
Robotic management of a large mullerian duct cyst: a case report and review of surgical options
Introduction
Mullerian duct cysts (MDC) are uncommon with a reported prevalence of 1–5%; in fact, the diagnosis is often one of exclusion or made incidentally (1). The peak age of incidence in adulthood is between 20 and 40 years old (2). Sixty percent of cysts are asymptomatic and are found with imaging or during surgery (2). MDCs are the consequence of focal incomplete Mullerian duct regression and saccular dilation of the duct, arising in the retroperitoneal space from the scrotum to the prostatic utricle without connection to the urethra (3). Additionally, some MDCs have presented in female patients in the mediastinum and cutaneous tissue of the lower extremities (4). In contrast, prostatic utricle cysts are an embryologic remnant of the Mullerian duct system but communicate with the urethra and do not extend above the base of the prostate (3,4). While MDCs are occasionally associated with renal agenesis, prostatic utricle cysts are associated with abnormalities of the external genitalia, including hypospadias and cryptorchidism (3,5).
Cystic lesions of the lower male urogenital tract are characterized into intraprostatic (Mullerian duct, prostatic utricle, ejaculatory duct) and extraprostatic (seminal vesicle) cysts; intraprostatic varieties are further subdivided into median, paramedian and lateral cysts (3). Unlike other forms of pelvic cysts, MDCs extend above the prostate and cause symptoms such as hematospermia, genitourinary tract infections (cystitis, prostatitis, epididymitis), urinary retention, pelvic-perineal pain, ejaculation pain, infertility (azoospermia, hypospermia, oligospermia), change in bowel habits, gross hematuria, and microscopic hematuria. In addition, MDCs can contain foci of carcinoma (endometrial, clear cell, or squamous cell) with a reported prevalence as high as 3% (6).
Management of MDCs can be difficult and often vary based on size and symptomatology. Initial identification of the cyst is often made via transrectal ultrasound (TRUS) or computerized tomography (CT), the latter of which is able to determine the size and visualize any connection to local structures (7). Magnetic resonance imaging (MRI) is often used to further characterize the cystic lesions of the epididymis and local structures following TRUS/CT (3,7). Historically, simple cysts have been treated with minimally invasive procedures (percutaneously, transrectal, or transurethral). Surgical intervention with open and laparoscopic methods are reserved for more complex cysts as the benefits outweigh the potential damage of local structures, such as the ureters, vas deferens, ejaculatory ducts and pelvic nerves (2,5). In this review of the management of MDCs, we present a case report of a robotic-assisted MDC excision in a patient who previously failed minimally invasive treatment modalities. Due to the uniqueness of this patient’s anatomy, our robotic approach, and the paucity of literature on this subject, we believe this case will serve as a learning tool to guide urologic surgeons in identifying and treating MDCs. A summary of the case is provided in Video 1. We present the following article in accordance with the CARE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-216/rc).
Case presentation
A 38-year-old male with no prior medical history was referred to our clinic for worsening obstructive lower urinary tract symptoms and recurrent episodes of acute urinary retention. He required tamsulosin daily to urinate and endorsed urologic symptoms including decreased ejaculatory volume, perineal discomfort while sitting, and constipation. There was no family history of urologic malignancy. He was married and had 3 children. On physical exam, a 15-cc prostate was palpated and found to have a large palpable cranial mass.
Due to his history of recurrent urinary retention and the physical exam findings, a CT urogram was ordered, and a large 15 cm cystic mass was detected compressing the posterior bladder. To further characterize the mass, an MRI Pelvis was performed and displayed a 11×15×14.7 cm noncalcified, thin walled, multilocular, pedunculated mass located between the rectum and the prostate without any obvious connection to the urethra. The decision was made to first perform a cystoscopy, retrograde urethrogram and retrograde pyelograms in the operating room to obtain more information for surgical planning followed by transperineal or transrectal aspiration.
Initially, a rigid cystoscope was unable to be advanced past the external sphincter due to the acute angle of the urethra at this juncture. Flexible cystoscopy showed a normal pendulous urethra and an elongated prostatic urethra with stretched verumontanum. The bladder was found to be enlarged and had been displaced anteriorly by mass effect. A retrograde urethrogram was performed and displayed an elongated prostatic urethra. Retrograde pyelogram showed no hydronephrosis. At the conclusion of the case, an attempt to aspirate fluid in the presumed area of the prostatic utricle was unsuccessful.
We then returned to the operating room at a later date to attempt transperineal aspiration of the cyst. One gram of vancomycin and 240 milligrams of gentamicin were given upon induction and the patient was extensively prepped with iodine and chlorohexidine. Transperineal aspiration was attempted using ultrasound guidance and was ultimately unsuccessful as the angulation of needle trajectory restricted our ability to reach the pelvic cyst/mass. After palpation of the large mass with a digital rectal exam, we were able to successfully perform a transrectal aspiration using ultrasound guidance, evacuating 800 cc of dark red/brown fluid. Pathologic analysis of the fluid revealed hemosiderin laden macrophages, red blood cells, and debris with no malignant cells or spermatozoa identified.
The patient had an uneventful recovery with minor improvement in lower urinary tract symptoms, perineal pain, erections, and ejaculate volume at the two weeks follow up visit. Repeat MRI at this time displayed a reduction in size of the mass to 11.6×8.4×11.5 cm. The patient was offered repeat transrectal aspiration or robotic excision and elected for the latter approach as an attempt for definitive treatment. We used a 6-port configuration similar to a robotic assisted laparoscopic prostatectomy (RALP). Two double J ureteral stents were placed. Additional trocars were then placed 10 centimeters laterally and curved slightly downwards towards the pelvis. Eight millimeter trocars were placed with two on the left and one on the right. A 12-millimeter assistant port was placed roughly 2 fingerbreadths from the right anterior superior iliac spine. A 5-millimeter assistant port was placed cranial to and between the camera port and the robotic arm on the same side as the 12 mm assistant port. The patient was placed in steep Trendelenburg position and the da Vinci Si was docked. The abdomen was insufflated and adhesions were lysed to visualize the large multiloculated pelvic cyst, which was densely adherent at the right lateral aspect to surrounding structures including the right ureter (Figure 1). Transurethral and robotic aspiration was performed and a small portion of the cyst wall was resected, both of which were found to be negative for malignancy. As the patient desired the more definitive treatment, we proceeded with maximal extraction of the cyst. The plane of dissection coursed close to the anterior rectal wall, and colorectal surgery was consulted intraoperatively due to concern for rectal perforation. After confirmation that there was no damage to either the ureters or bowel, the cyst was then dissected laterally and then anteriorly. The cyst was inadvertently entered and some of the contents of the cyst were introduced to the peritoneum due to the prior transperitoneal approach; however, there were no associated complications and there was no concern for contingent metastasis as the cyst was benign. The left vas deferens was identified and dissected free from the cyst. The origin of the cyst remained unclear at the conclusion of the dissection. One liter of fluid was drained from the cyst. About 90% of the cyst was excised and removed using a small endocatch bag. The remaining cyst wall was oversewn using 2-0 Vicryl and the remaining tissue was marsupialized. The total operative time was 301 minutes. The patient had an uneventful postoperative course and was able to be discharged the next day.
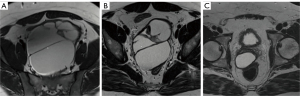
The specimen was sent to pathology which found that the mass was PAX-8 positive and lined with columnar epithelial tissue without malignancy, consistent with a MDC. One month postoperatively, the patient reported significant satisfaction and improvement in constipation, voiding and ejaculation; he no longer needed tamsulosin to void. His International Prostate Symptom Score (IPSS) was now 0, 0 (down from 13, 4). No complications were reported. A repeat MRI displayed a significant reduction in mass effect on the bladder and rectum. MRI images displaying the cyst at the initial presentation, post transrectal aspiration, and post robotic manipulation is shown in Figure 2A-2C, respectively. A small 5-centimeter cyst remained which corresponded to an area of cyst that coursed too close to the rectum to be safely dissected away. As a result, the risk of recurrence of symptoms remains, but the risk is low as the cyst continues to drain following marsupialization. After two years, the patient has not reported recurrence of symptoms.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
After a careful literature review, we were limited by only two reported retroperitoneal MDC cases that were performed using robotic excision (1,8). Furthermore, to our knowledge, this is only the second reported robotic surgical management of retroperitoneal MDC in an adult (8). Additionally, one case of a retroperitoneal MDC was reported by Parmentier in a 23-year-old woman; however, was treated by laparoscopic methods (4). A recent case report by Elsorougy et al. commented on the lack of guidelines in regards to the treatment of midline prostatic cysts, including MDCs (9). A summary of the robotic excision of MDC and prostatic utricle cyst is reported in Table 1. Our discussion focused on the various surgical methods for MDC treatment, a review of robotic surgical intervention, and histopathologic analysis for retropubic cysts. A summary of the pros and cons of each procedure is provided in Table 2.
Table 1
| Case characteristics | Our patient | Hong et al. (1) | Hong et al. (1) | Samueli et al. (8) | Goruppi et al. (10) | Nguyen et al. (11) |
|---|---|---|---|---|---|---|
| Patient age | 38 years | 13.75 years | 29 months | 20 years | 19 years | 3 years |
| Presentation | Progressive lower urinary tract symptoms, history of acute urinary retention, decreased ejaculatory volume, constipation | Urgency, frequency, hesitancy | Recurrent epididymitis | Abdominal pain, constipation, retention | Two-year follow-up for a previous retrovesical mass | Recurrent urinary tract infection and post void dribbling |
| Presumed diagnosis | Mullerian duct cyst | Mullerian duct cyst | Prostatic utricle cyst | Mullerian duct cyst | Prostatic utricle cyst | Prostatic utricle cyst |
| Mean operation time (minutes) | 301 | 215 | 215 | n/a | n/a | 168 |
| Days stayed after surgery | 1 | 2 | 1 | n/a | 7 | n/a |
| Complications | None | Hematoma-resolved 2 months post-op | Left epididymitis 2 months post-op | Prostatic urethral leak | None | None |
Table 2
| Pros or cons | Robotic | Laparoscopic | Open | Transperineal | Transrectal | Transurethral |
|---|---|---|---|---|---|---|
| Risks | Learning curve | Difficulty maneuvering the laparoscopic ports | Higher risk of damaging local structures | Increased incidence of relapse | Increased incidence of relapse | General anesthesia |
| Difficulty maneuvering the robotic ports within the compressed pelvic region | Increased risk of infection | Increased risk of infection | Urine regurgitation | |||
| Increased risk of sepsis | Increased risk of sepsis | Hematospermia | ||||
| Increased risk of lower urinary tract symptoms | ||||||
| Pain | ||||||
| Benefits | Decreased postoperative pain | Better instrument control | High success rate of complete cyst resection | Local anesthesia | Local anesthesia | High success rate |
| Fewer days in the hospital | Better precision | Beneficial for pediatrics | Can be discharged the same day | Can be discharged the same day | ||
| Reduced convalescence | Easier suturing | Low risk of damaging local structures | Low risk of damaging local structures | |||
| Higher reproducibility |
Robotic and laparoscopic methods
There is limited literature where MDCs were treated using robotic methods (7,8). To our knowledge, only one previous case report by Samueli et al. describes robotic management of a complex retroperitoneal MDC in an adult (8). However, the robotic approach has been used in prostatic utricle cysts cases presenting with extensive malformations, ineffective minimally invasive treatment, ureter defects, and vas deferens defects (1,10-12). These methods have been shown to have decreased postoperative pain, required fewer days in the hospital, and reduced convalescence than other methods in the treatment of retroperitoneal cysts (2,12,13). In comparison to laparoscopic methods, the robotic method allows for better instrument control in reduced spaces, better precision, easier suturing, and higher reproducibility (1,13). Limitations of the robotic approach include an increased learning curve and difficulty maneuvering the robotic or laparoscopic ports within a compressed pelvic region (11). When comparing our approach to the RALP, our case did not present any novel, major difficulties. The most difficult aspect of our case was dissecting the adhered aspects of the cyst from the right ureter and rectum, which may or may not have been easier to perform using other invasive approaches. We believe that the robotic approach allowed for better visualization during this procedure which allowed us to explore the delicate local anatomy.
Open
The open surgical method was the most commonly used approach until more technologically advanced methods were developed. This method utilizes a midline suprapubic incision with transperitoneal access (10). The use of open surgery has been shown to have a high success rate of complete cyst resection (10). A case series performed by Desautel et al. recorded complete resection in 12 out of 13 cases in one (8/13) or two (2/13) surgeries (14). Desautel et al. claimed that due to reduced pelvic space in children, it is recommended to perform an open procedure for increased visualization (14). While the success rate may be promising, there is a significant risk of damaging the local structures around the cyst, including vas deferens, seminal vesicles, and part of the prostate (10,13). Overall, while there may be a benefit in the pediatric population, there is a significantly higher risk of adverse effects in both the adult and pediatric population (10,13).
Transperineal and transrectal aspiration
Percutaneous aspiration utilizes TRUS guided needle aspiration using a brachytherapy template grid traditionally used for prostate biopsy (12). This method uses local anesthesia rather than global anesthesia and the patient can be discharged on the same day of surgery. These methods have been reported to have a lower risk of damaging pelvic anatomy than the more invasive methods of surgery (12). The entire cyst can potentially be evacuated in a single attempt; however, it has been found to have a low success rate of only 43% (2,12,14). Limitations of this method include: increased incidence of relapse of the cyst, increased risk of infection, and risk of sepsis due to contamination by the rectal flora which may also have antibiotic resistance (12,14,15).
Transurethral resection
Use of transrectal guided transurethral puncture of the cyst has been described by Coppens et al. (2). A urethral catheter is guided to the cystic region, and a cold knife is inserted through an internal channel (2). This procedure requires general anesthesia and prophylactic antibiotics. The trial performed by Coppens et al. of 17 cases showed a success rate of 82% in patients with small, uncomplicated cysts that initially presented with hematospermia, lower urinary tract symptoms, recurrent urinary tract infections and infertility (2). Additionally, the case review by Desautel et al. displayed reversal of azoospermia by transurethral drainage in 10 of 13 patients with small retropubic cysts (14). While the results were promising in these case reviews, it is uncertain if the cysts were simple or complex. Additionally, the risks of the procedure include urine regurgitation into the vas deferens through the epididymis, leading to epididymitis (15). Complications of this method include hematospermia (resolves after 6 weeks), increased risk of LUTS, and significant pain (15).
Histopathologic analysis
MDCs are defined by ciliated cuboidal or columnar epithelium lining the intraluminal surface, similar to the endometrium or fallopian tubes (4). The wall of the cyst may contain smooth muscle and lymphocytic aggregates but is not always present (4). Stains are often used to confirm the diagnosis. MDCs are usually positive for antibodies to nuclear estrogen/progesterone receptors, cytokeratin CK7, epithelial membrane antigen, PAX-8, and WT-1, while negative for cytokeratin CK5/6, CK20 (4,7). The most sensitive stains have been found to be PAX-8, WT-1, and estrogen/progesterone receptors (4). PAX-8 is a transcription factor that codes for products of the Mullerian tract, upper urogenital tract, and the thyroid (4,8). The role of WT-1 is not well known but is believed to be involved in the differentiation of mesenchymal and epithelial cells (8). Most importantly, these stains are able to differentiate MDCs from prostatic utricle cysts, as the latter stains negative with ER and WT-1 (8).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-216/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-216/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-216/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong YK, Onal B, Diamond DA, et al. Robot-assisted laparoscopic excision of symptomatic retrovesical cysts in boys and young adults. J Urol 2011;186:2372-8. [Crossref] [PubMed]
- Coppens L, Bonnet P, Andrianne R, et al. Adult müllerian duct or utricle cyst: clinical significance and therapeutic management of 65 cases. J Urol 2002;167:1740-4. [Crossref] [PubMed]
- Shebel HM, Farg HM, Kolokythas O, et al. Cysts of the lower male genitourinary tract: embryologic and anatomic considerations and differential diagnosis. Radiographics 2013;33:1125-43. [Crossref] [PubMed]
- Parmentier E, Valk J, Willemsen P, et al. A large retroperitoneal Mullerian cyst: case report and review of the literature. Acta Chir Belg 2021;121:278-85. [Crossref] [PubMed]
- Halpern EJ, Cochlin DL. Cysts and congenital anomalies of the prostate and ejaculatory ducts. In: Imaging of the prostate. London: Martin Dunitz, 2002:115-28.
- Curran S, Akin O, Agildere AM, et al. Endorectal MRI of prostatic and periprostatic cystic lesions and their mimics. AJR Am J Roentgenol 2007;188:1373-9. [Crossref] [PubMed]
- He J, Tang K. A Müllerian cyst in a male adolescent: a case report and literature review. J Int Med Res 2021;49:3000605211016663. [Crossref] [PubMed]
- Samueli B, Mazor E, Kamenev I, et al. Müllerian duct cyst: Case report with updates in cellular origin, corresponding immunohistochemistry, and contrast to prostatic utricle cyst. Pathol Res Pract 2021;228:153657. [Crossref] [PubMed]
- Elsorougy A, Farg H, Abdelhamid A, et al. Should asymptomatic small anteriorly located midline prostatic cyst in young adults be treated surgically or conservatively? A case report and review of the literature. Egyptian Journal of Radiology and Nuclear Medicine 2022;53. [Crossref]
- Goruppi I, Avolio L, Romano P, et al. Robotic-assisted surgery for excision of an enlarged prostatic utricle. Int J Surg Case Rep 2015;10:94-6. [Crossref] [PubMed]
- Nguyen A, Arora H, Reese J, et al. Robot-assisted laparoscopic excision of prostatic utricle in a 3-year old. J Pediatr Urol 2018;14:343-4. [Crossref] [PubMed]
- Yogeswaran C, Srinivasan V, Ekwueme KC. Mullerian duct cyst treated with template-guided transperineal aspiration: a case report and review of the literature. JRSM Open 2018;9:2054270417725497. [Crossref] [PubMed]
- Selli C, Cavalleri S, De Maria M, et al. Robot-assisted removal of a large seminal vesicle cyst with ipsilateral renal agenesis associated with an ectopic ureter and a Müllerian cyst of the vas deferens. Urology 2008;71:1226.e5-7. [Crossref] [PubMed]
- Desautel MG, Stock J, Hanna MK. Mullerian duct remnants: Surgical Management and fertility issues. Journal of Urology 1999;162:1008-13. [Crossref] [PubMed]
- Kobori Y, Sato R, Ashizawa Y, et al. Mullerian duct cyst: a curable entity of male infertility. Two case reports. Reprod Med Biol 2010;9:223-4. [Crossref] [PubMed]



