1) and renal vein injury (n=2). Open conversion, which may be most appropriate in certain cases, should be considered to increase resectability and decrease complications. Although it is not as widely applicable as open surgery, LRN for large RCC showed feasibility in carefully selected patients. Although the upper limit of tumor size acceptable for direct laparoscopic removal remains to be resolved based on available evidence and our research, tumor size was not the most important factor limiting surgical selection. Overall, LRN for large RCC was a challenging technique but a feasible one.
Our data showed a 78.8% 5-year CSS rate and a 68.2% 5-year PFS rate in the LRN group, which were not remarkably lower than the corresponding rates in the ORN group in the univariate analysis, without a statistically significant difference. The median follow-up length of 43.0 months following LRN for T2bN0M0 RCC resulted in a reliable tumor prognosis; however, it was limited by the relatively small number of patients and slightly different pathology. Quite a few studies have reported that LRN for stage pT1-T2 RCC, including a 5-year CSS rate of 90–98% and a 5-year OS rate of 81–95%, were equivalent to those following ORN (22-24). Jeon et al. reported that the LRN group had 5-year overall (93.5% vs. 89.8%, P=0.120) and recurrence-free (94.0% vs. 92.8%, P=0.082) survival rates comparable to those of the ORN group (21). Hemal et al. also reported that the LRN group had 5-year cancer-specific (95.12 vs. 94.36, P=0.79) and recurrence-free (92.6 vs. 90.1, P=0.91) survival rates equivalent to those of the ORN group (13). All these findings show comparable OS, CSS, and PFS between LRN and ORN, and the former has the same comparable outcome as the latter in T2 RCC. However, few studies have focused on oncological outcomes following LRN for T2b renal tumors. Both groups in our study with stage T2b RCC showed worse CSS and PFS, which was consistent with research conducted by Novara et al. (CSS 70%) (25). The rate of metastasis in this report was significantly higher than that reported for pT1-T2a RCC, in that pathological stage and tumor size had an extreme influence on oncological outcomes.
Our analysis had several inherent limitations. Firstly, although strict criteria were developed to standardize patient selection and pair matching, patients were selected for LRN or ORN at the discretion of the surgeons based on patient status, patient preference, and other immeasurable factors. However, this could not be completely avoided due to ethical requirements. Due to strict matching, the sample size of the study was small and the likelihood of random errors and potential selection bias in the propensity score match may be high. Secondly, the retrospective design of the study limited its objectivity, with innate biases related to data collection. Lastly, the follow-up time for some patients was too short to conclude the long-term oncological efficacy of laparoscopic nephrectomy.
Conclusions
The study confirmed that large renal tumors (greater than 10 cm) can be safely radically resected laparoscopically with less blood loss than ORN, with comparable surgical and oncological outcomes. LRN resulted in a 5-year CSS rate of 78.8% and a 5-year PFS rate of 68.2%, which were similar to the corresponding survival rates for ORN.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-449/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-449/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-449/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Changhai Hospital (No. CHEC2021-191). Written informed consent was obtained from each patient for publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991;146:278-82. [Crossref] [PubMed]
- Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010;58:398-406. [Crossref] [PubMed]
- Dursun F, Elshabrawy A, Wang H, et al. Survival after minimally invasive vs. open radical nephrectomy for stage I and II renal cell carcinoma. Int J Clin Oncol 2022;27:1068-76. [Crossref] [PubMed]
- Muaddi H, Hafid ME, Choi WJ, et al. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann Surg 2021;273:467-73. [Crossref] [PubMed]
- Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol 2000;164:1153-9. [Crossref] [PubMed]
- Ono Y, Kinukawa T, Hattori R, et al. The long-term outcome of laparoscopic radical nephrectomy for small renal cell carcinoma. J Urol 2001;165:1867-70. [Crossref] [PubMed]
- Chen S, He Z, Yao S, et al. Enhanced Recovery After Surgery Protocol Optimizes Results and Cost of Laparoscopic Radical Nephrectomy. Front Oncol 2022;12:840363. [Crossref] [PubMed]
- Shuford MD, McDougall EM, Chang SS, et al. Complications of contemporary radical nephrectomy: comparison of open vs. laparoscopic approach. Urol Oncol 2004;22:121-6. [Crossref] [PubMed]
- Gill IS, Meraney AM, Schweizer DK, et al. Laparoscopic radical nephrectomy in 100 patients: a single center experience from the United States. Cancer 2001;92:1843-55. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-26. [PubMed]
- Leiss F, Schindler M, Götz JS, et al. Superior Functional Outcome and Comparable Health-Related Quality of Life after Enhanced Recovery vs. Conventional THA: A Retrospective Matched Pair Analysis. J Clin Med 2021;10:3096. [Crossref] [PubMed]
- Hemal AK, Kumar A, Kumar R, et al. Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. J Urol 2007;177:862-6. [Crossref] [PubMed]
- Conley SP, Humphreys MR, Desai PJ, et al. Laparoscopic radical nephrectomy for very large renal tumors (> or =10 cm): is there a size limit? J Endourol 2009;23:57-61. [Crossref] [PubMed]
- Abbou CC, Cicco A, Gasman D, et al. Retroperitoneal laparoscopic versus open radical nephrectomy. J Urol 1999;161:1776-80. [Crossref] [PubMed]
- Guo X, Wang H, Xiang Y, et al. Safety and oncological outcomes for large (stage ≥T2b) and locally advanced renal cell carcinoma: comparison between laparoscopic and modified hand-assisted laparoscopic radical nephrectomy. J Int Med Res 2020;48:300060520961238. [Crossref] [PubMed]
- Pierorazio PM, Hyams ES, Lin BM, et al. Laparoscopic radical nephrectomy for large renal masses: critical assessment of perioperative and oncologic outcomes of stage T2a and T2b tumors. Urology 2012;79:570-5. [Crossref] [PubMed]
- Liu Z, Tang S, Tian X, et al. Laparoscopic conversion to open surgery in radical nephrectomy and tumor thrombectomy: causal analysis, clinical characteristics, and treatment strategies. BMC Surg 2020;20:185. [Crossref] [PubMed]
- Steinberg AP, Finelli A, Desai MM, et al. Laparoscopic radical nephrectomy for large (greater than 7 cm, T2) renal tumors. J Urol 2004;172:2172-6. [Crossref] [PubMed]
- Grimaud LW, Chen FV, Chang J, et al. Comparison of Perioperative Outcomes for Radical Nephrectomy Based on Surgical Approach for Masses Greater Than 10 cm. J Endourol 2021;35:1785-92. [Crossref] [PubMed]
- Jeon SH, Kwon TG, Rha KH, et al. Comparison of laparoscopic versus open radical nephrectomy for large renal tumors: a retrospective analysis of multi-center results. BJU Int 2011;107:817-21. [Crossref] [PubMed]
- Chan DY, Cadeddu JA, Jarrett TW, et al. Laparoscopic radical nephrectomy: cancer control for renal cell carcinoma. J Urol 2001;166:2095-9; discussion 2099-100. [Crossref] [PubMed]
- Colombo JR Jr, Haber GP, Jelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008;71:1149-54. [Crossref] [PubMed]
- Hemal AK, Kumar A, Gupta NP, et al. Oncologic outcome of 132 cases of laparoscopic radical nephrectomy with intact specimen removal for T1-2N0M0 renal cell carcinoma. World J Urol 2007;25:619-26. [Crossref] [PubMed]
- Novara G, Ficarra V, Antonelli A, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol 2010;58:588-95. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


