
Development of the prediction model for negative outcomes after primary laparoscopic pyeloplasty in children: a retrospective study of 535 patients
Introduction
Ureteropelvic junction obstruction (UPJO) is one of the most common abnormalities leading to hydronephrosis in infants and children. Open pyeloplasty (OP) proposed by Anderson and Hynes has remained the gold standard for surgical treatment of UPJO in children for decades (1). Since the first dismembered laparoscopy pyeloplasty (LP) was described by Peters et al. (2) in 1995, this minimally invasive approach, along with good functional outcomes, has currently become the preferred surgical modality. However, limited laparoscopic manipulation space and smaller ureteral caliber make LP more challenging in children than in adults (3). Previous studies demonstrated a wide complication rate of 6.7% to 37.5% (4-6), and some researchers further explored the relationship between LP complications and its risk factors (4,5). Compared with other types of complications, the negative outcomes, as proposed and defined by Clavien-Dindo often require secondary treatment, which may burden children and their parents (7,8). However, few studies focused on the negative outcomes after LP.
The present study aims to collect and analyze the characteristics of negative outcomes after LP, seek potential risk factors, and construct a prediction model. We anticipate that the prediction model can help clinicians assess patients with an individual bias, identify specific high-risk groups, and conduct an early intervention to minimize negative outcomes and adverse effects. The study was presented in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-327/rc).
Methods
Patients
The records for children who underwent LP were retrospectively reviewed between January 2016 and December 2020. UPJO was diagnosed based on the patient’s symptoms and clinical examinations. The indication for surgery at our center is the presence of any of the following conditions: (I) ultrasonography showed progression of hydronephrosis, (II) patients with symptomatic renal colic, urinary tract infection (UTI) combined with severe upper urinary tract dilatation (Society of Fetal Urology grade III or IV), (III) the renal function of the hydronephrotic kidney is less than 40%. The technetium-99m-diethylene triamine pentaacetic acid (99mTc-DTPA) renal dynamic imaging demonstrated an obstructive pattern (defined as T1/2 >20 min after administration of furosemide) for reference. Preoperative anteroposterior pelvic diameter (APD) values were recorded at the previous preoperative ultrasonography. Voiding cystourethrography was performed in patients with perioperative ureteral dilatation showed by ultrasonography, preoperative urinary infection, and repeated (more than two times) postoperative urinary infections. Prophylactic antibiotics [cephalosporin, 50 mg/(kg·d)] were used in patients before surgery.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained approval from our institutional ethical review board (No. 2019-k-12). Individual consent for this retrospective analysis was waived.
Surgical technique
LP was performed through a transperitoneal approach with three ports, with 5-0 absorbable monofilament running suture used in all cases. The double-J (DJ) stent was first tried by the surgeons. The perinephric drain and urethral catheter were placed simultaneously for those who had DJ stent well placed in an antegrade fashion. The appropriate catheter size was selected based on the patient’s age. For patients aged 0–2, 2–5, 5–10, and 10–16 years, 6, 8, 8–10, and 10–12 Fr catheters were selected, respectively. As for the DJ stent, a 4.7F stent is commonly chosen. If the age- and height-appropriate stent was difficult to place and changing to a smaller size still had significant resistance at the ureterovesical junction, this situation was considered a difficult process of inserting the DJ stent. In this case, externalized pyeloureteral (EPU) stent (9,10), which comprises a nephrostomy tube and an internal-external ureteral stent, was indwelled as an alternative drainage method. All operations were performed by physicians with experience in laparoscopic surgery. Surgeons were classified into chief physician and associate chief physician groups based on their experience.
Postoperative management
Postoperatively, oral feeding was given once the patient experienced flatulence, defecation, or reappearance of bowel sounds. The indication for perirenal drain removal was no increase in drainage volume or an increase of less than 10 mL/24 h. The Foley catheters and internal-external ureteral stents were removed before the patient’s discharge. The prophylactic antibiotics were administered for 1 to 2 weeks after discharge. DJ stents were removed 1 to 2 months after surgery. The nephrostomy tube was removed at discharge, usually 10 to 14 days after surgery. Routine follow-up for all patients included assessment in the clinic at 3, 6, 12, 18, and 24 months postoperatively under outpatient or telephone interview.
Data collection
Restenosis was defined as the need for redo dismembered pyeloplasty based on postoperative obstruction manifesting as persistent or worsening hydronephrosis, or symptomatic obstruction. The negative outcomes, as proposed and defined by Clavien-Dindo, were defined as restenosis and grade III and IV complications based on the Clavien-Dindo grading system (7,8). Patients were divided into negative and non-negative outcomes groups. The preoperative data, intraoperative parameters, and follow-up information were collected retrospectively. Preoperative data included the patient’s age, sex, weight, APD, renal malformation, preoperative presentation, surgical history, and values of creatinine, urea and albumin. Intraoperative parameters included operation time, operation side, surgeon, and difficulty of DJ stent insertion. Follow-up information included restenosis and postoperative complications. Patients converted to open pyeloplasty or with missing data were excluded from this study.
Development and validation of the prediction model
The binary univariate logistic regression analysis was conducted to seek risk factors for negative outcomes after LP, and those with P<0.15 were included in the multivariate logistic regression model. The multivariate logistic regression procedure was used to select variables with P<0.05 for inclusion in the nomogram. The probabilities of the negative outcomes were estimated from the nomogram. The patient’s total score was calculated by analyzing scores corresponding to each predictor variable in the nomogram, and the corresponding probability of negative outcomes was obtained.
Various methods were used to evaluate the predictive ability of the prediction model. The receiver operating characteristic (ROC) curve and decision curve analysis (DCA) were performed in R 4.0.3 with the “pROC” package and the “rmda” package. DCA is a method to assess the clinical benefit of substitute models and is combined with nomograms quantifying net gain at different threshold probabilities (11). The model was internally validated using the parametric bootstrapping method (B=1000). The calibration curve and the Brier score were used to assess calibration capability. The smaller the Brier score, the better the calibration effect. The area under the curve (AUC) of the ROC was used to evaluate the discriminative ability.
Statistical analysis
Continuous data were analyzed by t-test or Mann-Whitney U test and showed as mean ± standard deviation (SD) or median [interquartile range (IQR)], whereas categorical data were analyzed by Chi-square test or Fisher exact test and presented as N (%). All P values were 2-tailed, and P<0.05 was considered statistically significant. The R programming language and environment for Windows (version 4.0.3, http://www.r-project.org) were used for analysis.
Results
Patients characteristic
A total of 561 patients were included in the study. Three patients converted to open pyeloplasty were excluded. Twenty-three patients were excluded because of missing data. Finally, 535 patients were included in the study, of which 33 had negative outcomes, with an incidence of 6.2%. The clinical characteristics of the patients are shown in Table 1. The median age of the overall cohort at the time of LP was 51.4 (IQR, 21.3–94.0) months, while the median weight was 17.0 (IQR, 12.0–25.4) kg. Follow-up duration ranged from 12 to 60 months. Compared with the non-negative outcomes group, a larger APD was observed in the negative outcomes group [4.1 (IQR, 3.30–4.60) cm, P<0.001], and the largest one was 9.60 cm. More patients in the negative outcomes group experienced the difficulty of inserting the DJ stent during the surgery compared to the non-negative outcomes group (P<0.001). The operation time in the negative outcomes group was longer than that in the non-negative outcomes group [125 (IQR, 108–150) vs. 109 (IQR, 84.0–140) min], but it was not statistically significant (P=0.054).
Table 1
| Characteristics | Non-negative outcomes group (N=502) | Negative outcomes group (N=33) | P value |
|---|---|---|---|
| Age, months | 51.7 [21.6–94.7] | 48.1 [19.3–79.0] | 0.606 |
| Sex | 0.125 | ||
| Male | 411 (81.9) | 31 (93.9) | |
| Female | 91 (18.1) | 2 (6.06) | |
| Weight, kg | 17.0 [12.0–26.8] | 18.0 [11.0–21.0] | 0.089 |
| APD, cm | 2.80 [2.20–3.50] | 4.10 [3.30–4.60] | <0.001 |
| Renal malformation | 0.567 | ||
| Yes | 12 (2.39) | 1 (3.03) | |
| No | 490 (97.6) | 32 (97.0) | |
| Preoperative presentation | 0.910 | ||
| Yes | 215 (42.8) | 15 (45.5) | |
| No | 287 (57.2) | 18 (54.5) | |
| Surgery history | 0.507 | ||
| Yes | 10 (1.99) | 1 (3.03) | |
| No | 492 (98.0) | 32 (97.0) | |
| Creatinine, μmol/L | 30.5 [25.0–37.9] | 31.1 [27.5–36.1] | 0.531 |
| Urea, mmol/L | 4.63 [3.86–5.41] | 4.50 [3.57–5.36] | 0.431 |
| Albumin, g/L | 44.8 [42.7–46.8] | 44.2 [42.2–46.3] | 0.269 |
| Operation time, min | 109 [84–140] | 125 [108–150] | 0.054 |
| Operation side | 0.661 | ||
| Left | 386 (76.9) | 27 (81.8) | |
| Right | 116 (23.1) | 6 (18.2) | |
| Surgeon | 0.880 | ||
| Chief physician | 168 (33.5) | 12 (36.4) | |
| Associate chief physician | 334 (66.5) | 21 (63.6) | |
| Difficulty of inserting DJ stent | <0.001 | ||
| Yes | 26 (5.18) | 11 (33.3) | |
| No | 476 (94.8) | 22 (66.7) |
Data are shown as median [IQR] or number (percentage). IQR, interquartile range; APD, anteroposterior pelvic diameter; DJ, double-J.
Negative outcomes and management
As shown in Table 2, 33 patients developed negative outcomes after LP, and restenosis occurred in 10 (1.9%) patients. Seven (1.3%) patients were classified as grade IIIa based on the Clavien-Dindo grading system, whereas 16 (3%) patients were grade IIIb. UPJ leakage (two patients), the difficulty of urinating after the removal of the DJ stent (two patients), and ileus (two patients) were the most common grade IIIa complications. A urinary catheter was inserted for UPJ leakage and difficulty of urinating after removing the DJ stent, while fasting and gastrointestinal decompression were performed for ileus.
Table 2
| Negative outcomes | Management |
|---|---|
| UPJ restenosis (10 cases, 1.9%) | Redo open pyeloplasty |
| IIIa complications (7 cases, 1.3%) | |
| UPJ leakage (2 cases) | Insert a urinary catheter |
| Difficulty of urinating after removing the DJ stent (2 cases) | Insert a urinary catheter |
| Ileus (2 cases) | Fasting, gastrointestinal decompression |
| UTI because of VUR with DJ stent (1 case) | Insert a urinary catheter |
| IIIb complications (16 cases, 3.0%) | |
| Distal ureteral stricture (5 cases) | Ureteral-bladder reimplantation |
| Hernia formation (3 cases) | Debridement and suturing |
| Intra-abdominal bleeding, hematoma formation (1 case) | Hemostasis, hematoma evacuation |
| Prolapse of nephrostomy tube, recurrent UTI (1 case) | Performed nephrostomy |
| Delayed wound healing around the fistula (2 cases) | Fistula extraction, debridement and suturing |
| UPJ leakage (1 case) | Reset DJ stent |
| Infected allantois formation, perinephric empyema (1 case) | Performed nephrostomy |
| DJ stent migration (1 case) | Reset DJ stent |
| Nephrolithiasis (1 case) | Redo open pyeloplasty |
LP, laparoscopic pyeloplasty; UPJ, ureteropelvic junction; DJ, Double-J; UTI, urinary tract infection; VUR, vesicoureteral reflux.
For the ten patients with UPJ restenosis, their intraoperative diagnoses were: four UPJ stenosis, three high ureters, two crossing vessels, and one UPJ polypus.
Of the 16 patients who developed grade IIIb complications after LP, distal ureteral stricture (five patients), hernia formation (three patients), and delayed wound healing around the fistula (two patients) were the three most common grade IIIb complications. The redo of open pyeloplasty was performed in eleven patients, ten with UPJ restenosis and one with nephrolithiasis. Five patients with distal ureteral stricture underwent ureteral-bladder reimplantation. Hernia formation (three patients) and delayed wound healing around the fistula (two patients) were performed with debridement and suturing. One patient with intra-abdominal bleeding and hematoma formation was managed with hemostasis and hematoma evacuation. Nephrostomy was performed for prolapse of the nephrostomy tube combined with recurrent UTI (one patient) and infected allantois formation combined with perinephric empyema (one patient). UPJ leakage (one patient) and DJ stent migration (one patient) were handled by resetting the DJ stent.
Of the 33 patients with negative outcomes, 22 patients were drained with the DJ tube, and among them, 9 developed restenosis, 6 had grade IIIa complications, and 7 had grade IIIb complications after LP. The remaining eleven patients were drained with EPU, of which one developed restenosis, one had grade IIIa complication, and nine had grade IIIb complications.
Among the 33 patients with negative outcomes, 28 had records for the exact timing of postoperative complications. All negative outcomes occurred within 17 months after surgery. A proportion of 39.29% (11/28) of the negative outcomes occurred within a week after surgery, and 53.57% (15/28) occurred 3 months after surgery. Moreover, eight patients, mostly UPJ restenosis (7/8), occurred 6 months after surgery. The longest time for negative outcomes occurred in this study was 17 months postoperatively, where one child redid open pyeloplasty on account of UPJ restenosis.
Development and validation of the prediction model for negative outcomes
The clinical variables of the patients in Table 1 were used to fit the univariate logistic regression model, and the variables with P<0.15 were selected: sex, difficulty of inserting the DJ stent, weight, and APD (Table 3). Furthermore, we included these four variables in the multivariate logistic regression model. As shown in Table 3, the patient’s weight (OR: 0.93, 95% CI: 0.88–0.98), the difficulty of inserting DJ stent (OR: 3.16, 95% CI: 1.14–8.80) and APD (OR: 2.29, 95% CI: 1.65–3.19) were independent risk factors (P<0.05) for negative outcomes after LP. These three factors were used to fit the prediction model and construct the nomogram (Figure 1A).
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Sex | |||||||
| Male | 0.29 | 0.07–1.24 | 0.1 | 0.30 | 0.07–1.33 | 0.11 | |
| Female | 1.000 | 1.000 | |||||
| Difficulty of inserting DJ stent | |||||||
| Yes | 9.15 | 4.01–20.88 | <0.001 | 3.16 | 1.14–8.80 | 0.03 | |
| No | 1.000 | 1.000 | |||||
| Weight | 0.95 | 0.91–0.99 | 0.02 | 0.93 | 0.88–0.98 | 0.008 | |
| APD | 2.09 | 1.62–2.7 | <0.001 | 2.29 | 1.65–3.19 | <0.001 | |
OR, odds ratio; CI, confidence interval; DJ, Double-J; APD, anteroposterior pelvic diameter.
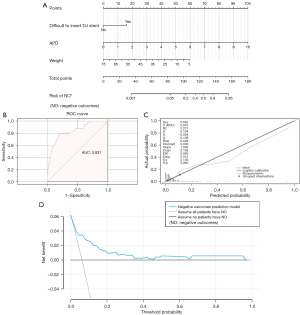
At internal validation, the nomogram was validated by the 1,000 repetitions of bootstrap sample corrections. The AUC of the ROC was 0.831 (95% CI: 0.756–0.906) for the prediction of negative outcomes (Figure 1B), indicating constructive discrimination by the nomogram. The calibration curve of the nomogram demonstrated great consistency between the predicted and observed negative outcomes probability (Figure 1C). The Brier score was 0.048, indicating perfect calibration. Furthermore, the DCA curve of the nomogram is shown in Figure 1D, illustrating that the predictive model had strong clinical utility.
Discussion
UPJO is the leading cause of hydronephrosis in infants and children, and surgical intervention is necessary when hydronephrosis worsens, clinical symptoms develop, and renal function deteriorates (12). LP was born in the 1990s, developed from OP, and is considered the new “gold standard” for treating UPJO in children (12). Previous studies demonstrated that LP is a minimally invasive measurement with a low complication rate and a high success rate (12,13). However, limited studies focused on the negative outcomes and their risk factors. The negative outcomes, defined as UPJ restenosis requiring secondary surgery and grade III and IV complications based on the Clavien-Dindo grading system (7,8), often require secondary treatment and intervention, which may seriously affect the prognosis of UPJO patients. In the present study, we explored risk factors of negative outcomes after LP and developed a prediction model with a view to an individualized assessment of patients.
Risk factors for negative outcomes
For pediatric patients, age and body weight have always been taken seriously since these two parameters are good indicators of the difficulty and complexity of the surgery. Based on our common sense, there is a strong link between age and weight. Kutikov et al. (14) suggested that laparoscopic surgery has no significant advantages for patients weighing less than 10 kg due to the narrow operating space and higher requirements for the operator, and the incisions for laparoscopic surgery in younger children are not much smaller than those for open surgery. However, Neheman et al. (15) argued that LP has a high success rate with low complication rates in infants and children weighing less than 10 kg. In the present study, 25 patients weighed less than 10 kg, accounting for 4.67% of the total. Our study revealed that weight was an independent risk factor for negative outcomes. From the nomogram we established, low body weight contributes a large proportion of the total score, which may lead to a higher probability of negative outcomes. We presumed that children of younger age and lower body weight might have poor tolerance to the effects of LP, tissue fragility, delicate anatomical structures, and limited intra-abdominal space for manipulation may lead to negative outcomes.
Progressive dilatation of preoperative APD is one of the risk factors influencing whether and when surgery is required (12). Grignon et al. (16) suggested that 94% of patients had an APD greater than 20 mm, 50% had an APD between 10 and 15 mm, less than 3% had an APD of less than 10 mm, and this proportion of patients had significant deformities after long-term follow-up and required surgery. Blachar et al. (17) focused on the mean value of APD and defined severe hydronephrosis as a mean of APD greater than 15 mm. In the current study, the mean value of APD in the negative outcomes group was 4.10 cm, which is larger than that in the non-negative outcomes group (P<0.01). Based on our analysis, preoperative APD was an independent risk factor for negative outcomes. The nomogram demonstrated that the larger the APD value, the higher the total score and the greater the probability of negative outcomes. The higher APD values may indicate more severe hydronephrosis, and the lowest anastomotic lesions were not easily detected through laparoscopy. Compression may still exist in patients with crossing vessels. Previously, Kim et al. (18) proposed that the crossing vessel was one of the factors that negatively affected the surgery outcome. In our study, 2 of 30 patients with crossing vessels had negative outcomes.
The best drainage method for LP surgery is controversial. Some physicians described the safety and feasibility of stent-less pyeloplasty and took it as one of the options for postoperative drainage. Kočvara et al. (9) clarified that stent-less LP resulted in good anastomotic conditions and avoided unnecessary further anesthesia and stent-related complications. Bayne et al. (19) analyzed 367 cases of pyeloplasty and concluded that the unstented method was as safe as the stented method. However, Liu et al. (20) concluded through a meta-analysis that stent-less pyeloplasty required more secondary procedures than stented pyeloplasty, mainly because of urinary leakage complications, although the overall complication rates were similar between stented and stent-less LP, 12% and 14%, respectively (21). Currently, most physicians prefer to place a stent through the anastomosis for drainage. Two types of stents are commonly reported: DJ stent (9,10,19) and EPU stent (9,10). In the present study, 498 patients were drained with the DJ stent, 37 patients encountered the difficulty of inserting the DJ stent during surgery, and the EPU approach was used as an alternative. Chu et al. (10) argued that the disadvantages of EPU stent insertion were mainly manifested in damage to the renal parenchyma, increased incidence of skin site infection, delayed healing, and urine leakage. In the current study, two patients developed postoperative skin infections around the stoma, leading to delayed healing. As for urine leakage, there were three cases in our study, one with the DJ stent and two with EPU stent. Besides, ten patients showed UPJ restenosis, nine with DJ stent, and one with EPU stent. Five patients developed distal ureteral obstruction and underwent ureteral reimplantation, of which four patients had a difficult process of inserting a DJ stent during the operation. We presumed that intraoperative insertion of the DJ stent could be difficult for two reasons. One is the iatrogenic injury during the attempt, and the other is the patient’s ureterovesical stenosis. Retrograde ureteropyelography is not routinely performed at our institution because it is invasive and prone to complications such as urinary tract infections. Therefore, the cause of distal ureteral obstruction still needs further study. The present study demonstrated that EPU drainage was more prone to negative outcomes than DJ drainage, with a probability of 29.73% (11/37) and 4.42% (22/498), respectively. Moreover, there were more grade IIIb postoperative complications in patients with EPU drainage (81.8%, 9/11) than DJ drainage (31.8%, 7/22), and thus required intervention under general anesthesia, which was a greater adverse impact on pediatric patients.
In our LP series, DJ stent was indwelled routinely to avoid puncturing the parenchyma (22). The DJ tube is the main site for bacterial colonization after surgery because the substances in the urine can be deposited on the surface of the stent tube, providing a suitable substrate for bacterial growth. At the same time, DJ stent allows bacteria to retrograde infection through the stent. It has been reported that the incidence of bacterial colonization of stents in patients with internal drainage tubes is as high as 42% to 90% (23,24). Kočvara et al. (9) and Chu et al. (10) have reported that artificial vesicoureteral reflux could occur with the use of the DJ stent. Furthermore, the effect of bacteria colonized on the stent and reflux may lead to UTIs. In our study, one patient developed UTI due to reflux with the DJ tube. In addition, the incidence of stone formation has been reported in the previous literature to be 0.5% to 10% (25,26). In addition to the patient may have a stone constitution, postoperative anastomotic obstruction caused by various reasons also leads to urine retention in the ureter and renal pelvis (26). Although foreign bodies placed intraoperatively, such as stents and sutures, may provide the core matrix required for stone formation, there are no reports on stents or sutures that induce postoperative stone formation. In the current study, one case with the DJ stent underwent redo open pyeloplasty 168 days after primary LP due to stone formation. The mechanisms need to be further explored.
The potential of the nomogram for predicting negative outcomes
A major strength of this study is that the three independent risk factors described above were incorporated into a predictive model. Although previous studies have reported risk factors for complications after LP, few studies focused on the risk factors of negative outcomes. More importantly, individualized prediction and assessment of pediatric patients are still lacking. To the best of our knowledge, this is the first study to develop a prediction model based on preoperative parameters and intraoperative conditions, quantifying the probability of negative outcomes after LP at the individual level. The prognosis nomogram demonstrated a strong prediction ability. The model was internally validated by the parametric bootstrapping (B=1,000) method. A relatively good Brier score and the calibration plot showed an agreement between predicted and observed consequences. A great AUC value revealed excellent discrimination of the nomogram in predicting negative outcomes after LP. As the postoperative complications rate reported in previous studies ranged from 6.7% to 37.5% (4-6), the negative outcomes rate in our study was 6.2%, both at the threshold of our study and in previous studies. The decision curve in the DCA plot lay above the none and all lines, illustrating that the predictive model had strong clinical utility. Based on our predictive model with strong clinical utility, we believe clinicians can assess and identify high-risk children, reduce the incidence of negative outcomes, and improve prognosis.
Limitation
The limitations of the present study should be emphasized. Firstly, although our institution is the referral center for UPJO treatment in China, the nature of the single-center study may lead to potential selection bias that can hardly be avoided. As China’s National Center for Children’s Health, our patients come from several parts of the country. We tried to ensure that the patients recover smoothly after the operation and then be discharged from the hospital to avoid unnecessary back and forth, which prolonged hospital stay. Secondly, seven surgeons performed operations separately, and we classified them into two groups based on their experience. Although there was no statistically significant difference between the two groups, bias was unavoidable because these physicians had slightly different preferences and approaches to some details during the surgery. Thirdly, it was a retrospective study. Despite the fact that we tried to avoid the omission of negative outcomes through telephone and outpatient follow-up, there may still be bias in recording, and the duration of follow-up varies. Moreover, our prediction model was internally validated with the bootstrapping method. Future studies require prospective, longer follow-up, more positive cases, and external validation for the model to avoid bias and improve the clinical utility of the model.
Conclusions
Primary LP is a safe and effective method for children and infants with UPJO. Patients with low weight, large preoperative APD, and the difficulty of inserting DJ sent during the surgery were more likely to develop negative outcomes after LP. Surgeons should adequately expose anatomy and perform careful manipulations while minimizing injury. Appropriate drainage methods should be selected based on the situation. We innovatively established and validated a prediction model for negative outcomes after LP. We believe this prediction model can help clinicians make individualized assessments of patients, identify patients at higher risk for negative outcomes as early as possible, and give them more attention and support, thereby minimizing the likelihood of negative outcomes after LP.
Acknowledgments
We would like to thank Mina Ayad Shamil for her help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-327/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-327/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-327/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained approval from our institutional ethical review board (No. 2019-k-12). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Szavay P, Zundel S. Surgery of uretero-pelvic junction obstruction (UPJO). Semin Pediatr Surg 2021;30:151083. [Crossref] [PubMed]
- Peters CA, Schlussel RN, Retik AB. Pediatric laparoscopic dismembered pyeloplasty. J Urol 1995;153:1962-5. [Crossref] [PubMed]
- Fuchs J, Luithle T, Warmann SW, et al. Laparoscopic surgery on upper urinary tract in children younger than 1 year: technical aspects and functional outcome. J Urol 2009;182:1561-8. [Crossref] [PubMed]
- Turner RM 2nd, Fox JA, Tomaszewski JJ, et al. Laparoscopic pyeloplasty for ureteropelvic junction obstruction in infants. J Urol 2013;189:1503-7. [Crossref] [PubMed]
- Badawy H, Saad A, Fahmy A, et al. Prospective evaluation of retroperitoneal laparoscopic pyeloplasty in children in the first 2 years of life: Is age a risk factor for conversion? J Pediatr Urol 2017;13:511.e1-4. [Crossref] [PubMed]
- Blanc T, Muller C, Abdoul H, et al. Retroperitoneal laparoscopic pyeloplasty in children: long-term outcome and critical analysis of 10-year experience in a teaching center. Eur Urol 2013;63:565-72. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kočvara R, Sedláček J, Drlík M, et al. Unstented laparoscopic pyeloplasty in young children (1-5 years old): a comparison with a repair using double-J stent or transanastomotic externalized stent. J Pediatr Urol 2014;10:1153-9. [Crossref] [PubMed]
- Chu DI, Shrivastava D, Van Batavia JP, et al. Outcomes of externalized pyeloureteral versus internal ureteral stent in pediatric robotic-assisted laparoscopic pyeloplasty. J Pediatr Urol 2018;14:450.e1-6. [Crossref] [PubMed]
- Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA 2015;313:409-10. [Crossref] [PubMed]
- Penn HA, Gatti JM, Hoestje SM, et al. Laparoscopic versus open pyeloplasty in children: preliminary report of a prospective randomized trial. J Urol 2010;184:690-5. [Crossref] [PubMed]
- Mei H, Pu J, Yang C, et al. Laparoscopic versus open pyeloplasty for ureteropelvic junction obstruction in children: a systematic review and meta-analysis. J Endourol 2011;25:727-36. [Crossref] [PubMed]
- Kutikov A, Resnick M, Casale P. Laparoscopic pyeloplasty in the infant younger than 6 months--is it technically possible? J Urol 2006;175:1477-9; discussion 1479. [Crossref] [PubMed]
- Neheman A, Noh PH, Piaggio L, et al. The role of laparoscopic surgery for urinary tract reconstruction in infants weighing less than 10 kg: a comparison with open surgery. J Pediatr Urol 2008;4:192-6. [Crossref] [PubMed]
- Grignon A, Filion R, Filiatrault D, et al. Urinary tract dilatation in utero: classification and clinical applications. Radiology 1986;160:645-7. [Crossref] [PubMed]
- Blachar A, Schachter M, Blachar Y, et al. Evaluation of prenatally diagnosed hydronephrosis by morphometric measurements of the kidney. Pediatr Radiol 1994;24:131-4. [Crossref] [PubMed]
- Kim EH, Tanagho YS, Traxel EJ, et al. Endopyelotomy for pediatric ureteropelvic junction obstruction: a review of our 25-year experience. J Urol 2012;188:1628-33. [Crossref] [PubMed]
- Bayne AP, Lee KA, Nelson ED, et al. The impact of surgical approach and urinary diversion on patient outcomes in pediatric pyeloplasty. J Urol 2011;186:1693-8. [Crossref] [PubMed]
- Liu X, Huang C, Guo Y, et al. Comparison of DJ stented, external stented and stent-less procedures for pediatric pyeloplasty: A network meta-analysis. Int J Surg 2019;68:126-33. [Crossref] [PubMed]
- Smith KE, Holmes N, Lieb JI, et al. Stented versus nonstented pediatric pyeloplasty: a modern series and review of the literature. J Urol 2002;168:1127-30. [Crossref] [PubMed]
- Tan HL. Laparoscopic Anderson-Hynes dismembered pyeloplasty in children. J Urol 1999;162:1045-7; discussion 1048. [Crossref] [PubMed]
- Silva MV, Levy AC, Finkelstein JB, et al. Is peri-operative urethral catheter drainage enough? The case for stentless pediatric robotic pyeloplasty. J Pediatr Urol 2015;11:175.e1-5. [Crossref] [PubMed]
- Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol 2004;18:891-6. [Crossref] [PubMed]
- Shoma AM, El Nahas AR, Bazeed MA. Laparoscopic pyeloplasty: a prospective randomized comparison between the transperitoneal approach and retroperitoneoscopy. J Urol 2007;178:2020-4; discussion 2024. [Crossref] [PubMed]
- Rassweiler JJ, Teber D, Frede T. Complications of laparoscopic pyeloplasty. World J Urol 2008;26:539-47. [Crossref] [PubMed]


