
Efficacy of compound aluminum sulfate injection as a monotherapeutic regimen in non-muscle invasive bladder cancer patients: a retrospective single-arm cohort study
Introduction
Bladder cancer is the most common malignant tumor of the urinary system and the 10th most common cancer worldwide (1), ranking 6th in men and 17th in women. There were about 573,000 new cases and 213,000 deaths in 185 countries worldwide in 2020 (2). In the USA, the lifetime risk of developing bladder cancer is approximately 2.3%, with a total of 712,644 cases with bladder cancer in 2019, and it was estimated that there would be 81,180 new cases in 2022 (3). The age-standardized incidence rate in China is 3.57/105, and new cases are estimated up to 79,600 per year (4). Non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of bladder cancer cases (5). The current first-line treatment for NMIBC is transurethral resection of bladder tumors (TURBT) followed by intravesical Mycobacterium bovis Bacillus Calmette-Guérin (BCG) immunotherapy. However, tumor recurrence rate is still high, ranging from 31–78% within 5 years (6). To inhibit recurrence, postoperative intravesical chemotherapy (IVC) following TURBT in patients with low-grade Ta/T1 NMIBC has been recommended by most guidelines for NMIBC worldwide (7-9). However, in practice, only a small percentage of patients receive intravesical chemotherapy (10). The main reasons for this discrepancy include high cost of IVC, inconvenience, possibility of serious side effects, and the belief that reducing small superficial bladder cancer recurrences is not clinically important. Some studies even report that postoperative IVC has no real benefit (11). Therefore, the protocol and advantages of IVC are still being investigated (12-15). Bladder cancer is considered to be the costliest solid tumor due to its high recurrence rate and the need for life-long routine monitoring and treatment (16).
To date, there has been no specific locally used drug to treat NMIBC. Compound aluminum sulfate injection (CASI) originated from a Chinese traditional medicine, “Kuzhiye”, and has been used in treating NMIBC (17-19). Studies showed that the clinical cure rate of NMIBC by submucosal injection of CASI at tumor root could reach 91.1–97.5%, the recurrence rate at 3.5 years was 23%, and the recurrence rate at 10 years was 30.5% (17,18). However, the TNM stage of the patient's tumors was unknown, and the recurrence rate of the tumor over 10 years was not further followed up. The study by Shi et al. showed that the recurrence rate (within 12 months) of NMIBC (below T2 stage) treated by CASI was significantly lower than that treated by TURBT (20), but the recurrence rate at more than 1 year or even longer was unknown. In addition, the above studies did not involve detailed adverse reactions of CASI, the absorption and accumulation of aluminum ions in the body, and the long-term safety of CASI. Therefore, we conducted a multicenter, retrospective, single-arm cohort study to assess the efficacy and safety of CASI in the treatment of NMIBC in Chinese patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-483/rc).
Methods
Study design, participants, and criteria
A multicenter, retrospective single-arm cohort study was conducted to investigate the tolerability, aluminum absorption, safety, and clinical response of CASI in patients with NMIBC (T1) and benign tumors. All patients were evaluated for clinical responses and safety. Safety, tolerability, and clinical response at 4 weeks following CASI administration were considered endpoints. Long-term follow-up of patients in Center 2 were performed to investigate tumor recurrence.
The inclusion criteria were as follows: (I) ≥18 years of age; (II) histologically proven NMIBC being concomitant to T1 lesions or benign tumors; and (III) pedicle or moss-like tumor with a diameter <5 cm. The exclusion criteria were as follows: (I) adenocarcinoma or squamous carcinoma (≥T2) confirmed by histological examination; (II) tumor with a diameter ≥5 cm or with a wide base; and (III) accompanied with severe cardiac, hepatic, or renal diseases.
Patients were treated from February 2006 to May 2009 at the following 3 clinical trial centers: the Department of Urology in Beijing Friendship Hospital (center 1, n=34), PLA General Hospital (center 2, n=43), and First Bethune Hospital of Jilin University (center 3, n=24). Since the absorption test and tolerance evaluation were carried out at center 2, patients with large tumors were included in this group. The selection of patients in center 2 could avoid the bias of “only small NMIBCs were treated”, so we conduct the follow-up study in center 2 until April 2022. A total of 113 patients were enrolled; of these, 101 patients passed the screening and were included in the study. Screening evaluations included a medical history, physical examination, vital signs, blood count, blood biochemistry, urinalysis, electrocardiogram, cystoscopic examination, and biopsies. Demographic characteristics of patients were obtained before treatment. Vital signs, electrocardiography, blood count, blood biochemistry, and urine analysis were re-examined on day 2 and day 14 after CASI, together with cystoscopic examination, 4 weeks after CASI treatment for all patients to assess the clinical activity and safety of CASI. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines of the Chinese Food and Drug Administration. The study was approved by the Ethics Committee of Beijing Friendship Hospital (No. 2005-15) and the other hospitals (PLA General Hospital and First Bethune Hospital of Jilin University) were informed and agreed the study. Informed consent was taken from all the patients.
Drug administration
CASI (20 mL/bottle, Chenguang Pharmaceutical Limited Liability Company, Changchun, China) was directly injected into the root of NMIBC using a catheter needle, as shown in Figure 1. In the efficacy and safety evaluation study, the injection dose was determined according to tumor size. For tumors <1 cm in diameter, 2–6 mL CASI was injected; for tumors 1–2 cm in diameter, 4–8 mL CASI was injected; for tumors 2–3 cm in diameter, 6–10 mL CASI was injected; and for tumors 3–5 cm in diameter, 8–16 mL CASI was injected. In the tolerance test, 4 patients were administered 16–20 mL CASI and 2 patients were administered 20–25 mL CASI. CASI should be given into the tumor root at multiple sites and the injection should be stopped when the whole tumor becomes pale and necrotic.
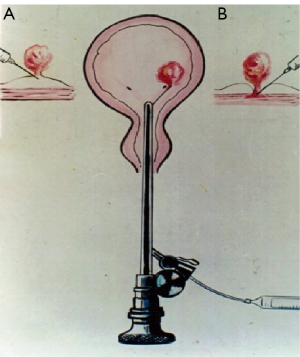
Comprehensive evaluation
Aluminum absorption
Eight patients were included in the absorption study. The patients were divided into 3 groups based on being administered low (<10 mL), medium (10–15 mL), and high (15–25 mL) doses of CASI, respectively. Blood samples (2 mL) were collected before CASI and 0.5 h, 1 h, 2 h, 4 h, 8 h, 1 day, 2 days, 4 days, 7 days, and 14 days after CASI administration. Blood aluminum concentration was determined by inductively coupled plasma-mass spectrometry. The relationship between aluminum blood concentration and CASI doses was studied.
Tolerance
Twenty-one patients participated in this tolerance test and were divided into 5 groups according to their tumor size, as shown in Table 1.
Table 1
| Group | Doses (mL) | Patients |
|---|---|---|
| 1 | ≤5 | 2 |
| 2 | 6–10 | 6 |
| 3 | 11–15 | 7 |
| 4 | 16–20 | 4 |
| 5 | 21–25 | 2 |
Patients were re-examined the day following drug injection and 14 days later. The re-examination included vital signs, electrocardiogram, blood count, blood biochemistry, urine analysis, and adverse effect monitoring.
Response evaluation
Clinical treatment effects of CASI were assessed according to a 4-grade response judgment: (I) complete response (CR), defined as complete tumor necrosis; (II) marked response (MR), defined as tumor necrosis over two-thirds of its volume; (III) partial response, defined as tumor necrosis between one-third to two-thirds of its volume; and (IV) no response (NR), defined as tumor necrosis less than one-third of its volume. The effective rate was applied to evaluate therapeutic effects, which was calculated according to the following equation: Effective rate (%) = (patients with complete tumor necrosis or tumor necrosis >2/3)/all patients × 100%.
Safety
Adverse events were monitored and recorded to achieve safety evaluation. Re-examinations were performed 4 weeks after CASI administration, including vital signs, electrocardiogram, blood count, blood biochemistry examination, and urine analysis, to assess the influence of CASI treatment.
Follow up
The recurrence of NMIBC, including recurrence time, location, pathological type, and treatment, as well as adverse reactions related to CASI treatment, were followed up to evaluate the long-term efficacy and safety of CASI.
Statistical analysis
Descriptive statistical analysis was used. Continuous variables with normal distribution were reported as mean and standard deviation. Continuous variables with abnormal distribution were reported using median and interquartile range. Categorical variables were reported with number and percentage. All data were input by EPI DATA 3.0 (EpiData Association, Odense, Denmark) and analyzed by SAS 9.13 (SAS Institute Inc., Cary, USA).
Results
Baseline characteristics of the patients
A total of 101 patients were enrolled in this study. The cohort was predominantly male (73.3%), and the mean age was 58.9±11.9 years. Demographic characteristics of patients in the 3 clinical centers are shown in Table 2. There were no significant differences among the 3 centers. There were 90 (89.11%) patients with primary tumor and 11 patients (10.89%) with recurrent tumor. Of the 101 patients, 89 patients were histologically proven NMIBC T1, 3 were cystitis glandularis, while the other 9 were unconfirmed.
Table 2
| Characteristics | Center 1 (n=34) | Center 2 (n=43) | Center 3 (n=24) | Total (n=101) |
|---|---|---|---|---|
| Age (years), mean (SD) | 61.8 (12.1) | 57.5 (11.3) | 57.3 (12.4) | 58.9 (11.9) |
| Males, n (%) | 22 (64.7) | 35 (81.4) | 17 (70.8) | 74 (73.3) |
| Height (cm), mean (SD) | 165.8 (4.4) | 170.0 (6.2) | 168.5 (7.1) | 168.2 (6.1) |
| Weight (cm), mean (SD) | 67.5 (6.1) | 70.7 (9.2) | 68.4 (10.2) | 69.1 (8.6) |
| Body temperature (℃), mean (SD) | 36.4 (0.3) | 36.5 (0.3) | 36.4 (0.2) | 36.5 (0.3) |
| Systolic blood pressure (mmHg), mean (SD) | 124.1 (8.4) | 128.4 (11.6) | 134.8 (12.8) | 128.5 (11.6) |
| Diastolic blood pressure (mmHg), mean (SD) | 76.32 (7.5) | 82.0 (6.2) | 87.2 (8.5) | 81.3 (8.3) |
| Respiratory rate (time/min), mean (SD) | 17.2 (0.8) | 17.6 (0.7) | 17.8 (1.2) | 17.5 (0.9) |
| Heart rate (time/min), mean (SD) | 73.5 (4.2) | 74.9 (6.2) | 80.0 (11.9) | 75.6 (7.8) |
SD, standard deviation.
Aluminum absorption
CASI dosage and patients’ blood aluminum concentration are shown in Table 3. After CASI, 6 of the 8 patients had slight elevation of their blood aluminum; the changes were not significant compared with their baseline aluminum concentration; therefore, pharmacokinetic parameters were not calculated. Two patients (S35 and S39) showed significantly increased aluminum concentration after CASI administration (S35: 0.5–8 h after injection; S39: 1 h to 1 day after injection), but then decreased to baseline level within the following 24 h.
Table 3
| Time | ≤10 mL group | 10–15 mL group | 15–25 mL group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | S04 | S35 | S38 | S39 | S40 | S42 | S43 | |||
| Before injection | 195 | 104.7 | 104.4 | 57.8 | 137.2 | 77.3 | 125.8 | 31.2 | ||
| 0.5 h | 278.2 | 190.2 | 992.8 | 112.4 | 67.8 | 89.7 | 47.7 | 18.3 | ||
| 1 h | 295.6 | 235.4 | 883.3 | 107.1 | 1,580.7 | 111 | 34.6 | 26 | ||
| 2 h | 246.5 | 201.1 | 634.8 | 123.8 | 3,729.9 | 127.4 | 35.9 | 17.9 | ||
| 4 h | 291.1 | 156.3 | 528.9 | 89.3 | 3,361.5 | 108.7 | 44.5 | 30.7 | ||
| 8 h | 263.7 | 133.2 | 369.4 | 106.6 | 2,529.3 | 80 | 83.3 | 79.8 | ||
| 1 day | 290.3 | 188.7 | 171.7 | 96.6 | 910.8 | 137.7 | 219.5 | 65.7 | ||
| 2 days | 268.4 | 239 | 182 | 91 | 188.4 | 45.1 | 170 | 51.3 | ||
| 4 days | 320.1 | 214 | 280.8 | 59.9 | 150.9 | 38.9 | 92.4 | 82.1 | ||
| 7 days | 406.9 | 233.2 | 112.4 | 79.8 | 174.2 | 58.1 | 93.3 | 18.7 | ||
| 14 days | 266.4 | 241.6 | 109.8 | 148.5 | 160.8 | 84 | 99.5 | 57.2 | ||
Blood biochemistry test results indicated that all the biochemistry indicators of S35 had no obvious abnormality. For S39, blood urea nitrogen and total bilirubin increased to a somewhat higher lever, but returned to normal 2 weeks later. Biochemistry results are shown in Table 4.
Table 4
| Indicator | Normal range | Unit | S39 | S35 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Day after injection | 2 weeks after injection | Initial | Day after injection | 2 weeks after injection | ||||
| Ca | 2.25–2.75 | mmol/L | 2.40 | 2.39 | 2.50 | 2.27 | 2.38 | 2.43 | |
| K | 3.5–5.5 | mmol/L | 3.87 | 4.21 | 3.86 | 3.60 | 3.95 | 4.21 | |
| Na | 130–150 | mmol/L | 142.4 | 140.8 | 144.9 | 139.3 | 141.3 | 141.5 | |
| Cr | 30–110 | μmol/L | 85.7 | 83.6 | 75 | 79.7 | 83 | 83.1 | |
| BUN | 1.8–7.5 | mmol/L | 7.06 | 7.54 | 7.35 | 8.55 | 5.18 | 8.20 | |
| Glu | 3.4–6.2 | mmol/L | 4.57 | 4.81 | 4.93 | 5.44 | 5.17 | 7.86 | |
| Tbil | 0–21 | μmol/L | 18.70 | 28.0 | 11.60 | 7.40 | 14.80 | 11.0 | |
| ALT | 0–40 | U/L | 26.2 | 29.1 | 28.1 | 15.20 | 14.80 | 26.0 | |
| AST | 0–40 | U/L | 21.4 | 24.5 | 28.4 | 18.20 | 16.30 | 17.1 | |
| TP | 55–80 | g/L | 71.3 | 71.7 | 72.5 | 63.8 | 63.10 | 65 | |
| CHO | 3.1–5.7 | mmol/L | 4.92 | 4.82 | 5.29 | 7.62 | 6.60 | 7.55 | |
| TG | 0.4–1.7 | mmol/L | 1.50 | 1.3 | 1.5 | 16.2 | 9.89 | 8.28 | |
BUN, blood urea nitrogen; Glu, glucose; Tbil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; CHO, cholesterol; TG, triglyceride.
Both S35 and S39 were in middle dosage group, with 12 mL CASI administration. Their tumors and injection sites were located in the neck of the bladder, where blood flow is abundant. Rich blood supply might explain why aluminum was absorbed more. luminum absorption was not dose-dependent, but administration site dependent. This finding indicated that CASI administration should be done carefully to avoid blood vessels, and be injected slowly in the bladder wall with abundant blood flow to inhibit aluminum absorption.
Tolerance
All 21 patients included in the tolerance test exhibited stable vital signs during the test. There were no visible electrocardiogram changes after CASI treatment. Experimental biochemical examination primarily showed some mild changes without any clinical significance. The significant change was mainly the increase of red blood cells, white blood cells, and protein in the urine test, which might be caused by the cystoscopic operation and abscission of the tumor. The adverse events rate was 47.62%. All adverse events were mild and tolerable, and relieved spontaneously in 1–3 days without any treatment. CASI-related adverse events occurred in 3 cases (14.28%). CASI-related adverse events included local pain at the injection site and abdominalgia, and were due to drug stimulation caused by an improper injection into the bladder muscle. Therefore, it is important to control the CASI depth to avoid these adverse effects.
The maximum single dose of CASI was 21 mL in the tolerance test. One of the 2 patients administered 21 mL CASI had mild and tolerable infection. The infection self-resolved in the following days and was as a result of physical injury caused by the cystoscope, rather than CASI.
Efficacy
When CASI was administered only once to each patient (no repeated administration of the drug), the overall effective rate was 96.04% (97/101 patients), and 92.08% (93/101) of the patients achieved CR. The effective rates (CR + MR) at centers 1, 2, and 3 were 100% (34/34 patients), 93.02% (40/43 patients), and 95.83% (23/24 patients), respectively. As 1 patient at center 2 had repeated CASI administration, CR at center 2 was 95.35% (41/43 patients), leading to an overall effectiveness of 97.03% (98/101 patients), with CR being 93.07% (94/101 patients) (Table 5). Typical necrosis and abscission process of NMIBC during CASI treatment is shown in Figure 2.
Table 5
| Therapeutic effects | Center 1 (n=34) | Center 2 (n=43) | Center 3 (n=24) | Total (n=101) |
|---|---|---|---|---|
| Without repeated injection | ||||
| CR, n (%) | 34 (100.00) | 40 (93.02) | 19 (79.17) | 93 (92.08) |
| MR, n (%) | 0 (0) | 0 (0) | 4 (16.66) | 4 (3.96) |
| PR, n (%) | 0 (0) | 3 (6.98) | 0 (0) | 3 (2.97) |
| NR, n (%) | 0 (0) | 0 (0) | 1 (4.17) | 1 (0.99) |
| With repeated injection | ||||
| CR, n (%) | 34 (100.00) | 41 (95.35) | 19 (83.33) | 94 (93.07) |
| MR, n (%) | 0 (0) | 0 (0) | 4 (12.50) | 4 (3.96) |
| PR, n (%) | 0 (0) | 2 (4.65) | 0 (0) | 2 (1.98) |
| NR, n (%) | 0 (0) | 0 (0) | 1 (4.17) | 1 (0.99) |
CR, complete response; MR, marked response; PR, partial response; NR, no response.
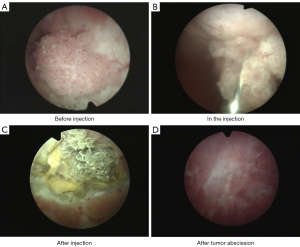
Safety
Treatment-related adverse events occurred in 20 patients (19.80%) (Table 6), of which 9 (8.91%) experienced adverse events related to drug injection, including local pain in 5 patients (4.95%), abdominal pain in 4 patients (3.96%), and anal irritation in 1 patient (0.99%). Other adverse events were related to urethral injury caused by cystoscopy. All adverse events were endurable and disappeared within 2 weeks without any treatment. No significant differences were found in electrocardiogram or hematological examination before and after drug administration. In the urine analysis, elevation of red blood cells and white blood cells in part of the patients was due to urethral injury caused by cystoscopic operation and following tumor abscission.
Table 6
| Adverse events | Center 1 (n=34) | Center 2 (n=43) | Center 3 (n=24) | Total (n=101) |
|---|---|---|---|---|
| Total, n (%) | 9 (26.47) | 10 (23.26) | 1 (4.17) | 20 (19.80) |
| Local pain, n (%) | 2 (5.88) | 3 (6.98) | 0 (0) | 5 (4.95) |
| Frequent urination, n (%) | 1 (2.94) | 0 (0) | 1 (4.17) | 2 (1.98) |
| Urgent urination, n (%) | 2 (5.88) | 0 (0) | 1 (4.17) | 3 (2.97) |
| Odynuria, n (%) | 1 (2.94) | 2 (4.65) | 1 (4.17) | 4 (3.96) |
| Hematuria, n (%) | 1 (2.94) | 5 (11.63) | 0 (0) | 6 (5.94) |
| Abdominal pain, n (%) | 3 (8.82) | 1 (2.32) | 0 (0) | 4 (3.96) |
| Infection, n (%) | 0 (0) | 3 (6.98) | 0 (0) | 3 (2.97) |
| Anal irritation, n (%) | 1 (2.94) | 0 (0) | 0 (0) | 1 (0.99) |
Combination
CASI was the only drug administrated to treat NMIBC in this study. All adverse events were mild and tolerable, and therefore, there was no combined drug treatment in the process.
Recurrence
The follow-up survey was carried out in 2012, 2019, and 2022. Among the 43 patients in Center 2, 3 patients who switched to other treatment regimens due to high pathological grades were excluded from this follow-up study, and the other 40 patients were included. By April 2022, 19 of the 40 patients had no recurrence; however, 3 patients did not relapse but died of other disease. Seven patients were diagnosed with bladder cancer after 3, 3, 4, 5, 6, 9, 11 years, respectively. Among these 29 patients were followed up and 8 were pathologically diagnosed as papillary urothelial carcinoma (PUC) high grade [transitional cell carcinoma (TCC) G2 and above], 18 were diagnosed as PUC low grade (TCC G1), 1 had cystitis glandularis, and the other 2 were unknown. The other 11 patients were lost to follow up. Of these patients, 1 patient did not relapse for at least 6 years in 2012. In these 11 patients, 9 were pathologically confirmed with TCC G1 and below, and 2 with G1-G2. We are still trying to contact these patients and will report the follow-up results in a future study.
Discussion
Until now, there is no other drug for bladder cancer by locally submucosal injection, so there is no suitable drug as control. Moreover, the pathological stage and severity of the patient's tumor in the early studies on CASi are not very clear, the therapeutic dose and the maximum tolerated dose of CASI through submucosal injection, the absorption and accumulation of aluminum ions after injection in body, the acute and long-term toxicity of CASI require further studies to determine. Therefore, we conducted a multicenter single-arm cohort clinical study to determine the safety, and efficacy of CASI.
CASI originated from a Chinese traditional medicine, “Kuzhiye”, and has been used in treating NMIBC (17-19). Aluminum sulfate is the key component of CASI. Compared with cytotoxic chemotherapeutic drugs, CASI treats NMIBC through a unique mechanism. Aluminum ions reduce cell permeability by promoting protein precipitation on the cell surface and in the interstitium, causing tumor necrosis from its base. In addition, in situ administration of aluminum sulfate can also lead to the closure of central vascular necrosis at the base of NMIBC, thereby inhibiting the blood and nutritional support of tumor tissue and accelerating tumor necrosis. (17). A similar drug, aluminum potassium sulfate and tannic acid injection (ALTA), which is derived from the traditional Chinese medicine “Xiaozhiling”, was approved to market in Japan in 2005 and in Korea in 2007 for internal and mixed hemorrhoid treatment (21-23).
At the Chinese People’s Liberation Army General Hospital, CASI, together with its former formulation, has been applied to treat 381 patients with bladder cancer and achieved a complete tumor necrosis rate of 91.9% in the 1980s (18). Kang obtained a 100% cure in 69 NMIBC patients at the Dandong Hospital of Traditional Chinese Medicine (19). Good efficacy and safety of CASI in the treatment of Chinese patients with NMIBC were demonstrated in our study, with an effective rate of 97.03%, while adverse events were mild and tolerable and disappeared spontaneously without any treatment.
The safety of CASI can be explained as follows: (I) low absorption after in situ injection. As seen in the absorption study, patients had low aluminum absorption. Similar results were also obtained in an animal experiment (24,25). (II) Almost no delayed absorption. The in situ injection caused necrosis of tissue and blocking of blood flow, so further drug absorption was prevented. As a result, systemic adverse effects, such as increases of alanine transaminase, were transient. (III) No aluminum accumulation after injection. In most cases, CASI needed to be injected only once with complete removal of the tumor. The injection caused the local tissue necrosis rapidly which detached subsequently. Therefore, there was no risk of adverse effects from aluminum accumulation.
High blood concentration of aluminum is toxic. Aluminum in serum is mainly excreted by the kidney (26). Pathological aluminum accumulation occurs when aluminum is taken in large quantities or continuously, or when patients have renal insufficiency. Aluminum toxicosis can be associated with encephalopathy, bone abnormalities, and neurological disorders (27). Therefore, we suggest that CASI should be used with caution in patients with renal insufficiency.
Recurrence is one of the most important aspects of NMIBC treatment. Our former practice in CASI clinical application demonstrated that CASI treatment was accompanied by a relatively lower recurrence rate (5-year recurrence <30.5% without any in situ recurrence) compared with TURBT (18). Shi et al. discussed the management of bladder cancer at PLA General Hospital from February 1982 to July 2004 (20). From a total of 1,908 patients, 1,174 cases were treated with CASI, 700 cases were treated by TURBT, and 34 cases were treated with holmium laser. The retrospective analysis results showed that there is no in situ tumor recurrence for CASI and holmium laser regimen, whereas TURBT was associated with tumor recurrence in the original site. CASI is the most economic treatment option among the 3 regimens. Based on the follow-up outcome of our CASI clinical study in 2022, the recurrence rate after CASI treatment was low, with a 10-year recurrence of 20.7% (6/29) and 5-year recurrence of 13.8% (4/29). Studies showed that when treated by TURBT, 5-year recurrence of early stage (Ta–T1) NMIBC was up to 70% (28); treated by TURBT followed a total of 27 intravesical instillations for 3 years, recurrence was 43% within 9.2 years (29); even when treated by TURBT plus an immediate instillation, 2-year recurrence of NMIBC could be as high as 31.4% (30). Treatment and recurrence in recent key reports are compared and listed in Table 7. Since one of the possible mechanisms of early recurrence of NMIBC after TURBT is the implantation of floating cancer cells in the bladder urothelium after resection (4), the low recurrence rate after CASI treatment is reasonable because injection in the tumor pedicle results in tumor necrosis and shedding, with no live cancer cells roaming the bladder. CASI clinical experiences have demonstrated relatively low recurrence; however, the data are inconclusive due to the lack of randomized control trials.
Table 7
| Items | RCT phase III study 30911 (29) | CASI study (center 2) | Retrospective survey (30) |
|---|---|---|---|
| Treatment period | Jan 1992–Feb 1997 | Feb 2006–May 2009 | Jan 2012–Dec 2016 |
| Total cases | 957 | 43 | 477 |
| Evaluable cases | 837 (87.5) | 40 (93.0) | 477 |
| Age (year) | 67 (median) | 61.8±12.1 (mean ± SD) | 64 (median) |
| Tumors, n (%) | |||
| Single | 121 (15.0) | 36 (90.0) | 112 (23.5) |
| Multiple | 688 (85.0) | 4 (10.0) | 365 (76.5) |
| Unknown | 28 | ||
| Tumor size, n (%) | |||
| ≤1 cm | 397 (50.6) | 12 (30.0) | 193 (40.5) |
| >1 cm and ≤3 cm | 320 (40.8) | 28 (70.0) | 148 (31.0) |
| >3 cm | 68 (8.7) | 0 | 136 (28.5) |
| Median | 1 cm | 1.5 cm | |
| T stage, n (%) | |||
| Ta | 521 (63.6) | 0 | 75.3 |
| T1 | 298 (36.4) | 95 | 24.7 |
| WHO 1973 grade, n (%) | |||
| G1 | 312 (38.3) | 22 (55.0) | 318 (66.7) |
| G2 | 401 (49.2) | 8 (20.0) | 106 (22.2) |
| G3 | 102 (12.5) | 2 (5.0) | 53 (11.1) |
| 8 uncertified | |||
| Follow-up time (years) | |||
| Median | 9.2 | 13.8 | – |
| Maximum | 14.3 | 16.2 | – |
| Intravesical instillation (times) | 27 (BCG or chemotherapy) | 0 | 1 (immediate chemotherapy) |
| Recurrence, n (%) | |||
| No | 477 (57.0) | 22 (55.0) | 327 (68.6) |
| Yes | 360 (43.0) | 7 (17.5) | 150 (31.4) |
| Unknown | 0 | 11 (27.5) | 0 |
| Survival, n (%) | |||
| Unknown | 0 | 11 (27.5) | – |
| Alive | 559 (66.8) | 26 (65.0) | – |
| Deceased | 278 (33.2) | 3 (7.5) | – |
| Bladder cancer | 38 (4.5) | 0 | |
| Cardiovascular | 106 (12.7) | 0 | |
| Other | 92 (11.0) | 1 (MODS) | |
| Cause unknown | 42 (5.0) | 2 (5.0) |
Unknown indicates that the patient was lost to follow up. NMIBC, non-muscle invasive bladder cancer; RCT, randomized controlled trial; CASI, compound aluminum sulfate injection; SD, standard deviation; BCG, Bacillus Calmette-Guérin; MODS, multiple organ dysfunction syndrome.
However, because our trial was a single-arm, open-label design and lacked controls, the results may have been affected by confounding factors. Also, due to the small sample size, there may be selection bias. Therefore, a larger randomised controlled trial is needed for further study.
Conclusions
As a monotherapeutic regimen for NMIBC, CASI has the following characteristics in the treatment of NMIBC. First, it has high efficiency. In most cases, just a single injection of CASI will cause the tumor tissue to necrotic and slough off and be excreted in the urine. Second, it is well-tolerated. Treatment-related adverse events were mild and relatively rare, and patients self-recovered quickly. Third, it is convenient. Treatment of NMIBC with CASI requires only cystoscopy. Fourth, it is associated with low recurrence, based on available literature. Therefore, CASI could be considered a new potential method for NMIBC management; however, further research and controlled clinical studies are warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-483/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-483/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-483/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines of the Chinese Food and Drug Administration. The study was approved by the Ethics Committee of Beijing Friendship Hospital (No. 2005-15) and the other hospitals (PLA General Hospital and First Bethune Hospital of Jilin University) were informed and agreed the study. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol 2020;38:1895-904. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- seer.cancer.gov [Internet]. NIH: National Cancer Institute; c2022 [cited 2022 Jul 3]. Available online: https://seer.cancer.gov/statfacts/html/urinb.html
- Li HZ, Zheng R, Du L, et al. Bladder cancer incidence, mortality and temporal trends in China. Zhonghua Zhong Liu Za Zhi 2021;43:293-8. [PubMed]
- Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 2022;81:75-94. [Crossref] [PubMed]
- Li Y, Youssef SF, Buanz AB. Intravesical combination therapies for non-muscle invasive bladder cancer: Recent advances and future directions. Eur J Pharmacol 2022;926:175024. [Crossref] [PubMed]
- Power NE, Izawa J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2016;2:27-36. [Crossref] [PubMed]
- Gregg JR, Dahm P, Chang SS. Guideline-based management of non-muscle invasive bladder cancer. Indian J Urol 2015;31:320-6. [Crossref] [PubMed]
- nhc.gov.cn [Internet]. National Health Commission of the People's Republic of China; Guideline for bladder cancer diagnosis and treatment (2022 edition). c2022 [cited 2022 Jul 3]. Available online: http://www.nhc.gov.cn/yzygj/s2911/202204/a0e67177df1f439898683e1333957c74.shtml.
- Yap SA, Brunson A, Pugashetti N, et al. Immediate intravesical chemotherapy for low-grade bladder tumors in California: An underutilized practice and its impact on recurrence. Urol Oncol 2018;36:498.e1-7. [Crossref] [PubMed]
- Holmäng S. Early single-instillation chemotherapy has no real benefit and should be abandoned in non-muscle-invasive bladder cancer. Eur Urol Suppl 2009;8:458-63. [Crossref]
- Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, et al. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur Urol 2018;73:226-32. [Crossref] [PubMed]
- Messing EM, Tangen CM, Lerner SP, et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence SWOG S0337 randomized clinical trial. JAMA 2018;319:1880-8. [Crossref] [PubMed]
- Bosschieter J, van Moorselaar RJA, Vis AN, et al. The Effect of Timing of an Immediate Instillation with Mitomycin C after Transurethral Resection in 941 Non Muscle-invasive Bladder Cancer Patients. BJU Int 2018;122:571-5. [Crossref] [PubMed]
- Tanimoto R, Saika T, Ebara S, et al. Prospective randomized controlled trial of postoperative early intravesical chemotherapy with pirarubicin (THP) for solitary non muscle invasive bladder cancer comparing single and two time instillation. World J Urol 2018;36:889-95. [Crossref] [PubMed]
- Michaeli JC, Boch T, Albers S, et al. Socio-economic burden of disease: Survivorship costs for bladder cancer. J Cancer Policy 2022;32:100326. [Crossref] [PubMed]
- Wang X, Wei S, Zhou G, et al. In situ injection of Compound Aluminum Sulfate injection in bladder cancer treatment. Chin J Urol 1982;3:54-6.
- Wang X, Hong B, Zeng X, et al. Use of aluminum sulfate co. in treatment of bladder tumors: experimental study and 10 years clinical experience. Chin J Urol 1989;10:162-4.
- Kang Y. Treatment of bladder cancer by compound aluminum sulfate injection injected under cystoscope. Chin J Min Inv Surg 2005;5:453-5.
- Shi L, Xu A, Hong B, et al. Three managements of bladder tumors under cystoscopy. Chinese Medical Equipment Journal 2005;26:11-2.
- Lim SW. Aluminum potassium sulfate and tannic Acid injection for hemorrhoids. J Korean Soc Coloproctol 2012;28:73-7. [Crossref] [PubMed]
- Hachiro Y, Kunimoto M, Abe T, et al. Aluminum potassium sulfate and tannic acid (ALTA) injection as the mainstay of treatment for internal hemorrhoids. Surg Today 2011;41:806-9. [Crossref] [PubMed]
- Abe T, Kunimoto M, Hachiro Y, et al. Long-term Outcomes of Aluminum Potassium Sulfate and Tannic Acid Sclerotherapy for Prolapsed Hemorrhoids: A Single-Center, Observational Study. Dis Colon Rectum 2022;65:271-5. [Crossref] [PubMed]
- Xu F, Chen Y, Fang Y, et al. Pharmacokinetic study on compound aluminum sulfate injection after iv and in situ administration. Chin J Clin Pharmacol Ther 2006;11:691-5.
- Xu F, Fu W, Zhang Z, et al. Experimental studies and evaluation on safety of compound aluminum sulfate injection. Chin J Clin Pharmacol Ther 2006;11:510-4.
- DeVoto E, Yokel RA. The biological speciation and toxicokinetics of aluminum. Environ Health Perspect 1994;102:940-51. [Crossref] [PubMed]
- Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol 2019;12:45-70. [Crossref] [PubMed]
- Loras A, Trassierra M, Sanjuan-Herráez D, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci Rep 2018;8:9172. [Crossref] [PubMed]
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57:766-73. [Crossref] [PubMed]
- Lu M, Chen S, Zhou Q, et al. Predicting recurrence of nonmuscle-invasive bladder cancer (Ta-T1): A study based on 477 patients. Medicine (Baltimore) 2019;98:e16426. [Crossref] [PubMed]
(English Language Editor: R. Scott)


