
Comparison of efficacy of roxadustat and erythropoietin for the treatment of renal anemia in patients with chronic kidney disease: a retrospective study
Highlight box
Key findings
• Roxadustat is superior to rhEPO owing to its real clinical effects on anemia in patients with CKD.
What is known and what is new?
• There are limitations on application extension in clinical practice of roxadustat due to their strict inclusion criteria in RCTs.
• This study includes more types of participants.
What is the implication, and what should change now?
• This study provides clear evidence for the efficacy of roxadustat in clinical practice and further investigation will be required to firmly establish the safety and efficacy profile of roxadustat in wider population.
Introduction
In China, there are an estimated 120 million people with chronic kidney disease (CKD) (1), of which 2% will likely progress to end-stage renal disease (ESRD). Anemia is a common complication of CKD caused by impaired oxygen sensing during renal failure. It decreases erythropoiesis, influences the survival of red blood cells, induces inflammation, and blocks metabolism. More than 90% of dialysis patients have been reported to suffer from renal anemia (2), which is associated with an increased risk of cardiovascular events (3,4) and all-cause mortality (5).
Conventionally, recombinant human erythropoietin (rhEPO) is used to treat anemia in patients with CKD. It not only improves hemoglobin (Hb) levels but also delays the progression of CKD, reduces hospitalization and all-cause mortality, and improves the quality of life of patients with CKD (6). However, the increase in adverse events associated with disease progression is disturbing. Owing to the frequent incidence of treatment-related adverse events, such as tumors, endocrine system dysfunction, and cardiovascular events, rhEPO doses are often reduced (7). In addition, erythropoietin resistance can occur in some patients, resulting in treatment failure, the risk of needle-stick injury for nurses, and the inconvenience of frequent subcutaneous injections (8).
As a hypoxia-inducible factor prolyl hydroxylase inhibitor, the novel agent roxadustat may increase endogenous erythropoietin concentrations by mediating the hypoxia-inducible factor, which is regulated via the inhibition of certain activated domains, such as pan-prolyl hydroxylase (9). Subsequently, it has been incorporated into the therapeutic regimen for anemia in CKD (10). Recent randomized, multicenter, double-blind clinical studies conducted in China have shown that roxadustat significantly improves the Hb levels in patients with CKD with or without dialysis (11,12). Other studies have also shown that roxadustat can effectively treat renal anemia and reduce the need for blood transfusions and intravenous iron supplementation (13-15).
Although some high-quality randomized controlled trials (RCTs) had demonstrated the effectiveness of roxadustat in the treatment of renal anemia, there were limitations on application extension in clinical practice of roxadustat due to their strict inclusion criteria (12,16). These RCTs somehow limited more types of participants to some extent, such as higher age and lower baseline Hb level. These patients were encountered in real clinical settings frequently. The concrete difference of real clinical efficacy of rhEPO and roxadustat was still uncertain. Therefore, this retrospective study included more types of participants and aimed to assess the real clinical efficacy of roxadustat in the treatment of renal anemia in patients with CKD and provide further insight. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-709/rc).
Methods
Data sources and processing
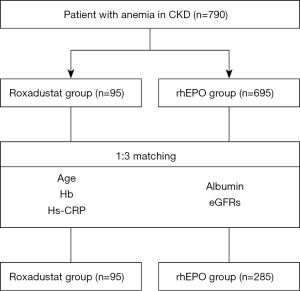
This was a single-center observational longitudinal cohort study. The study design is depicted as a flowchart in Figure 1. We performed a retrospective comparative study, enrolling a total of 790 patients with renal anemia, including 95 patients who received roxadustat therapy for more than 4 weeks and 695 patients who received rhEPO treatment for more than 4 weeks. And baseline characteristics were compared in the two groups. After propensity-score matching at a 1:3 ratio, we compared the Hb level of roxadustat and rhEPO groups, mainly at weeks of 4, 12 and 24. The concrete dose of roxadustat capsules initially administered to each patient was decided based on their weight: either 100 mg (weight between 45 and 60 kg) or 120 mg (weight ≥60 kg) three times a week. rhEPO was subcutaneously administered once or twice per week, and the dose was adjusted according to the Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Guidelines. Both groups were followed up for ≥24 weeks.
The primary endpoints were the changes in the median Hb level from baseline to weeks 4, 12, and 24. The secondary endpoints were the maximum Hb level during the follow-up period, time to reach the maximum Hb level, Hb response rate, and the proportion of patients achieving the target Hb levels between 100 and 120 g/L.
The basic information of the included patients was collected from the medical records. The baseline characteristics of the participants included: (I) history of underlying diseases: hypertension and diabetes mellitus (DM); (II) personal history: age, sex, smoking and drinking habits; (III) medication history: oral iron, oral folic acid, oral iron sucrose, and sevelamer hydrochloride; (IV) anemia-related parameters: Hb, hematocrit (HCT), red blood cell count, and platelet count; (V) nutritional and inflammatory indicators: high-sensitivity C-reactive protein (Hs-CRP), serum albumin, white blood cell count, and glucose; and (VI) renal function and serum electrolytes: estimated glomerular filtration rates (eGFRs), serum calcium, and phosphorus concentrations. We measured the biomedical parameters using a biochemical automatic enzyme analyzer at the Clinical Laboratory of Zhejiang Provincial People’s Hospital. All covariates were measured once at baseline. The patients underwent routine blood tests at weeks 4, 12, and 24. The number of days from treatment initiation to maximum Hb level was recorded during the follow-up period.
The Ethics Committee of Zhejiang Provincial People’s Hospital approved the study (No. 2022QT346) and it was carried out according to the Declaration of Helsinki (as revised in 2013). The need for informed consent was waived due to the retrospective study design. All data were analyzed anonymously to protect patient privacy.
Eligibility criteria
The inclusion criteria were as follows: (I) age >18 years; (II) anemia in patients with CKD, defined as Hb values from 60–100 g/L during the screening period; (III) administration of rhEPO for ≥4 weeks or administration of roxadustat for ≥4 weeks. The exclusion criteria were as follows: (I) anemia that was not CKD-related, including myelodysplastic syndrome, multiple myeloma, hereditary hematologic disease such as thalassemia, sickle cell anemia, pure red cell aplasia, or other diseases that interfere with blood cell metabolism; (II) follow-up time <12 weeks; and (III) patients with pre-existing malignancy.
Definitions
According to the 2018 Chinese Guidelines for the Prevention and Treatment of Hypertension, hypertension was defined as systolic and diastolic blood pressure ≥140/90 mmHg after 15 min of rest (17). DM was defined as fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test, glycosylated hemoglobin A1C ≥6.5% (48 mmol/mol), or random plasma glucose ≥200 mg/dL (11.1 mmol/L), according to the American Diabetes Association: Standards of Medical Care in Diabetes-2021 (18). Renal function was calculated using the following formula: eGFRs (mL/min−1/1.73 m2) = 186 × serum creatinine − 1.154 × age − 0.203 (× 0.742, if female), which was derived from the simplified Modification of Diet in Renal Disease study for Chinese people (19).
Statistical analyses
Imputation was performed if the missing values were <20%. We used predictive mean matching to impute the numeric features. Categorical data are presented as numbers (%) and were analyzed using the χ2 or Fisher’s exact tests. Normally distributed continuous variables are expressed as mean ± standard deviation and were compared between groups using independent two-sample t-tests; continuous data not conforming to a normal distribution are expressed as median and interquartile ranges and were compared between groups using the Mann-Whitney U test.
All statistical analyses were performed using R software (version 3.6.2, R Foundation for Statistical Computing., Auckland, NZ) and IBM SPSS Statistics (version 26.0, IBM Corp., Armonk, NY, USA). Statistical significance was set at P<0.05. To minimize the influence of selection bias and potential confounders, a 1:3 propensity-score matching (PSM) analysis was performed to balance the differences between the groups. Propensity scores were calculated using an R language model; the variables used as covariates were age, Hb, albumin, Hs-CRP, and eGFRs, and the matching tolerance was set to 0.1 (Figure 2).
Results
The serum albumin, Hs-CRP, glucose, HCT, red blood cell count, white blood cell count, age, and median baseline Hb level covariates were different between the two groups before PSM. After PSM, the matched cohort included 95 and 285 patients in the roxadustat and rhEPO groups, respectively. In addition, no differences in clinical parameters existed between the two groups, except for the proportions of patients with hypertension and DM, and patients on medications e.g., oral iron, sucrose, and folic acid (Table 1). The propensity score distribution and changes in the density of the propensity scores are shown in Figure 2.
Table 1
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Roxadustat group (N=95) | rhEPO group (N=695) | P value | Roxadustat group (N=95) | rhEPO group (N=285) | P value | ||
| Gender (male), n (%) | 64 (58.7) | 409 (58.8) | 0.979 | 54 (56.8) | 167 (58.6) | 0.764 | |
| Age (years) | 57 [46–71] | 67 [54–82] | <0.001 | 57 [45–69] | 58 [47–71] | 0.454 | |
| Smoking habit, n (%) | 29 (26.6) | 204 (29.4) | 0.557 | 26 (27.4) | 81 (28.4) | 0.843 | |
| Drinking habit, n (%) | 26 (23.9) | 136 (19.8) | 0.328 | 21 (22.1) | 55 (19.3) | 0.554 | |
| Hypertension, n (%) | 57 (52.3) | 332 (47.8) | 0.38 | 53 (55.8) | 123 (43.2) | 0.032 | |
| Diabetes mellitus, n (%) | 27 (24.8) | 143 (20.6) | 0.319 | 25 (26.3) | 45 (15.8) | 0.022 | |
| Oral iron, n (%) | 65 (59.6) | 426 (61.3) | 0.741 | 56 (58.9) | 173 (60.7) | 0.762 | |
| Oral folic acid, n (%) | 53 (48.6) | 475 (68.3) | <0.001 | 46 (48.4) | 186 (65.3) | 0.004 | |
| Oral iron sucrose, n (%) | 12 (11.0) | 236 (34.0) | <0.001 | 10 (10.5) | 93 (32.6) | <0.001 | |
| Use of sevelamer hydrochloride, n (%) | 8 (7.3) | 29 (4.2) | 0.142 | 8 (8.4) | 10 (3.5) | 0.094 | |
| White blood cell count, ×109/L | 5.69 [4.73–7.29] | 6.2 [4.73–8.24] | 0.043 | 5.69 [4.78–7.3] | 6.25 [4.83–8.30] | 0.074 | |
| Red blood cell specific volume, L/L | 0.257 [0.224–0.285] | 0.235 [0.206–0.27] | 0.001 | 0.257 [0.229–0.279] | 0.26 [0.223–0.281] | 0.963 | |
| Platelet, ×109/L | 170 [136–233.5] | 163 [116–216] | 0.061 | 170 [137–233] | 164 [115.5–215.5] | 0.089 | |
| Red blood cells count, ×1012/L | 2.94 [2.52–3.24] | 2.59 [2.29–3] | <0.001 | 2.94 [2.6–3.22] | 2.81 [2.52–3.18] | 0.264 | |
| Hemoglobin, g/L | 85 [74–96] | 77 [67–87] | <0.001 | 85 [76–93] | 83 [73–91] | 0.164 | |
| Albumin, g/L | 34.1 [31.1–38.4] | 32 [28.3–35.4] | <0.001 | 34.4 [31.1–38.6] | 33.7 [30.2–37.4] | 0.183 | |
| Glucose, mmol/L | 5.05 [4.52–6.03] | 5.48 [4.68–7.2] | 0.004 | 5.03 [4.48–6] | 5.20 [4.54–6.85] | 0.075 | |
| Ca, mmol/L | 2.145 [2.03–2.27] | 2.08 [1.94–2.22] | 0.01 | 2.15 [2.04–2.29] | 2.11 [1.98–2.24] | 0.115 | |
| P, mmol/L | 1.65 [1.41–1.98] | 1.56 [1.20–1.98] | 0.047 | 1.65 [1.37–1.98] | 1.55 [1.20–1.98] | 0.128 | |
| eGFRs, mL/min | 8.42 [4.5–14.11] | 7.538 [4.86–11.86] | 0.376 | 9.18 [4.75–15.57] | 7.65 [4.93–13.14] | 0.287 | |
| Hs-CRP, mg/L | 4.2 [2.2–11.39] | 12.3 [3–39.7] | <0.001 | 3.90 [2.20–10.8] | 5.90 [1.925–18.2] | 0.138 | |
Data are presented as, n (%) or median [interquartile range]. PSM, propensity-score matching; Ca, serum calcium; P, serum phosphorus; eGFRs, estimated glomerular filtration rates; rhEPO, recombinant human erythropoietin; Hs-CRP, high-sensitivity C-reactive protein.
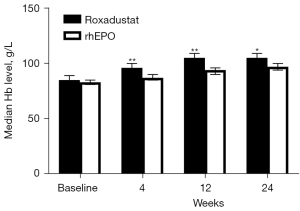
In the matched cohort, the baseline Hb levels were 85 g/L in the roxadustat group and 83 g/L in the rhEPO group, which did not represent a significant difference (P=0.164). After 4 weeks of treatment, the median Hb level was 96 g/L in the roxadustat group and 87 g/L in the rhEPO group (P<0.001). After 12 weeks of treatment, the Hb level was 105 g/L in the roxadustat group, which was significantly higher than that in the rhEPO group (94 g/L, P<0.001). Similar results were observed after 24 weeks of treatment. The Hb level during week 24 was 105 g/L in the roxadustat group, which was higher than the median Hb level in the rhEPO group (97 g/L, P=0.001) (Figure 3).
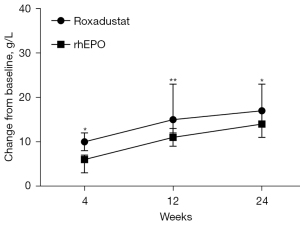
After 4 weeks of treatment, the change from baseline (CFB) in the median Hb level was 10 g/L in the roxadustat group and 6 g/L in the rhEPO group, with a treatment difference of 5 g/L [95% confidence interval (CI): 2–9 g/L; P=0.002] (Figure 4). At 12 weeks, there was a distinction between the levels of Hb exhibited in the roxadustat and rhEPO groups (15 vs. 11 g/L, P=0.001), and the treatment difference was 7 g/L (95% CI: 3–11 g/L). In addition, at 24 weeks, the CFB in the median level of Hb was 17 g/L in the roxadustat group and 14 g/L in the rhEPO group, with an estimated difference of 5 g/L (95% CI: 1–9 g/L; P=0.025) between the two groups (Figure 4).
Secondary endpoints
In the matched cohort, the median maximum Hb level was 108 g/L in the roxadustat group and 101 g/L in the rhEPO group (P<0.001). Compared to the rhEPO group (16 g/L), roxadustat treatment increased the Hb level to 22 g/L, and the difference between the two groups was 6 g/L (95% CI: 2–10 g/L). However, the time from treatment initiation to maximum Hb level was 81 days in the roxadustat group and 86 days in the rhEPO group, which does not represent a significant distinction (P=0.738). A Hb increase of 10.0 g/L was considered to indicate a response to the medication, resulting in a 12-week response rate of 65.3% in the roxadustat group and 50.2% in the rhEPO group (P=0.011) (Figure 5). Furthermore, the proportion of patients who achieved the target Hb levels in the roxadustat group was significantly higher than that in the rhEPO group after 24 weeks of treatment (52.6% vs. 41.4%, P=0.001) (Table 2).
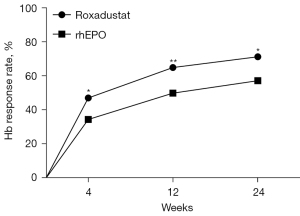
Table 2
| Endpoint | Roxadustat group | rhEPO group | P value |
|---|---|---|---|
| The median maximum Hb level (g/L) | 108 [96–118] | 101 [89–113] | <0.001 |
| The time for the maximum Hb level (days) | 81.29 [46.64–123.38] | 85.83 [35.55–152.03] | 0.738 |
| The compliance rate of the target Hb level of 100–120 g/L | 50 (52.6) | 118 (41.4) | 0.001 |
Data are presented as n (%) or median [interquartile range]. PSM, propensity-score matching; rhEPO, recombinant human erythropoietin; Hb, hemoglobin.
Discussion
The present study demonstrated that oral roxadustat was more effective than the subcutaneous administration of rhEPO in patients with renal anemia during a 24-week follow-up period. The median Hb level and CFB at 12 weeks were 105 and 15 g/L in the roxadustat group and 94 and 11 g/L in the rhEPO group, respectively. Despite similar baseline levels, the Hb levels in the roxadustat group were markedly higher than those in the rhEPO group at all three time points. The compliance rate for the target Hb level in the roxadustat group was significantly higher than that in the rhEPO group. Furthermore, patients in the roxadustat group had higher maximum Hb levels than those in the rhEPO group at similar time points, suggesting that roxadustat could treat anemia more rapidly and efficiently than rhEPO. The use of the PSM analysis increased the credibility of the results. In previous preliminary studies, roxadustat showed promising efficacy for the treatment of CKD patients with anemia (11,12), which is consistent with our current study.
Owing to uremic toxins, volume overload, nutrient loss through dialysis, oxidative stress, and other factors, a high proportion of patients with ESRD develop malnutrition-inflammation complex syndrome (20), which is considered an important etiology for erythropoietin hyporesponsiveness (21). Other possible causes involve insufficient iron absolution and iron metabolism dysfunction, and even nutritional conditions. In the current study, there were no differences in the baseline characteristics of the two groups, including medication history (such as oral iron or sevelamer hydrochloride) and nutritional and inflammatory indicators (such as Hs-CRP and glucose). Our research methods reduced the incidence of erythropoietin hyporesponsiveness to some extent by excluding the effects of other factors on anemia, making the two treatment groups comparable.
However, there were differences in the proportions of patients with hypertension and DM between the two groups. Hypertension and DM accounted for a slightly higher proportion of the population in the roxadustat group than in the rhEPO group. This could be explained by the numerous studies confirming hypertension as a common but frequently overlooked adverse effect of rhEPO therapy (22,23). Furthermore, as an independent influencing factor of inflammation, DM seems to be less sensitive than Hs-CRP and has been reported to cause erythropoietin hyporesponsiveness (24,25). Therefore, in clinical practice, patients with hypertension or DM often avoid rhEPO injections.
As a hypoxia-inducible factor prolyl hydroxylase inhibitor, roxadustat stabilizes hypoxia-inducible factors and stimulates the expression of the erythropoietin gene. It promotes increases in the concentrations of erythropoietin in certain organs, including the kidneys and liver, which is beneficial for maintaining Hb levels (26). In our study, the convenience of orally administered roxadustat resulted in high treatment compliance and led to more effective treatment than rhEPO for improving anemia. This may provide an advantage for both dialysis-dependent and non-dialysis-dependent patients with renal anemia by reducing the need for frequent hospital visits.
This study has two main advantages over previous studies. Firstly, 790 consecutive patients with renal anemia were recruited from Zhejiang Provincial People’s Hospital; thus, the study included more types of participants, which improved the statistical power for detecting differences between groups and provided reference for clinical application in wider population. Secondly, PSM analysis was performed to reduce the effect of selection bias and potential confounding factors between the two groups, which increased the credibility of the results.
However, it is important to acknowledge that our study has certain limitations. Firstly, this was a retrospective study, and some biases may not have been completely eliminated, despite performing PSM to reduce the effects of selection bias and potential confounding factors. Secondly, the single center limits the validity of the conclusions. Thirdly, due to the limitation of research condition, the concrete data of safety was not complete, which was also a limitation of our current study. Fourthly, indicators related to iron metabolism, blood lipids, and other nutritional and inflammatory indicators, including serum interleukin-6 and procalcitonin, were not detected owing to the limited objective conditions. Thus, to refine the measurement of the related indicators, further prospective, multicenter, larger sample-size, randomized studies are needed to explore the effects of roxadustat on iron metabolism, nutritional and inflammatory indicators, and blood lipids throughout treatment. In addition, the safety of roxadustat in the treatment of renal anemia should not be ignored. More results from real-world practice are also needed in the future.
Conclusions
In summary, the results of the present study suggest that roxadustat is superior to rhEPO owing to its therapeutic effects on anemia in patients with CKD. Our study provides clear evidence for the efficacy of roxadustat in clinical practice. Further investigation will be required to firmly establish the safety and efficacy profile of roxadustat in this patient population.
Acknowledgments
We would like to thank our colleagues at Zhejiang Provincial People’s Hospital for their valuable contributions to this study. We are grateful for the technical support and data platform from Yidu Cloud Technology Company Ltd.
Funding: This work was supported by the Medical and Health Technology Program of Zhejiang Province (Nos. 2021KY448 and 2023KY454), and the Zhejiang Traditional Chinese Medicine Science and Technology Program (No. 2018ZA010).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-709/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-709/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-709/coif). The authors report technical support and data platform from Yidu Cloud Technology Company Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815-22. [Crossref] [PubMed]
- Nakhoul G, Simon JF. Anemia of chronic kidney disease: Treat it, but not too aggressively. Cleve Clin J Med 2016;83:613-24. [Crossref] [PubMed]
- Ma JZ, Ebben J, Xia H, et al. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999;10:610-9. [Crossref] [PubMed]
- Walker AM, Schneider G, Yeaw J, et al. Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. J Am Soc Nephrol 2006;17:2293-8. [Crossref] [PubMed]
- Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 1996;28:53-61. [Crossref] [PubMed]
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019-32. [Crossref] [PubMed]
- Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf 2016;15:1021-30. [Crossref] [PubMed]
- Hirai K, Nonaka H, Ueda M, et al. Effects of Roxadustat on the Anemia and Iron Metabolism of Patients Undergoing Peritoneal Dialysis. Front Med (Lausanne) 2021;8:667117. [Crossref] [PubMed]
- Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis 2017;69:815-26. [Crossref] [PubMed]
- Fishbane S, Pollock CA, El-Shahawy M, et al. Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study. J Am Soc Nephrol 2022;33:850-66. [Crossref] [PubMed]
- Chen N, Hao C, Peng X, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 2019;381:1001-10. [Crossref] [PubMed]
- Chen N, Hao C, Liu BC, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med 2019;381:1011-22. [Crossref] [PubMed]
- Akizawa T, Otsuka T, Reusch M, et al. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial 2020;24:115-25. [Crossref] [PubMed]
- Akizawa T, Iwasaki M, Otsuka T, et al. Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther 2019;36:1438-54. [Crossref] [PubMed]
- Fishbane S, El-Shahawy MA, Pecoits-Filho R, et al. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J Am Soc Nephrol 2021;32:737-55. [Crossref] [PubMed]
- Akizawa T, Iwasaki M, Yamaguchi Y, et al. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol 2020;31:1628-39. [Crossref] [PubMed]
- Al-Makki A, DiPette D, Whelton PK, et al. Hypertension Pharmacological Treatment in Adults: A World Health Organization Guideline Executive Summary. Hypertension 2022;79:293-301. [Crossref] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44:S15-S33. Erratum in: Diabetes Care 2021;44:2182. [Crossref] [PubMed]
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-44. [Crossref] [PubMed]
- Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77-90. [Crossref] [PubMed]
- Young P, Lombi F, Finn BC, et al. Malnutrition-inflammation complex syndrome" in chronic hemodialysis. Medicina (B Aires) 2011;71:66-72. [PubMed]
- Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol 2009;4:470-80. [Crossref] [PubMed]
- Cernaro V, Lacquaniti A, Buemi A, et al. Does erythropoietin always win? Curr Med Chem 2014;21:849-54. [Crossref] [PubMed]
- Eriguchi R, Taniguchi M, Ninomiya T, et al. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol 2015;28:217-25. [Crossref] [PubMed]
- Kuwahara M, Arai Y, Takehara E, et al. Early response to erythropoiesis-stimulating agents in non-dialysis chronic kidney disease patients. Clin Exp Nephrol 2016;20:585-94. [Crossref] [PubMed]
- Macdougall IC. Hypoxia-inducible factor prolyl hydroxylase enzyme inhibitors: ready for primetime? Curr Opin Nephrol Hypertens 2022;31:399-405. [Crossref] [PubMed]


