
A morphology-based nephrometry score to predict pathological upstaging to T3 renal cell carcinoma
Introduction
The last few years have witnessed the rapidly expanding application of partial nephrectomy (PN) for localized, complex renal cell carcinoma (RCC), owing to advancements in surgical techniques and equipment such as robotic surgical systems. Complex RCC refers to the completely endogenous tumor, and those being close to the renal hilum or renal sinus. Various treatment options for small renal masses including cryoablation, microwave ablation, and radiofrequency ablation have continued to emerge in addition to increasing application of PN (1). The purpose of PN is to completely remove the tumor while preserving the surrounding structures, making less excision of the peritumoral tissue. However, it has led to increasing numbers of missing cases with adverse pathological features, such as sinus fat, calyx or venous infiltration (2).
The diagnostic issue of upstaging clinical tumor stage 1 to 2 (cT1-2) to pathological stage 3 (pT3) has attracted extensive attention and the prognosis of patients with pathological upstaging remains controversial. Some reported that patients with pathologically upstaging renal masses were subject to inferior survival outcomes compared to those without upstaging (3,4), whereas others suggested that pathological upstaging did not result in worse oncological outcomes (5,6). In addition, a recent meta-analysis investigating over 100,000 cases strongly supported that cT1 RCC patients with pT3a upstaging after surgery had a poorer recurrence-free survival and cancer-specific survival than those without pathological upstaging (7). Thus, an accurate prediction for pathological upstaging of cT1-2 RCC is an unmet need to be addressed.
In current study, we retrospectively measured different nephrometry scores through preoperative enhanced computed tomography (CT) images among patients with cT1-2 RCC who underwent surgery. Based on the selected risk factors of pathological upstaging, the M-Index, a novel morphology-based nephrometry scoring system, was developed to predict pathological upstaging to T3 of RCC. Finally, the predictive accuracy and net benefit of M-Index was compared with R.E.N.A.L (radius, exophytic/endophytic, nearness, anterior/posterior, location), PADUA (preoperative aspects and dimensions used for an anatomic classification), DAP (diameter, axial, polar) and C-Index scores. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-430/rc) for prediction models of risk of disease development or progression.
Methods
Study cohort
From December 2020 to May 2021, 431 patients clinically diagnosed as RCC in Changhai Hospital were enrolled. Patients whose preoperative digital images could not be obtained or tumor histological type was benign or sarcoma were excluded. In total, 200 patients with cT1-2 RCC were enrolled in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Changhai Hospital (No. CHEC2021-191) and informed consent was taken from all the patients.
The baseline characteristics of included patients were retrospectively collected from medical records: age, gender, body mass index (BMI), tumor laterality, and pathological outcomes (tumor pathological type and TNM stage). The renal tumor characteristics were achieved based on preoperative enhanced CT images: tumor diameter, morphology, depth, and location.
Nephrometry scoring system
According to the protocols described for these nephrometry scoring systems, the R.E.N.A.L., PADUA, DAP, and C-Index scores were determined through enhanced CT images performed within one month before surgery (8-11). The R.E.N.A.L. score consists of radius (tumor size as maximal diameter), exophytic/endophytic properties of the tumor, nearness of tumor deepest portion to the collecting system or sinus, anterior/posterior descriptor and the location relative to the polar line. The preoperative aspects and dimensions used for an anatomical PADUA score were generated to predict the risk of complications by evaluating anterior or posterior, longitudinal, and rim tumor location; tumor relationships with renal sinus or urinary collecting system; and percentage of tumor deepening into the kidney. The DAP score consists of tumor diameter, axial, and polar parameters. Centrality index (C-Index) is described to quantify the proximity of kidney tumors to the renal central sinus. The R.E.N.A.L. score was categorized into low (score 4–6), moderate (score 7–9), and high (score ≥10). The PADUA score was categorized into low (score 6–7), moderate (score 8–9), and high (score ≥10). The DAP score was categorized into low (score 3–5) and high (score 6–9). C-Index score was categorized into low (score ≤1) and high (score >1).
Univariate and multivariate logistic regression analyses were performed to identify independent predictors of pathological upstaging. A stepwise selection method was applied to select predictors to construct the M-Index, a novel nephrometry scoring system based on tumor morphology. Receiver-operating characteristic (ROC) analyses were applied to distinguish the predictive power of M-Index, R.E.N.A.L, PADUA, DAP, C-Index scores. Decision curve analysis (DCA) was used to examine the net benefit of these nephrometry scoring systems in clinical decision-making at different threshold probabilities of pathological upstaging.
Statistical analysis
All data processing and statistical tests were performed with SPSS 18.0 (SPSS, IL, USA) and Stata v12.0 (StataCorp., TX, USA). The continuous parametric or nonparametric variables were compared using Student’s t-test or Mann-Whitney U-test, respectively. The categorical variables were compared using Pearson’s Chi-square test. The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of the selected predictors of pathological upstaging were presented. Statistically significant P value was set at 0.05 with two sides.
Results
Baseline characteristics of included patients
Strictly conforming to the inclusion and exclusion criteria, 200 cT1-2 RCC patients who underwent surgical treatment between December 2020 and May 2021 at our center were enrolled. Of the included patients, 34 (17%) were upstaged to pT3 and 166 (83%) were not upstaged (pT1-2). The baseline characteristics and renal tumor characteristics of included patients are listed in Table 1. There was no significant difference in distributions of age, gender, tumor laterality, and pathological type between upstaging and non-upstaging groups. Moreover, we compared the independent tumor features, as recorded by the R.E.N.A.L, PADUA, DAP and C-Index scores, between the patients with pathological upstaging or not. The tumors with pathological upstaging tended to have a larger diameter (5.0 vs. 4.0 cm, P=0.016) and a more irregular morphology (23.5% vs. 6.0%, P<0.001), which were not regular shapes such as round, oval, or lobular. Furthermore, they were located closer to the center of kidney (52.9% vs. 19.9%, P<0.001), and nearer to the collecting system (70.6% vs. 50.0%, P=0.038) or renal sinus (31.9% vs. 52.9%, P=0.020).
Table 1
| Variable | Without upstage (n=166) | With upstage (n=34) | P value |
|---|---|---|---|
| Age, years, median [IQR] | 56 [51–64] | 61 [54–68] | 0.085 |
| Gender | 0.770 | ||
| Female | 38 | 7 | |
| Male | 128 | 27 | |
| BMI (kg/m2), median (IQR) | 24.4 (22.3–27.0) | 24.5 (22.6–27.1) | 0.985 |
| Surgical approach | 0.046 | ||
| Open | 4 | 4 | |
| Laparoscopic | 126 | 26 | |
| Robot-assisted laparoscopic | 36 | 4 | |
| Tumor laterality | 0.223 | ||
| Left | 69 | 18 | |
| Right | 97 | 16 | |
| Maximum tumor diameter (cm), median (IQR) | 4.0 (3.0–5.0) | 5.0 (4.2–6.1) | 0.016 |
| Tumor morphology | <0.001 | ||
| Round | 144 | 14 | |
| Lobular | 12 | 12 | |
| Irregular | 10 | 8 | |
| Tumor depth | 0.522 | ||
| ≥50% exophytic | 105 | 18 | |
| <50% exophytic | 52 | 14 | |
| Endophytic | 9 | 2 | |
| Tumor longitudinal location | 0.068 | ||
| Upper/lower | 92 | 13 | |
| Middle | 74 | 21 | |
| Polar distance | 0.003 | ||
| Distance to polar lines >2 cm | 51 | 1 | |
| Distance to polar lines ≤2 cm | 34 | 10 | |
| Overlap renal hilum level | 81 | 23 | |
| Tumor rim location | <0.001 | ||
| Outer | 98 | 10 | |
| Inner | 35 | 6 | |
| Renal hilar lesion | 33 | 18 | |
| Tumor lateral location | 0.001 | ||
| Anterior | 60 | 9 | |
| Posterior | 71 | 7 | |
| Touching renal artery or vein | 35 | 18 | |
| Axial distance | 0.015 | ||
| Distance to axial midline >1.5 cm | 62 | 4 | |
| Distance to axial midline ≤1.5 cm | 37 | 11 | |
| Overlap axial renal midline | 67 | 19 | |
| Nearness of tumor to collecting system | 0.038 | ||
| Yes | 83 | 24 | |
| No | 83 | 10 | |
| Nearness of tumor to renal sinus | 0.020 | ||
| Yes | 53 | 18 | |
| No | 113 | 16 | |
| Pathological type | 0.488 | ||
| Clear cell | 154 | 31 | |
| Papillary | 7 | 3 | |
| Chromophobe | 5 | 0 |
BMI, body mass index; IQR, interquartile range.
The tumors with pathological upstaging had higher median R.E.N.A.L. score than those with non-upstaging (9 vs. 7, P<0.001). The R.E.N.A.L score, which consists of radial width, exophytic/endophytic growth, nearness to renal sinus, anterior/posterior hilar, and location relative to polar lines could assess the complexity of nephrometry. According to this categorical standard, renal tumors were categorized as low complexity in 73 (36.5%) cases, moderate in 92 (46%) cases, and high in 34 (17%) cases. The other nephrometry scores are also listed in Table 2. It showed that the tumors in the pathological upstaging group had higher PADUA scores (10 vs. 9, P=0.003), higher DAP scores (8 vs. 7, P<0.001) and lower C-Index scores (1 vs. 2, P<0.001).
Table 2
| Variable | Without upstage (n=166) | With upstage (n=34) | P value |
|---|---|---|---|
| R.E.N.A.L. score, median [IQR] | 7 [5–9] | 9 [8–10] | <0.001 |
| R.E.N.A.L. risk | <0.001 | ||
| Low [4–6] | 70 | 3 | |
| Moderate [7–9] | 72 | 20 | |
| High [≥10] | 24 | 11 | |
| PADUA score, median [IQR] | 9 [7–10] | 10 [9–11] | 0.003 |
| PADUA risk | 0.005 | ||
| Low [6–7] | 51 | 3 | |
| Moderate [8–9] | 54 | 9 | |
| High [≥10] | 61 | 22 | |
| DAP score, median [IQR] | 7 [5–8] | 8 [7–9] | <0.001 |
| DAP risk, n (%) | 0.001 | ||
| Low [3–5] | 48 | 1 | |
| High [6–9] | 118 | 33 | |
| C-Index score, median [IQR] | 2 [2–4] | 1 [1–2] | <0.001 |
| C-Index risk, n (%) | <0.001 | ||
| Low (≤1) | 37 | 21 | |
| High (>1) | 129 | 13 |
R.E.N.A.L., radius, exophytic/endophytic, nearness, anterior/posterior, location; IQR, interquartile range; PADUA, preoperative aspects and dimensions used for an anatomic classification; C-Index, centrality index; DAP, diameter, axial, polar.
Risk factors for pathological upstaging
Firstly, the tumor characteristics with significant difference between two groups were regarded as potential risk factors. Secondly, the parameter criteria (Score 1–3) of these eight potential risk factors were set according to Table S1. Thirdly, we performed univariate logistic regression analysis to determine independent risk factors for pathological upstaging to T3 of RCC. The results indicated that the tumor diameter and morphology, polar and axial distances, tumor rim and lateral locations, adjacency of tumor to collecting system and renal sinus may constitute independent risk factors for pT3 upstaging (all P<0.05) (Table 3).
Table 3
| Variable | Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Maximum tumor diameter | 1.97 | 1.06–3.68 | 0.033 | 0.98 | 0.46–2.07 | 0.952 | |
| Tumor morphology | 3.47 | 2.08–5.80 | <0.001 | 3.26 | 1.79–5.97 | <0.001 | |
| Polar distance | 2.20 | 1.27–3.84 | 0.005 | 1.92 | 0.92–4.03 | 0.084 | |
| Tumor rim location | 2.33 | 1.50–3.63 | <0.001 | 2.95 | 1.16–7.46 | 0.023 | |
| Tumor lateral location | 2.03 | 1.23–3.34 | 0.006 | 0.70 | 0.30–1.64 | 0.413 | |
| Axial distance | 1.81 | 1.13–2.90 | 0.014 | 1.43 | 0.75–2.74 | 0.282 | |
| Nearness of tumor to collecting system | 2.40 | 1.08–5.33 | 0.032 | 0.68 | 0.22–2.14 | 0.514 | |
| Nearness of tumor to renal sinus | 2.40 | 1.13–5.07 | 0.022 | 0.42 | 0.09–1.94 | 0.267 | |
OR, odds ratio; CI, confidence interval.
Based on the independent risk factors identified, we performed multivariate logistic regression analysis to construct a novel nephrometry scoring system to predict pT3 upstaging. Finally, the tumor morphology (P<0.001) and tumor rim location (P=0.023) were found to be significantly associated with pT3 upstaging (Table 3). Consisting of two non-complex parameters such as tumor morphology and rim location, a novel nephrometry score special for predicting pT3 upstaging was developed. Due to its morphology-based features, the novel nephrometry scoring system was named as M-Index.
Predictive performance of nephrometry scoring systems
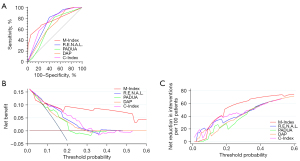
The performance of predicting pathological upstaging for M-Index was compared with previously reported nephrometry scoring systems including R.E.N.A.L., PADUA, DAP, and C-Index. The M-Index [area under curve (AUC): 0.756] came out to be the greatest accurate predictor and outperformed other nephrometry scores including R.E.N.A.L. (AUC: 0.728, P=0.617), PADUA (AUC: 0.641, P=0.026), DAP (AUC: 0.661, P=0.100) and C-Index (AUC: 0.743, P=0.778) (Figure 1A). Furthermore, the DCA showed that the M-Index was clearly superior to the other nephrometry scoring systems with a higher net benefit for all threshold probabilities greater than 10% for patients with cT1-2 RCC undergoing surgical treatment (Figure 1B). For example, if a pathological upstaging risk of 10% to 20% is considered as the threshold probability for cT1-2 RCC, decision based on M-Index score would reduce 24.5% to 52.5% of missing prediction (Figure 1C and Table S2).
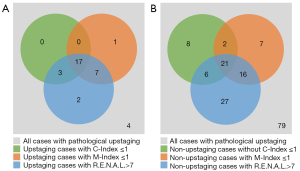
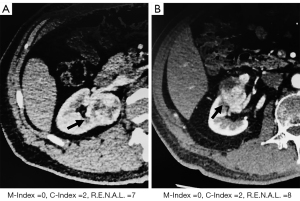
Among the total 34 patients with pathological upstaging, there were, respectively, 25 and 29 cases with M-Index less than or equal to 1 and R.E.N.A.L. higher than 7, more than the 20 cases with C-Index less than or equal to 1 (Figure 2A). However, as for non-upstaging patients, R.E.N.A.L. score misidentified the most number of non-upstaging cases than the M-Index and C-Index scores (70 vs. 46 vs. 37) (Figure 2B). Moreover, the typical CT images of upstaging patients with M-Index equal to 0 are shown in Figure 3, while C-Index and R.E.N.A.L. scores might predict them as non-upstaging cases.
Discussion
Over the past decades, the incidence of RCC has increased significantly (12). Due to the wide use of new diagnostic technology and the strengthening awareness of cancer screening, most RCCs were diagnosed at their cT1-2 stages (13). Those patients could benefit from surgical treatment, such as radical nephrectomy (RN) and PN. There was no statistically significant difference in survival prognosis comparing PN to RN (14). However, 4% to 25% of these tumors were found to have occult adverse pathological features at final pathology report, such as perirenal or sinus fat invasion or tiny tumor thrombus, and these cases would be diagnosed as pT3 RCC (1,15,16). It has been demonstrated that cT1-2 RCC patients upstaging to pT3 after surgery seem to have a worse oncological outcome than those non-upstaging patients (4,17). Among these patients, PN presented significantly inferior recurrence-free survival and worse oncologic outcomes relative to RN, such as distant metastasis (18,19). Therefore, preoperative identification of those cT1 RCC patients who are most likely to be pathologically upstaged is extremely important, and this may help clinicians in decision-making and patient counseling. In current study, we developed a morphology-based nephrometry scoring system for predicting pT3 upstaging of RCC.
The nephrometry scoring system was developed in 2009 originally to quantify anatomic characteristics of RCC to overcome the dilemma of surgical decision on PN or RN because of the tumor complexity. The R.E.N.A.L., PADUA, DAP, and C-Index scores were determined through enhanced CT images performed within one month before surgery, and were all successfully used to predict warm ischemia time, blood loss, complications including urine leak, length of hospital stay, and functional recovery (9,10). More recently, there have been attempts to correlate these nephrometry scoring systems with tumor pathology and biology, declaring that cT1 RCC with higher scores were more likely to be of a higher pathological stage (20,21). Although frequently used, controversy does exist regarding the possible role of R.E.N.A.L. score as a predictor of malignancy and aggressiveness of RCC (22). These scoring systems were originally conceived for the evaluation of surgical complexity and morbidity of PN, which were suboptimal in predicting pathological upstaging (23).
The applications and limitations of previous studies were conspicuous. Multiple and overlapped parameters caused the inefficiency of nephrometry scoring system, resulting in a decrease in specificity and sensitivity (17,19). Due to the accuracy and immediacy, the R.E.N.A.L. score is the most widely used nephrometry scoring system for assessing the complexity of RCC. However, its accessibility is reduced due to its large number of parameters (17). By contrast, the C-Index score is simple in parameters, but complex in calculation. More importantly, it regards the kidney as an approximate ellipsoid, ignoring the irregularity of tumor (24). The DAP score is a synthesis and simplification of R.E.N.A.L. and C-Index, but it did not describe the relationship between tumor and collecting system (25). There remains an unmet demand for a simple and specialized tool to better characterize pathological upstaging in the preoperative setting.
Given this clinical need, we sought to identify risk factors of pT3 upstaging in RCC. In our research, tumor morphology and tumor rim location were significantly associated with pT3 upstaging, as a novel nephrometry scoring system M-Index. Due to the proliferative activity and heterogeneity of renal tumor, our study confirmed that irregular tumor morphology was related to the aggressiveness of tumor, which could lead to tumor progression. This finding also seems to be congruent with the previous report (1). On the other hand, the renal hilar location is a relatively composite parameter. According to the results of our own and other researchers, the renal hilar location is not only related to the histology of tumor progression, but also related to the difficulty of surgical treatment (26,27).
The current study is not without limitations. Firstly, 34/200 (17%) of our cohort had their RCC upstaged to pT3a, which was relatively greater than the rates reported by other researchers (5,28). An explanation for these discordant results might have been that our cohort included patients who underwent RN and PN for the cT1-2 tumors. Secondly, we did not perform the survival analysis due to short follow-up time. Thus it was unverifiable that pT3 upstaging could affect prognosis of RCC. Thirdly, the associations between M-Index and pathological upstaging risk should be validated in external cohorts.
Conclusions
In summary, we have demonstrated that patients with cT1-2 RCC upstaging to pT3 tended to have large tumor diameter, irregular tumor morphology, inner tumor location, and short polar and axial distance, compared to those without upstaging. Consisting of fewer non-complex parameters (tumor morphology and rim location), the M-Index is intuitive, practical, and outperformed R.E.N.A.L, PADUA, DAP, C-Index in predicting pathological upstaging to T3 in RCC patients undergoing surgery.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (82203134 to Xiaolei Shi); Naval Medical University Sailing Program (2021 to Wei Zhang); Changhai Hospital Basic Medical Research Program (2021JCMS04 to Wei Zhang); and National Natural Science Foundation of China (81802581 to Wei Zhang).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-430/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-430/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-430/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-430/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Changhai Hospital (No. CHEC2021-191) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teishima J, Hayashi T, Kitano H, et al. Impact of radiological morphology of clinical T1 renal cell carcinoma on the prediction of upstaging to pathological T3. Jpn J Clin Oncol 2020;50:473-8. [Crossref] [PubMed]
- Liu H, Wang Z, Peng E, et al. Added Value of Systemic Inflammation Markers in Predicting Clinical Stage T1 Renal Cell Carcinoma Pathologically Upstaged to T3a. Front Oncol 2021;11:679536. [Crossref] [PubMed]
- Guo P, Wang Y, Han Y, et al. Oncological Outcomes of Patients With Different Pathological Features of pT3a Renal Tumor: A Systematic Review and Quantitative Synthesis. Front Oncol 2021;11:678459. [Crossref] [PubMed]
- Lee H, Lee M, Lee SE, et al. Outcomes of pathologic stage T3a renal cell carcinoma up-staged from small renal tumor: emphasis on partial nephrectomy. BMC Cancer 2018;18:427. [Crossref] [PubMed]
- Ramaswamy K, Kheterpal E, Pham H, et al. Significance of Pathologic T3a Upstaging in Clinical T1 Renal Masses Undergoing Nephrectomy. Clin Genitourin Cancer 2015;13:344-9. [Crossref] [PubMed]
- Lee C, You D, Yoo S, et al. Oncological outcomes of patients with incidental pathological T3a stage small renal cell carcinoma after partial nephrectomy. J Cancer Res Clin Oncol 2016;142:1651-7. [Crossref] [PubMed]
- Chen L, Deng W, Liu X, et al. Impact of pathological T3a upstaging on oncological outcomes of clinical T1 renal cell carcinoma: a meta-analysis. J Cancer 2019;10:4998-5006. [Crossref] [PubMed]
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. [Crossref] [PubMed]
- Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. [Crossref] [PubMed]
- Simmons MN, Hillyer SP, Lee BH, et al. Diameter-axial-polar nephrometry: integration and optimization of R.E.N.A.L. and centrality index scoring systems. J Urol 2012;188:384-90. [Crossref] [PubMed]
- Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol 2010;183:1708-13. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:706-20. [Crossref] [PubMed]
- Mir MC, Derweesh I, Porpiglia F, et al. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol 2017;71:606-17. [Crossref] [PubMed]
- Beksac AT, Paulucci DJ, Gul Z, et al. Risk factors and prognostic implications for pathologic upstaging to T3a after partial nephrectomy. Minerva Urol Nefrol 2019;71:395-405. [Crossref] [PubMed]
- Patel SH, Uzzo RG, Larcher A, et al. Oncologic and Functional Outcomes of Radical and Partial Nephrectomy in pT3a Pathologically Upstaged Renal Cell Carcinoma: A Multi-institutional Analysis. Clin Genitourin Cancer 2020;18:e723-9. [Crossref] [PubMed]
- Veccia A, Antonelli A, Minervini A, et al. Upstaging to pT3a disease in patients undergoing robotic partial nephrectomy for cT1 kidney cancer: Outcomes and predictors from a multi-institutional dataset. Urol Oncol 2020;38:286-92. [Crossref] [PubMed]
- Shah PH, Moreira DM, Patel VR, et al. Partial Nephrectomy is Associated with Higher Risk of Relapse Compared with Radical Nephrectomy for Clinical Stage T1 Renal Cell Carcinoma Pathologically Up Staged to T3a. J Urol 2017;198:289-96. [Crossref] [PubMed]
- Russell CM, Lebastchi AH, Chipollini J, et al. Multi-institutional Survival Analysis of Incidental Pathologic T3a Upstaging in Clinical T1 Renal Cell Carcinoma Following Partial Nephrectomy. Urology 2018;117:95-100. [Crossref] [PubMed]
- Mouracade P, Kara O, Dagenais J, et al. Perioperative morbidity, oncological outcomes and predictors of pT3a upstaging for patients undergoing partial nephrectomy for cT1 tumors. World J Urol 2017;35:1425-33. [Crossref] [PubMed]
- Veccia A, Falagario U, Martini A, et al. Upstaging to pT3a in Patients Undergoing Partial or Radical Nephrectomy for cT1 Renal Tumors: A Systematic Review and Meta-analysis of Outcomes and Predictive Factors. Eur Urol Focus 2021;7:574-81. [Crossref] [PubMed]
- Ball MW, Gorin MA, Bhayani SB, et al. Preoperative predictors of malignancy and unfavorable pathology for clinical T1a tumors treated with partial nephrectomy: a multi-institutional analysis. Urol Oncol 2015;33:112.e9-14. [Crossref] [PubMed]
- Fonseca RB, Straub Hogan MM, Kapp ME, et al. Diagnostic renal mass biopsy is associated with individual categories of PADUA and RENAL nephrometry scores: Analysis of diagnostic and concordance rates with surgical resection. Urol Oncol 2021;39:371.e7-371.e15. [Crossref] [PubMed]
- Hu C, Sun J, Zhang Z, et al. Parallel comparison of R.E.N.A.L., PADUA, and C-index scoring systems in predicting outcomes after partial nephrectomy: A systematic review and meta-analysis. Cancer Med 2021;10:5062-77. [Crossref] [PubMed]
- Veccia A, Antonelli A, Uzzo RG, et al. Predictive Value of Nephrometry Scores in Nephron-sparing Surgery: A Systematic Review and Meta-analysis. Eur Urol Focus 2020;6:490-504. [Crossref] [PubMed]
- Correa AF, Yankey H, Li T, et al. Renal Hilar Lesions: Biological Implications for Complex Partial Nephrectomy. Urology 2019;123:174-80. [Crossref] [PubMed]
- Shim M, Song C, Park S, et al. Hilar location is an independent prognostic factor for recurrence in T1 renal cell carcinoma after nephrectomy. Ann Surg Oncol 2015;22:344-50. [Crossref] [PubMed]
- Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: a multi-institutional analysis. J Urol 2013;190:1907-11. [Crossref] [PubMed]


