
Evaluation of the surgical and functional outcomes of secondary vaginoplasties with free skin mesh graft in patients following transfeminine genital reconstructive surgery
Introduction
Transgender or Gender Incongruence (ICD-11) is characterized by the behavioral and social identification of the opposite gender to the patient’s genotype, differing from the external sexual anatomy that the patient has at birth. Gender Dysphoria (GD) (DSM-5) is defined as feeling dissonance between one’s natal sex and gender identity (1).
The Hospital de Clínicas de Porto Alegre (HCPA) provides free public assistance to transgender people since 1998 through the Gender Identity Program (PROTIG), the first in Brazil and one of the pioneers in South America. Our programs offer multidisciplinary support and, whenever indicated, gender-affirmation surgery (GAS) (2).
In our institution, we perform the classic penile inversion vaginoplasty, with an inverted flap of the penis skin used for the neovagina (2,3). The advantages of this method include hairless penile skin, less likelihood of contracture of the flap, and the maintenance of the innervation intact (4). One of the disadvantages is that this technique may not promote adequate depth in patients who have a small amount of penoscrotal skin, such as those who are circumcised, who have genital hypoplasia due to hormonal treatment, or who have already undergone previous procedures (5). In these patients whose vaginal conduit became short or stenotic in the postoperative period of GAS, we routinely propose to perform secondary vaginoplasty using an abdominal free skin mesh graft.
The objective of this study is to analyze demographic data, postoperative complications, and neovagina functionality with adequate sexual intercourse in patients who underwent secondary free skin mesh graft vaginoplasty. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-215/rc).
Methods
We performed a retrospective cohort study of transgender patients who underwent penile inversion vaginoplasty and required secondary free skin mesh graft vaginoplasty due to neovagina stenosis or inadequate length at HCPA. We analyzed all patients from January 2000 to July 2017. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by our institutional ethics committee (Comitê de Ética em Pesquisa do Hospital de Clínicas de Porto Alegre) under the CAAE number 90344318.0.0000.5327, and informed consent was obtained from participants who returned to the outpatient follow-up consultation during the data collection period.
All male-to-female transgenders are followed up in our institution’s multidisciplinary program to this day. All surgeries were performed by one of two surgeons and assisted by residents.
A retrospective review of the medical record was performed, recording patient demographic data, intra and postoperative complications, and patient satisfaction in the postoperative period. A functional vaginal conduit was defined as the ability to maintain satisfactory sexual intercourse, with adequate penetration, and without dyspareunia, since the average vaginal depth was not collected. Patients without sufficient data or with follow-up loss were excluded.
Surgical technique
In our institution, patients submitted to classic vaginoplasty that evolved to non-function neovagina were submitted to a secondary vaginoplasty using an abdominal free skin mesh graft.
The procedure is performed with general and neuraxial anesthesia. We routinely use 1st generation cephalosporin as antibiotic prophylaxis. The patient is placed in a lithotomy position and two surgical teams work separately: one on the lower abdomen and one on the perineum.
After bladder catheterization with a Foley catheter, we perform an opening of the neovaginal area previously created, with the release of adhesions and tissue fibrosis. Blunt digital dissection is performed between the bladder and the rectum, aiming for a cavity of approximately 10 cm × 5 cm.
We perform an elliptical incision to remove a full skin graft of 12 cm × 6 cm from the lower abdomen (Figure 1A,1B). The graft is prepared in the usual way with the removal of the adipose tissue (Figure 1C). Subsequently, multiple small incisions are made using a scalpel blade, to increase its final area by at least twice (Figure 1D). The donor site is closed primarily, just like the concept of aesthetic abdominoplasty. The graft is then sutured over a 12 cm rigid silicone structure (mold) using a 4-0 absorbable monofilament suture (polydioxanone) (Figure 2A,2B).
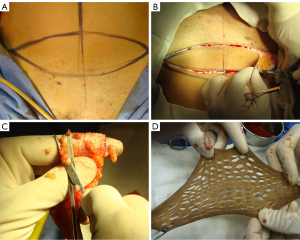
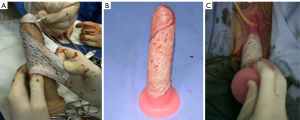
The silicon mold with the graft is inserted into the created perineal space and the outer edge of the graft is sutured to the native genital skin (Figure 2C). External fixation points are performed so that there is no displacement of the mold, optimizing graft adherence in the desired area.
Postoperative follow-up
Patients are maintained with prophylactic antibiotics for 24 hours and discharged 48 hours after surgery. The foley catheter and the vaginal mold are removed 7 days after the procedure in an outpatient consultation.
Patients are instructed to daily use vaginal dilators during the night for at least 8 hours for 4 weeks after removing the mold. The dilator used is the largest possible to be gently inserted. After these 4 weeks, we recommend using the dilator for 2 hours daily for 3 months.
Sexual intercourse is allowed after 6 weeks if the findings are normal (no visual or digital sign of any local complication and adequate length in digital examination) on physical examination.
Outpatient consultations are carried out on 14, 30, 90, and 180 days after hospital discharge. Afterward, annual consultations are considered sufficient in most cases.
Statistical analysis
The data were revised and analyzed using the SPSS program, version 18.0. Demographic, surgical (operative time, surgical technique, complications) and aesthetic (personal satisfaction), and functional (ability to maintain sexual intercourse) data were analyzed.
Qualitative variables were described as absolute (n) and relative (n%) frequencies. Quantitative variables were expressed as means and standard deviations or medians and interquartile ranges (25th and 75th percentiles, P25 – P75), depending on the Shapiro Wilk normality test. Spearman correlations were conducted between the variables of interest and the outcomes of postoperative complications. For all analyzes, the significance level was 5%.
Results
During the study period, 186 patients were submitted to primary vaginoplasty by penile inversion and 36 (19.3%) had complications in the neovagina such as introitus or conduit stenosis (refractory to self-dilatation) or inadequate length and patients. These 36 patients underwent secondary vaginoplasty using a free skin mesh graft and 35 patients followed the proposed postoperative follow-up and formed our sample (one patient with missing data and uncompleted follow-up was excluded). Most patients were Caucasian (82%). The most prevalent comorbidities were HIV infection in 6 patients (17%), active smoking in 4 patients (11.4%), systemic arterial hypertension, and diabetes mellitus in 2 patients each (5.7%). Mean BMI was 23.7 (Table 1).
Table 1
| Characteristics | n=35 (%) |
|---|---|
| Age (years), median [interquartile range] | 41 [37–50.5] |
| Caucasian | 29 (82.9%) |
| HIV | 6 (17.1%) |
| Hypertension | 2 (5.7%) |
| Diabetes mellitus | 2 (5.7%) |
| Smoking | 4 (11.4%) |
| Syphilis | 2 (5.7%) |
| Hormonal replacement therapy | 25 (71.4%) |
| Time to vaginoplasty (months), median [interquartile range] | 6.0 [4.0–24.0] |
| Success rates | |
| Functional neovagina | 27 (77.1%) |
| Personal satisfaction | 27 (77.1%) |
| Complications | |
| Any postoperative complications | 10 (28.6%) |
| Suture dehiscence | 1 (2.9%) |
| Urethral stenosis | 0 (0.0%) |
| Urethral fistula | 1 (2.9%) |
| Rectal fistula | 3 (8.6%) |
| Neovagina stenosis | 8 (22.9%) |
HIV, human immunodeficiency virus.
The median age at the time of surgery was 41 years (interquartile range: 37 to 50.5 years). The median surgical time was 120 minutes (interquartile range: 69.2–162 minutes). The median multidisciplinary follow-up was 62 (31 to 112) months with a urologist and 46 (7 to 88) months with a psychiatrist.
Twenty-seven patients (77.1%) reported functioning neovagina. Postoperative complications were reported in 10 patients (28.6%). The most common was new neovagina stenosis in 8 cases (22.9%), followed by rectal fistula in 3 cases (8.6%), urethral fistula in one patient (2.9%), and graft dehiscence in one case (Table 1).
The variables of interest were correlated with the complications presented by patients with the Spearman test (Table 2). There was a positive correlation with: HIV and vaginal stenosis (P=0.020); depression and vaginal stenosis (P=0.039), urethral fistula (P=0.002) and dehiscence (P=0.002); and smoking and rectal fistula (P=0.002).
Table 2
| Variable | Complications, P (rs) | ||||
|---|---|---|---|---|---|
| Complications | Urethral fistula | Rectal fistula | Neovagina stenosis | Dehiscence | |
| Ethnics | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| HIV | P=0.092, rs=0.324 | P>0.10 | P>0.10 | P=0.020*, rs=0.438 | P>0.10 |
| Hypertension | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| Diabetes | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| Tabagism | P>0.10 | P>0.10 | P=0.002*, rs=0.556 | P=0.369, rs=0.054 | P>0.10 |
| Depression | P>0.10 | P=0.002*, rs=0.556 | P>0.10 | P=0.039*, rs=0.386 | P=0.002*, rs=0.556 |
| Syphilis | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| Hormonal therapy | P>0.10 | – | P>0.10 | P>0.10 | – |
| Age | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| Surgical time | P>0.10 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
| Time to vaginoplasty | P=0.056, rs=0.341 | P>0.10 | P>0.10 | P>0.10 | P>0.10 |
*, correlation with statistical significance. HIV, human immunodeficiency virus; rs, Spearman rank correlation.
Discussion
Gender-affirming surgery is an increasingly performed surgery in the world. The gold standard for vaginoplasty is inverted penile skin surgery and the gold standard for secondary procedures is sigmoidoplasty (6). We are proposing a less aggressive, less invasive, and faster technique for secondary vaginoplasty, using a variation of McIndoe’s vaginoplasty, but using the mesh principle. Compared to the traditional McIndoe technique, which is a skin-free graft, the mesh technique uses a smaller donor area.
Secondary vaginoplasty using an intestinal segment had long-term sexual satisfaction rates ranging from 78% to 91% in some studies (7,8). However, these procedures involve major surgeries with violation of the abdominal cavity, possible complications such as dehiscence of anastomosis, adhesions and intestinal obstructions, risk of long-term carcinoma, excessive mucus production, prolapse, and a 25% chance of needing a second operation. In a study that evaluated long-term follow-up, 88% of the patients maintained adequate vaginal depth, however, 79% presented meatal stenosis with the need for introitusplasty additionally after the initial surgery (5).
Regardless of the chosen technique, it must be one that the surgeon and the team are well trained to obtain the best results. Our patients presented a recurrence rate of vaginal stenosis with dyspareunia in 22.9% of the sample. This complication was not found in another group of patients in our service who underwent the same surgery, but primarily, due to vaginal agenesis. Of the 7 patients followed up in the other study, all had a functional vaginal canal with 100% satisfaction (9). We believe that the main reason for restenosis is due to the retraction of the graft placed on the fibrotic area of previous surgery. The surgery manipulation on the primary sexual affirming surgery promotes more risks of fibrosis and tissue contractures in the subsequent manipulations.
Previous studies also have reported the use of full-thickness skin, myocutaneous grafts, or peritoneal vaginoplasty with more extensive donor areas (10-13). However, in these patients, the scars often became more visible, associated with contractures and deformities of abdominal skin. With the technique proposed in the present study, the resulting scar in the donor area (lower abdominal incision) was more aesthetical, as already known in other studies due to being widely used for patients who perform aesthetic abdominoplasty surgeries. The use of the mesh technique (skin mesh) allowed the use of a much smaller donor area (almost half the size of the usual donor area). This grafting technique was also performed in thin patients without the need for other flaps or expanders.
The multiple small scalpel incisions created in the donated tissue facilitate drainage of secretion, avoiding chances of bruises and collections that can compromise good graft adherence (14).
The statistically significant increase in the incidence of complications in active smokers or HIV patients could be associated with humoral immunity changes that mediate the processes of body healing. Review studies did not correlate postoperative complications with characteristics of the population and with previous pathologies of patients (7,10,15-18). Further studies are needed to better understand the healing behavior and a larger sample could confirm and strengthen these findings. Other studies may be needed to address the external validity of this study.
Conclusions
Surgical vaginal reconstruction with a free skin mesh graft is feasible and shows positive functional results with minimal morbidity and associated complications. Most patients (77%) described an active and satisfactory sex life. Objective parameters and comparative studies are needed to assess the possible complications and long-term results of this technique.
Acknowledgments
We would like to thank Professor Charles F. Ferreira from the Universidade Federal do Rio Grande de Sul for helping with the statistics of this article. Partial results of this study were presented at the 34º Congresso Brasileiro de Urologia (Brazilian Urology Society) in November 16th, 2013.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-215/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-215/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-215/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by our institutional ethics committee (Comitê de Ética em Pesquisa do Hospital de Clínicas de Porto Alegre) under the CAAE number 90344318.0.0000.5327, and informed consent was obtained from participants who returned to the outpatient follow-up consultation during the data collection period.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coleman E, Bockting W, Botzer M, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. International Journal of Transgenderism 2012;13:165-232. [Crossref]
- Massie JP, Morrison SD, Van Maasdam J, et al. Predictors of Patient Satisfaction and Postoperative Complications in Penile Inversion Vaginoplasty. Plast Reconstr Surg 2018;141:911e-21e. [Crossref] [PubMed]
- Pan S, Honig SC. Gender-Affirming Surgery: Current Concepts. Curr Urol Rep 2018;19:62. [Crossref] [PubMed]
- Selvaggi G, Ceulemans P, De Cuypere G, et al. Gender identity disorder: general overview and surgical treatment for vaginoplasty in male-to-female transsexuals. Plast Reconstr Surg 2005;116:135e-45e. [Crossref] [PubMed]
- van der Sluis WB, Bouman MB, de Boer NK, et al. Long-Term Follow-Up of Transgender Women After Secondary Intestinal Vaginoplasty. J Sex Med 2016;13:702-10. [Crossref] [PubMed]
- Moisés da Silva GV, Lobato MIR, Silva DC, et al. Male-to-Female Gender-Affirming Surgery: 20-Year Review of Technique and Surgical Results. Front Surg 2021;8:639430. [Crossref] [PubMed]
- van der Sluis WB, Pavan N, Liguori G, et al. Ileal vaginoplasty as vaginal reconstruction in transgender women and patients with disorders of sex development: an international, multicentre, retrospective study on surgical characteristics and outcomes. BJU Int 2018;121:952-8. [Crossref] [PubMed]
- Van der Sluis WB, Bouman MB, Buncamper ME, et al. Revision Vaginoplasty: A Comparison of Surgical Outcomes of Laparoscopic Intestinal versus Perineal Full-Thickness Skin Graft Vaginoplasty. Plast Reconstr Surg 2016;138:793-800. [Crossref] [PubMed]
- Motta GL, Tavares PM, Burttet LM, et al. Vaginoplasty With Full-thickness Mesh Skin Graft for Vaginal Agenesis. Urology 2016;98:200-3. [Crossref] [PubMed]
- Sadove RC, Horton CE. Utilizing full-thickness skin grafts for vaginal reconstruction. Clin Plast Surg 1988;15:443-8. [Crossref] [PubMed]
- Akn S. Experience with neovaginal construction using the full-thickness skin graft in vaginal agenesis. Ann Plast Surg 2004;52:391-6; discussion 397. [Crossref] [PubMed]
- Li JS, Crane CN, Santucci RA. Vaginoplasty tips and tricks. Int Braz J Urol 2021;47:263-73. [Crossref] [PubMed]
- Fernandez N, Chavarriaga J, Pérez J. Complete corporeal preservation clitoroplasty: new insights into feminizing genitoplasty. Int Braz J Urol 2021;47:861-7. [Crossref] [PubMed]
- Miraliakbari R, Mackay DR. Skin grafts. Operat Tech Gen Surg 2006;8:197-206. [Crossref]
- van der Sluis WB, Bouman MB, Buncamper ME, et al. Clinical Characteristics and Management of Neovaginal Fistulas After Vaginoplasty in Transgender Women. Obstet Gynecol 2016;127:1118-26. [Crossref] [PubMed]
- Horbach SE, Bouman MB, Smit JM, et al. Outcome of Vaginoplasty in Male-to-Female Transgenders: A Systematic Review of Surgical Techniques. J Sex Med 2015;12:1499-512. [Crossref] [PubMed]
- Revol M, Servant JM, Banzet P. Surgical treatment of male-to-female transsexuals: a ten-year experience assessment. Ann Chir Plast Esthet 2006;51:499-511. [Crossref] [PubMed]
- Bizic M, Kojovic V, Duisin D, et al. An overview of neovaginal reconstruction options in male to female transsexuals. ScientificWorldJournal 2014;2014:638919. [Crossref] [PubMed]


