Oral therapy for Peyronie’s disease, does it work?
Introduction
Peyronie’s disease (PD) is a localized, wound-healing, connective tissue disorder of the penis characterized by scarring of the tunica albuginea. This fibrous inelastic scar leads to penile pain, penile deformity and erectile dysfunction (ED) with difficulty in performing coitus. Francois de la Peyronie first described the condition in 1743 while he was the surgeon for Louis XIV of France. Although earlier studies reported an incidence of 0.3–0.7%, recent publications have shown an overall incidence of 3.2–8.9%, with more than 75% of cases occurring in men between 45 and 65 years of age (1,2). Of note, 10% of patients experience onset of symptoms before 40 years of age (2). Furthermore, the incidence of PD following radical prostatectomy is reported to be as high as 15.9%, and a recent study showed that penile curvature is a common finding (38.6%) at the time of inflatable penile prosthesis implantation surgery for ED after prostate cancer treatment (3,4).
The treatment for PD begins with a focused history and physical exam. A detailed history should assess information about the onset and duration of disease, associated traumatic etiology, degree of penile curvature, loss of length during erection, and subjective level of sexual function. With regards to subjective sexual evaluation, standardized questionnaires such as the International Index of Erectile Function (IIEF) allow for objective and subjective initial assessments and a tool for measuring efficacy during treatment. Physical examination of the genitourinary system should include penile length while stretched, plaque location, and size. The degree of curvature can be assessed by the patient taking photographs of the erect phallus or by vasoactive injections in combination with penile duplex Doppler ultrasound.
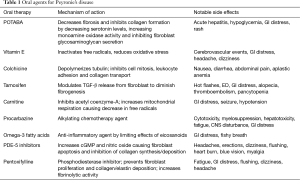
Understanding the natural history and pathogenesis of PD is important to selecting appropriate treatment regimens. While the pathophysiology is not completely understood, it is hypothesized that the inciting event is a subclinical traumatic tear to the tunica albuginea. The tear leads to a proliferative fibrotic reaction, resulting in an inelastic scar. The treatment for PD is separated into those within the acute phase and those in the chronic stabilization phase. The acute phase time is loosely defined as less than 12 months from the onset of symptoms. During this time, the patient’s scar is continuing to modify and change. The chronic phase is determined once the patient’s curvature has remained stable for at least 6 months. While surgical treatment remains the gold standard for PD, it is mostly offered to males in the chronic phase of the disease. For those patients in the acute phase, pharmacotherapy provides the potential to improve function, reduce deformity, and stabilize scar progression (5). Herein, we review the current available knowledge regarding oral treatment options for PD. It is important to note that while certain recommendations listed are based on evidence-based findings; others mirror expert opinion (Table 1).
Full table
Potassium para-aminobenxoate (POTABA)
POTABA is a compound known for its antifibrotic and anti-inflammatory properties that aid in treatment for fibrotic disorders such as dermatomyositis and scleroderma. Its efficacy is thought to be due to a reduction in collagen formation via decreasing serotonin levels, increasing monoamine oxidase activity, and inhibiting fibroblast glycosaminoglycan secretion (6). The first proposed use of POTABA to treat PD was in 1959 by Zarafonetis and Horrax (7). In 2005, Weidner et al. performed a multi center, randomized, double blind placebo-controlled trial of POTABA (51 patients) versus placebo (52 patients) in treatment naïve PD patients with non-calcified plaque. The trial arm received 3 g of POTABA orally, 4 times per day for 12 months. After 12 months, patients on POTABA had significant improvement in penile plaque size compared to those on placebo. In addition, patients receiving placebo were noted to have worsening of their curvature. The authors concluded that POTABA may aid in the stabilization of scar tissue, which would prevent progression of curvature (6). There have been no further randomized control trials to evaluate the efficacy of POTABA. This is perhaps due to its side-effect profile, which includes gastrointestinal distress, diarrhea, acute hepatitis, and hypoglycemia (8).
Vitamin E
Vitamin E, a fat-soluble antioxidant, inactivates free radicals that saturate nitric oxide (NO), thereby keeping active NO levels elevated to allow proper wound healing. By limiting oxidative stress, it potentially also offers an anti-inflammatory effect. It was first described for the treatment of PD in 1948 (9) and, due to its low cost and availability, remains the most frequently prescribed oral agent for PD (10). Despite its use over the past seven decades, multiple placebo-controlled designs have shown no significant improvement in pain, degree of curvature, plaque size, or ability to have intercourse. In 1983, Pryor and Farrell completed a double-blind, placebo-controlled trial of vitamin E in 40 PD patients, and found no significant improvement in plaque size or penile curvature (11). Furthermore, a more recent study in 2007 by Safarinejad et al. provided the largest trial to date evaluating vitamin E for PD. The authors compared vitamin E alone and in combination with L-carnitine to placebo in 236 men with early chronic PD; classified as patient with pain during erections, penile curvature not interfering with vaginal penetration, non-painful palpable scar, hyperechoic lesion on penile ultrasound, absence of calcification, and total plaque area <2 cm2. Group 1 (58 men) received vitamin E 300 mg orally twice a day. Group 2 (59 men) received propionyl-L-carnitine, 1 g orally twice a day. Group 3 (60 men) received vitamin E 300 mg orally twice a day as well as propionyl-L-carnitine 1 g orally twice daily. Lastly group 4 received a similar regimen of placebo during the 6-month trial period. After therapy, there was no significant change in reduction of penile curvature between the four groups (P=0.9), nor any decrease in plaque size (P=0.1) (12).
Despite lack of evidence proving vitamin E efficacy, it is still frequently prescribed and oftentimes given concurrently with other treatment modalities with hopes of a synergistic role. Common adverse effects from vitamin E use include nausea, vomiting, diarrhea, and increased risk for prostate cancer and cerebrovascular events (13).
Colchicine
Although colchicine is mostly known for its treatment of gout, it also exhibits properties that aid in the treatment of PD. Colchicine depolymerizes tubulin, thereby inhibiting cell mitosis, leukocyte adhesion, and collagen transport. Applying this property, colchicine should theoretically diminish wound contraction by inhibiting collagen deposition (14). Most studies evaluating colchicine have described some improvement in curvature, but these studies were not randomized, nor did they provide objective measurement for definition of improvement. Safarinejad et al. proved to be one of the few trials that evaluated the therapeutic effects of colchicine by completing a single center, randomized, double-blind, placebo-controlled trial of 84 PD patients without calcified plaques (15). Patients in the colchicine arm were treated with 0.5–2.5 mg colchicine daily for 4 months. At the end of the trial, the colchicine group did not demonstrate objective improvement in penile curvature or plaque size. Other studies have evaluated the synergistic effects of colchicine with vitamin E. Prieto Castro et al. reported significant improvement in plaque size and penile curvature in patients using daily vitamin E and colchicine compared to ibuprofen alone (16). Of note, there were only 45 patients in this trial and there was no placebo arm. However, a subsequent retrospective study of 100 men exposed no statistically significant differences in efficacy for pain relief, penile curvature, or plaque size between colchicine and colchicine combined with vitamin E (17).
Tamoxifen
Tamoxifen is a non-steroidal estrogen receptor antagonist. In the treatment of PD, it has been shown to diminish fibrogenesis in the tunica albuginea via modulating the release of TGF-β released from fibroblast (18). Ralph et al. first proposed its treatment in 1992 as a result of an uncontrolled study that demonstrated improvement in penile deformity for 11 out of 31 men. However, these finding were not reproducible when tamoxifen was evaluated in a prospective, placebo-controlled trial in 25 patients with PD without calcified plaques. Despite the use of 20 mg tamoxifen twice daily, there was no significant improvement in pain, curvature, or plaque size when compared to placebo (19).
Carnitine
Carnitine is an inhibitor of acetyl coenzyme-A that allows for the decrease of free radical formation during times of cell stress. In 2001, Biagiotti et al. performed a randomized trial of 96 PD patients to L-carnitine versus tamoxifen. Results showed significant penile curvature improvement in the L carnitine group (20). As aforementioned, the Safarinejad 2007 4-arm trial (vitamin E, carnitine, vitamin E and carnitine, and placebo) proved no significant improvement in penile curvature, plaque size, or pain (12).
Omega 3 fatty acid
Similar to other oral agents, omega-3 fatty acids have been evaluated in the treatment of PD due to its known anti-inflammatory properties. In 2009, Safarinejad et al. published their results on a prospective, randomized double-blind omega-3 fatty acids versus placebo trial. A total of 224 patients with early chronic stage PD were randomized to 1.84 g of daily omega-3 supplementation versus placebo for 6 months. Patients were assessed with IIEF-5 and PDDU before and after the 6 months of medication. Unfortunately, there was no significant improvement with regard to plaque volume, penile curvature, pain during erection, and erectile function (21). Currently, there is a lack of data to support a beneficial effect of omega-3 supplementation in early-chronic stage of PD.
Procarbazine
Procarbazine is an alkylating chemotherapy drug often used to treat CNS lymphoma, Hodgkin’s lymphoma, and high-grade gliomas. In 1968, Aron et al. noted a regression of Dupuytren’s disease in patients undergoing treatment of Hodgkin’s disease with procarbazine, thereby suggesting its use in a disease with proliferation of connective tissue (22). With this premise, in the 1970s, Byström proposed procarbazine for the treatment of PD, but subsequent studies did not reveal any objective benefit. In addition, studies unveiled significant side effects of the cytotoxic medication, and it was recommended to not be used in the benign disease of PD. Side effects include myelosuppression, hepatotoxicity, fatigue, GI distress, and CNS disturbance (23,24).
Phosphodiesterase type 5 inhibitors (PDE-5 inhibitors)
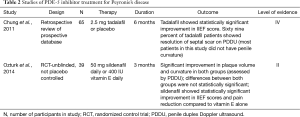
Although PDE-5 inhibitors are commonly thought as a treatment for ED, recent studies have shown its potential use in the treatment of PD in patients with or without ED. PDE-5 inhibitors increase cyclic guanosine monophosphate (cGMP) by inhibiting the degradation of cGMP to GMP. With the increase of cGMP and NO, collagen synthesis and deposition are inhibited and apoptosis of fibroblast and myofibroblast occurs (25). Due to this property, PDE-5 inhibitors may prove to be advantageous for scar remodeling. In a rat model of PD, sildenafil was shown to cause significant reduction in the collagen-to-fibroblast ratio in the tunica albuginea as well as plaque size (25). Chung and colleagues study the use of tadalafil for treatment of PD in human subjects. They reported that 2.5 mg tadalafil daily for 6 months resulted in resolution of septal scar in 69% (24/35) of patients without palpable penile plaque. Only 10% of the non-treatment arm noted resolution of scar. It is important to mention that the septal scar was not clinically palpable and most patients in this study did not have curvature (26). More recently, a 2014 study reported treatment outcomes of patients with PD using 50 mg sildenafil daily or 400 IU vitamin E for 3 months. After 12 weeks, both groups showed similar reduction in plaque volume and penile curvature that was statistically significant. The differences between the two groups, however, were not statistically significant. The sildenafil cohort did show statistically significant improvement in IIEF scores and pain reduction compared to vitamin E alone (27). A large-scale double blind placebo trial would need to be done and validate the clinical benefit of PDE-5 inhibitor, but current research is promising (Table 2).
Full table
L-arginine
L-arginine is a NO precursor that stimulates NO synthase. This causes an increase in NO, as well as a reduction in fibroblast due to apoptosis. These two properties are thought to be the mechanisms behind L-arginine as an antifibrotic agent (25). As aforementioned, Valente et al. evaluated penile plaques in rat models treated with PDE-5 inhibitors, but also with l-arginine. Similarly to PDE-5 inhibitors, L-arginine exhibited significant reduction in plaque size as well as collagen to fibroblast ratio (25). A later study by Medeiros et al. demonstrated that arginine also has a protective effect against scar tissue formation when submitting the penis of rats to pelvic radiation (28). In 2012, Abern et al. noted a trend toward curvature improvement in PD patients treated with penile traction therapy in conjunction with intralesional verapamil, oral L-arginine and oral pentoxifylline (PTX) (29). While this study has numerous variables that could contribute to curvature improvement, it proves to be one of the few human trials in which L-arginine was used to treat PD. Given these findings, L-arginine may prove to be a valuable treatment options for PD, but further human trials are needed.
Pentoxifylline (PTX)
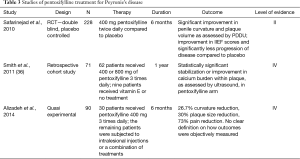
Although most of the oral medications investigated for the treatment of PD have shown poor or indeterminate outcomes in controlled studies, PTX has fared much better. PTX is a xanthine derivative that functions as a nonspecific phosphodiesterase inhibitor with known anti-inflammatory and anti-fibrogenic properties (30). It has been proposed for treatment of PD due to an in vitro study showing it prevents tunica albuginea fibroblast proliferation, attenuates TGF-B mediated deposition of collagen, reduces deposition of elastin, and increases fibrinolytic activity (31-33). Given these properties, a 2010 double blind, placebo-controlled study sought to determine the effect of PTX in patients with early chronic PD (30). Two hundred twenty-eight patients were randomized to receive 400 mg of PTX sustained release versus placebo for 6 months. Most participants had failed at least one previous PD treatment. The placebo group was 4 times more likely (42%) to have disease progression compared to the treatment arm (11%). Furthermore, the PTX group showed significant improvement in both objective and subjective measures; (I) improvement in penile curvature as measured by duplex ultrasound before and after corporal injection with prostaglandin E1; (II) plaque volume and (III) IIEF scores (30). More recent studies have evaluated oral PTX in conjunction with oral antioxidants, intralesional PTX and intralesional verapamil with varying results (34,35). While PTX remains a strong competitor for PD treatment, further large multi-center trials will need to be concluded to ensure results are reproducible (Table 3).
Full table
Conclusions
Despite decades of PD research, the definitive etiology and pathophysiology have not been completely elucidated. While surgical therapy remains the gold standard for chronic severe PD, there is a myriad of other less invasive treatment options, namely oral and intralesional medications. Herein, we have reviewed the mostly commonly prescribed or discussed oral agents for treatment of PD. Although a number of well-designed studies have discovered positive responses with oral medication, their small sample size limits the power and reproducibility of the study. Currently, the American Urological Association guidelines state clinicians should not offer oral therapy with vitamin E, tamoxifen, omega-3 fatty acid or combination of vitamin E with L-carnitine. Similarly, the 2010 International Consultation on Sexual Medicine did not support the routine clinical use of oral agents for PD (5). Two oral medications not restricted by the guidelines are PDE-5 inhibitors and PTX. Further research on PDE-5 and PTX, to include large multi center double blind, randomized-control trials, are needed to determine their efficacy. Both medication classes give hope to the absence of allowed oral medications for the treatment of PD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mulhall JP, Creech SD, Boorjian SA, et al. Subjective and objective analysis of the prevalence of Peyronie's disease in a population of men presenting for prostate cancer screening. J Urol 2004;171:2350-3. [Crossref] [PubMed]
- Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie's disease. J Urol 2006;175:2115-8; discussion 2118. [Crossref] [PubMed]
- Tal R, Heck M, Teloken P, et al. Peyronie's disease following radical prostatectomy: incidence and predictors. J Sex Med 2010;7:1254-61. [Crossref] [PubMed]
- Lin H, Alba F, Romero C, et al. 1815 penile curvature is a common finding at the penile prosthesis implantation for patients with erectile dysfunction after radical prostatectomy. J Urol 2011;185:e728-e729. [Crossref]
- Ralph D, Gonzalez-Cadavid N, Mirone V, et al. The management of Peyronie's disease: evidence-based 2010 guidelines. J Sex Med 2010;7:2359-74. [Crossref] [PubMed]
- Weidner W, Hauck EW, Schnitker J, et al. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol 2005;47:530-5; discussion 535-6. [Crossref] [PubMed]
- Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium para-aminobenzoate (potaba). J Urol 1959;81:770-2. [PubMed]
- Roy J, Carrier S. Acute hepatitis associated with treatment of Peyronie's disease with potassium para-aminobenzoate (Potaba). J Sex Med 2008;5:2967-9. [Crossref] [PubMed]
- Scott WW, Scardino PL. A new concept in the treatment of Peyronie's disease. South Med J 1948;41:173-7. [Crossref] [PubMed]
- Levine LA, Burnett AL. Standard operating procedures for Peyronie's disease. J Sex Med 2013;10:230-44. [Crossref] [PubMed]
- Pryor JP, Farrell CF. Controlled clinical trial of vitamin E in Peyronie's disease. Prog Reprod Biol 1983;9:41-5.
- Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol 2007;178:1398-403; discussion 1403. [Crossref] [PubMed]
- Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549-56. [Crossref] [PubMed]
- El-Sakka AI, Bakircioglu ME, Bhatnagar RS, et al. The effects of colchicine on a Peyronie's-like condition in an animal model. J Urol 1999;161:1980-3. [Crossref] [PubMed]
- Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res 2004;16:238-43. [Crossref] [PubMed]
- Prieto Castro RM, Leva Vallejo ME, Regueiro Lopez JC, et al. Combined treatment with vitamin E and colchicine in the early stages of Peyronie's disease. BJU Int 2003;91:522-4. [Crossref] [PubMed]
- Cortés-González JR, Glina S. Conservative treatment of Peyronie's disease: colchicine vs. colchicine plus vitamin E. Actas Urol Esp 2010;34:444-9. [Crossref] [PubMed]
- Ralph DJ, Brooks MD, Bottazzo GF, et al. The treatment of Peyronie's disease with tamoxifen. Br J Urol 1992;70:648-51. [Crossref] [PubMed]
- Teloken C, Rhoden EL, Grazziotin TM, et al. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol 1999;162:2003-5. [Crossref] [PubMed]
- Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int 2001;88:63-7. [Crossref] [PubMed]
- Safarinejad MR. Efficacy and safety of omega-3 for treatment of early-stage Peyronie's disease: A prospective, randomized, double-blind placebo-controlled study. J Sex Med 2009;6:1743-54. [Crossref] [PubMed]
- Aron E. Medical treatment of Dupuytren's disease with a cytostatic agent (methylhydrazine). Presse Med 1968;76:1956. [PubMed]
- Byström J, Johansson B, Edsmyr F, et al. Induratio penis plastica (Peyronie's disease). The results of the various forms of treatment. Scand J Urol Nephrol 1972;6:1-5. [Crossref] [PubMed]
- Oosterlinck W, Renders G. Treatment of Peyronie's disease with procarbazine. Br J Urol 1975;47:219-20. [Crossref] [PubMed]
- Valente EG, Vernet D, Ferrini MG, et al. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide 2003;9:229-44. [Crossref] [PubMed]
- Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med 2011;8:1472-7. [Crossref] [PubMed]
- Ozturk U, Yesil S, Goktug HN, et al. Effects of sildenafil treatment on patients with Peyronie's disease and erectile dysfunction. Ir J Med Sci 2014;183:449-53. [Crossref] [PubMed]
- Medeiros JL Jr, Costa WS, Felix-Patricio B, et al. Protective effects of nutritional supplementation with arginine and glutamine on the penis of rats submitted to pelvic radiation. Andrology 2014;2:943-50. [Crossref] [PubMed]
- Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie's disease. J Sex Med 2012;9:288-95. [Crossref] [PubMed]
- Safarinejad MR, Asgari MA, Hosseini SY, et al. A double-blind placebo-controlled study of the efficacy and safety of pentoxifylline in early chronic Peyronie's disease. BJU Int 2010;106:240-8. [Crossref] [PubMed]
- Raetsch C, Jia JD, Boigk G, et al. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut 2002;50:241-7. [Crossref] [PubMed]
- Schandené L, Vandenbussche P, Crusiaux A, et al. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) by monocytes and T cells. Immunology 1992;76:30-4. [PubMed]
- Shindel AW, Lin G, Ning H, et al. Pentoxifylline attenuates transforming growth factor-β1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. J Sex Med 2010;7:2077-85. [Crossref] [PubMed]
- Paulis G, Barletta D, Turchi P, et al. Efficacy and safety evaluation of pentoxifylline associated with other antioxidants in medical treatment of Peyronie's disease: a case-control study. Res Rep Urol 2015;8:1-10. [Crossref] [PubMed]
- Alizadeh M, Karimi F, Fallah MR. Evaluation of verapamil efficacy in Peyronie's disease comparing with pentoxifylline. Glob J Health Sci 2014;6:23-30. [Crossref] [PubMed]
- Smith JF, Shindel AW, Huang YC, et al. Pentoxifylline treatment and penile calcifications in men with Peyronie's disease. Asian J Androl 2011;13:322-5. [Crossref] [PubMed]


