
Identification of risk factors and clinical model construction of abdominal distension after radical cystectomy
Introduction
Bladder cancer (BCa) is one of the ten most common cancers worldwide. It is one of the tumors with the greatest impact on postoperative quality of life, most notably muscle-invasive bladder cancer (MIBC) (1). Treatments for MIBC include neoadjuvant therapy followed by radical cystectomy (RC), pelvic lymph node dissection, urinary diversion, or a bladder-sparing protocol in selected patients (2). The common methods of urinary diversion are an ileal conduit, an orthotopic neobladder, or a cutaneous diversion (3). Postoperative ileus (POI) is one of the most common complications following RC, leading to prolonged length of hospital stay (LOS) and increased costs (4). A previous study revealed that factors for ileus post-cystectomy include obesity and older age (5). Increased intravenous fluids is associated with prolonged POI and longer LOS in patients who underwent robot-assisted radical cystectomy (RARC) (6). Xue et al. reported that factors for ileus post-cystectomy include chronic constipation and increased dosage of laxatives (7). POI is the most common cause of prolonged LOS after RC (8). It usually appears three to five days after surgery, and is often accompanied by abdominal distention of varying degrees (9). Therefore, it is necessary to pay attention to abdominal distension after surgery and take corresponding measures.
Very few studies have investigated the factors leading to abdominal distension after RC thoroughly. Moreover, no studies have combined laparoscopic radical cystectomy (LRC) with RARC. Therefore, we retrospectively analyzed the clinical data of 139 patients who underwent RC in our hospital. We then developed a nomogram to predict abdominal distension in patients with RC and its accuracy was encouraging. Our study provides a theoretical basis for the prevention and nursing of abdominal distension after RC. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-455/rc).
Methods
Data collection and inclusion/exclusion criteria
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Second Hospital of Tianjin Medical University (No. KY2022K080) and informed consent was taken from all the patients. The clinical information of 139 BCa patients who underwent RC in the second hospital of Tianjin Medical University from January 2020 to August 2021 was collected, including gender, age, the stomach tube insertion before the operation, postoperative water fasting time, body mass index (BMI), as well as the history of smoking, hypertension, diabetes, cardiovascular disease, abdominal surgery history, and operation method.
Study inclusion criteria included: (I) patients who underwent RC could articulate their true feelings; (II) obtain informed consent. Exclusion criteria: there were intestinal lesions before surgery or insanity.
Abdominal distension was defined as a subjective sensation of gassiness, trapped gas, or a feeling of pressure or being distended without obviously visible distension (10).
Statistical analysis
In this study, R x64 4.1.2 statistical software was used to process the data. Chi-square and Hypergeometric tests were used to describe the distribution of categorical variables, respectively. Univariate or multivariable logistic regression was used to analyze risk factors associated with abdominal distention after RC by utilizing rms R package. A nomogram was established based on logistic regression and stepwise regression and verified by calibration curves. The specificity and sensitivity of its prediction were assessed using receiver operating characteristic (ROC) curves by using ROCR R package. For all analyses, P<0.05 was considered statistically significant.
Results
A total of 139 patients conformed to our inclusion criteria. Firstly, we summarize the baseline characteristics of the patients (Table 1). Postoperative abdominal distension occurred in 35 patients. No significant differences were found between the two groups in terms of demographics such as age and gender. To explore the distribution of different clinical information, we calculated the percentage of variables between abdominal distension and the non-distension group. We found the distribution of the variables (including the stomach tube insertion before the operation, diversion mode of urinary flow, lymphadenectomy, postoperative water fasting time, and abdominal surgery history group) showed a statistically significant difference between the abdominal distension and non-distension groups (Figure 1A). We found the odds of patients with the stomach tube insertion before the operation, postoperative water fasting time, and abdominal surgery history were higher than those who did not undergo these treatments.
Table 1
| Characteristics | Abdominal distension/non-distension | ||||
|---|---|---|---|---|---|
| n | No | Yes | χ2 | P | |
| Gender | |||||
| Female | 21 | 17 | 4 | 0.185 | 0.667 |
| Male | 118 | 87 | 31 | ||
| Age (years) | |||||
| <65 | 46 | 32 | 14 | 0.634 | 0.426 |
| ≥65 | 93 | 72 | 21 | ||
| Completed high school | |||||
| No | 42 | 33 | 9 | 0.210 | 0.647 |
| Yes | 97 | 71 | 26 | ||
| The stomach tube insertion | |||||
| No | 101 | 84 | 17 | 12.094 | 0.001 |
| Yes | 38 | 20 | 18 | ||
| Postoperative water fasting time (days) | |||||
| <4 | 83 | 76 | 7 | 28.500 | 0.000 |
| ≥4 | 56 | 28 | 28 | ||
| BMI (kg/m2) | |||||
| <24 | 54 | 37 | 17 | 0.843 | 0.359 |
| ≥24 | 79 | 61 | 18 | ||
| Smoking | |||||
| No | 48 | 38 | 10 | 0.425 | 0.514 |
| Yes | 91 | 66 | 25 | ||
| Hypertension | |||||
| No | 74 | 58 | 16 | 0.698 | 0.404 |
| Yes | 65 | 46 | 19 | ||
| Diabetes | |||||
| No | 111 | 82 | 29 | 0.072 | 0.789 |
| Yes | 28 | 22 | 6 | ||
| Cardiovascular disease | |||||
| No | 107 | 81 | 26 | 0.042 | 0.837 |
| Yes | 32 | 23 | 9 | ||
| Abdominal surgery history | |||||
| No | 110 | 87 | 23 | 4.076 | 0.044 |
| Yes | 29 | 17 | 12 | ||
| Lymphadenectomy | |||||
| No | 35 | 22 | 13 | 2.756 | 0.097 |
| Yes | 104 | 82 | 22 | ||
| Operation method | |||||
| Laparoscopic | 78 | 54 | 24 | 3.492 | 0.175 |
| Open | 3 | 3 | 0 | ||
| Robot-assisted | 58 | 47 | 11 | ||
| Diversion mode of urinary flow | |||||
| Ileal conduit | 86 | 62 | 24 | 1.231 | 0.540 |
| Neobladder | 8 | 7 | 1 | ||
| Ureterocutaneostomy | 45 | 35 | 10 | ||
BMI, body mass index.
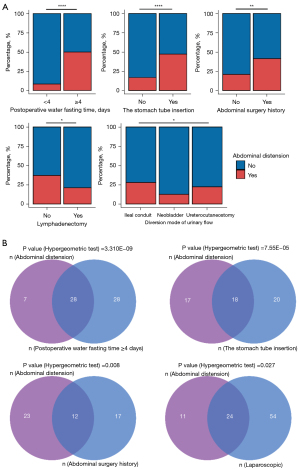
Among these, 28 of 56 patients who underwent postoperative water fasting time longer than or equal to 4 days experienced abdominal distension (P value =3.310E-09), 18 of 38 patients who underwent the stomach tube insertion before operation had abdominal distension (P value =7.55E-05), 12 of 29 patients who experienced abdominal surgery before occurred abdominal distension (P value =0.008), and 24 of 78 patients who underwent a laparoscopic operation developed abdominal distension (P value =0.027), the hypergeometric test indicated the occurrence of these outcomes were not random events (Figure 1B).
Then, we conducted the univariate logistic regression to excavate effective factors for the clinical outcome (Table 2). The results revealed a statistically significant association between the stomach tube insertion before surgery [yes vs. no: odds ratio (OR) 4.447; P<0.001], postoperative water fasting time (≥4 vs. <4 days: OR 10.857; P<0.001), and abdominal surgery history (yes vs. no: OR 2.670; P<0.05) for abdominal distension. In contrast, other variables showed no significant association with clinical outcomes.
Table 2
| Variable | Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Gender (male vs. female) | 1.514 | 0.513−5.569 | 0.485 | − | − | − | |
| Age (years), (≥65 vs. <65) | 0.667 | 0.302−1.494 | 0.317 | − | − | − | |
| The stomach tube insertion (yes vs. no) | 4.447 | 1.964−10.268 | 0.000379 | 1.023 | 0.342−3.011 | 0.968 | |
| Postoperative water fasting time (days), (≥4 vs. <4) | 10.857 | 4.472−29.621 | 5.72E−07 | 11.401 | 3.71−38.846 | 4.11E-05 | |
| BMI (kg/m2), (≥24 vs. <24) | 0.642 | 0.294−1.104 | 0.265 | − | − | − | |
| Smoking (yes vs. no) | 1.439 | 0.638−3.438 | 0.393 | − | − | − | |
| Hypertension (yes vs. no) | 1.497 | 0.695−3.263 | 0.304 | − | − | − | |
| Diabetes (yes vs. no) | 0.771 | 0.263−1.993 | 0.609 | − | − | − | |
| Cardiovascular disease (yes vs. no) | 1.219 | 0.483−2.902 | 0.662 | − | − | − | |
| Abdominal surgery history (yes vs. no) | 2.67 | 1.105−6.376 | 0.027 | 3.14 | 1.123−9.099 | 0.0304 | |
| Lymphadenectomy (yes vs. no) | 0.454 | 0.198−1.056 | 0.0628 | − | − | − | |
| Robot-assisted vs. laparoscopic | 0.527 | 0.226−1.167 | 0.122 | − | − | − | |
| Ureterocutaneostomy vs. others | 0.789 | 0.329−1.786 | 0.579 | − | − | − | |
OR, odds ratio; CI, confidence interval; BMI, body mass index; others, Ileal Conduit and Neobladder.
In multivariate logistic regression (Table 2), we took the variables with statistical significance in the univariate logistic regression into the analysis and explore the relationship between them and abdominal distension. Forward and backward stepwise logistic regression algorithms and the Akaike information criterion (AIC) were systematically applied to screen the factors. Two factors were identified as effective predictors of abdominal distension after RC, including postoperative water fasting time (≥4 vs. <4 days: OR 11.401; P=4.11E-05) and abdominal surgery history (yes vs. no: OR 3.14; P=0.0304).
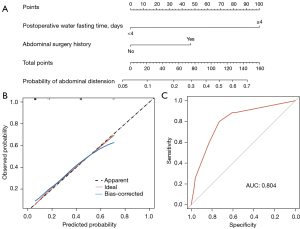
To provide the clinician with a quantitative method to predict a patient’s probability of abdominal distension after RC, we constructed a nomogram that integrated the risk factors from the multiple logistic regression (Figure 2A). Calibration plots showed that the nomogram did well compared to an ideal model. The predictive accuracy of the nomograms is shown in Figure 2B,2C with the area under the curve (AUC) of 0.804.
Finally, we collected other data (including data on postoperative hospital stay and intestinal obstruction) for these 139 patients. We found that majority of abdominal distension patients (77%) had a postoperative hospital stay of longer than or equal to 10 days (P=0.002, Chi-square). Moreover, among the abdominal cohort, seven patients (20%) developed intestinal obstruction, whereas non-distension cohort, no patients developed intestinal obstruction (P=2.303E-05, Chi-square) (Table 3). Among all patients, 7 patients developed POI, all of them had mechanical intestinal obstruction, and the average time of appearance was 10 days after operation. One patient died due to aspiration without gastrointestinal decompression, one patient underwent intestinal adhesion release, and the remaining five patients were discharged with gastrointestinal decompression combined with traditional Chinese medicine. The mean number of postoperative hospital days was longer in the patient with intestinal obstruction than in the other patients (16.71 vs. 10.42; P=0.095); we considered the difference was not statistically significant because the number of patients was small.
Table 3
| Variables | Abdominal distension/non-distension, n [%] | ||||
|---|---|---|---|---|---|
| n | No | Yes | χ2 | P value | |
| Postoperative hospital stays (days) | |||||
| <10 | 65 [47] | 57 [55] | 8 [23] | 9.49 | 0.002 |
| ≥10 | 74 [53] | 47 [45] | 27 [77] | ||
| Intestinal obstruction | |||||
| No | 132 [95] | 104 [100] | 28 [80] | 17.92 | 2.303E-05 |
| Yes | 7 [5] | 0 [0] | 7 [20] | ||
Discussion
RC is the surgical golden standard for MIBC (11). In most circumstances, laparoscopic surgery is an alternative to open surgery (12) because it reduces morbidity and speeds recovery (13). In addition, with the development of science and technology, a retrospective study recently showed that RARC is technically feasible and may reduce the incidence of blood loss, complications, and labor costs (14). POI is one of the most common complications after RC, with an incidence of 2% to 32% (15). Many factors could increase the incidence of POI, including male sex, infection, and increased intravenous fluids (6,16). For ileus, one of the most frequently reported problems after RC, follow-up control mainly depends on early recognition and subsequent treatment (15). Of note, abdominal distention is one of the main features of POI (17). However, evidence on the predictors of abdominal distention after RC is limited, and few retrospective studies were performed for LRC and RARC.
In our study, we retrospectively analyzed 139 patients who underwent the RC to determine the risk factors for postoperative abdominal distention and effective prevention strategies for managing abdominal distention complications. We did not find a significant difference between LRC and RARC in univariate/multivariate regression analysis, this may be caused by the small sample size. The univariate analysis represented that the stomach tube insertion before surgery was associated with abdominal distention after RC. The stomach tube insertion is used to prevent postoperative complications, such as nausea and vomiting, stomach content aspiration, and intestinal anastomotic leakage (18). However, it is reported that stomach tube might prolong gastrointestinal recovery and increase the duration of hospitalization (19). The stomach tube insertion was not statistically significant in the multivariate regression analysis. This could be the effect of postoperative water fasting time on it. Furthermore, abdominal surgery history was an independent risk factor in univariate/multivariate logistic regression analysis. Rybakov et al. show that previous abdominal surgery is a significant risk factor for POI (20), this may be caused by increased intraperitoneal adhesions in patients (21). Enhanced Recovery After Surgery (ERAS) was first introduced in colorectal surgery in the 1990s to reduce the perioperative burden and speed up patient recovery (22). It has recently been used in patients undergoing RC (23). In our study, patients whose postoperative water fasting time was longer than 4 days had a greater risk of abdominal distention than the others, this further illustrates the necessity to promote ERAS after surgery.
Finally, we used a nomogram to calculate the individual patient’s probability of abdominal distention after surgery, which is the first nomogram used to predict the probability of abdominal distention after RC. Our nomogram represents that the higher the score of a risk factor received, the greater its association with the occurrence of abdominal distention after surgery. As with other retrospective studies, the main limitation of our study is its ability to produce causality and control for all possible confounders. Furthermore, the expression ability of each patient also affects the outcome. Further validation is therefore required in a prospective randomized controlled trial.
Conclusions
Our study confirmed the risk factors for abdominal distention after RC. We recommend that the stomach tube should not be routinely used in the preoperative management of the patient undergoing RC. In addition, we provide a model to predict the probability of abdominal distention after RC so that physicians can take preventive measures in advance for high-risk patients. A stomach tube is feasible for high-risk patient and should be removed as soon as possible after the recovery of postoperative intestinal function. Meanwhile, we need to continue refining our model and perform more external validation to identify the patients.
Acknowledgments
Funding: This work was supported by The National Natural Science Foundation of China (No. 82070725).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-455/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-455/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-455/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-455/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Second Hospital of Tianjin Medical University (No. KY2022K080) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Panebianco V, Pecoraro M, Del Giudice F, et al. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J Magn Reson Imaging 2022;55:23-36. [Crossref] [PubMed]
- Lenis AT, Lec PM, Chamie K. Bladder Cancer. JAMA 2020;324:2006. [Crossref] [PubMed]
- Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol 2008;53:834-42; discussion 842-4. [Crossref] [PubMed]
- Maffezzini M, Campodonico F, Canepa G, et al. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol 2008;17:41-8. [Crossref] [PubMed]
- Ramirez JA, McIntosh AG, Strehlow R, et al. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: a systematic review. Eur Urol 2013;64:588-97. [Crossref] [PubMed]
- Shim JS, Noh TI, Ku JH, et al. Effect of intraoperative fluid volume on postoperative ileus after robot-assisted radical cystectomy. Sci Rep 2021;11:10522. [Crossref] [PubMed]
- Xue X, Wang D, Ji Z, et al. Risk factors of postoperative ileus following laparoscopic radical cystectomy and developing a points-based risk assessment scale. Transl Androl Urol 2021;10:2397-409. [Crossref] [PubMed]
- Chang SS, Baumgartner RG, Wells N, et al. Causes of increased hospital stay after radical cystectomy in a clinical pathway setting. J Urol 2002;167:208-11. [Crossref] [PubMed]
- Vilz TO, Stoffels B, Strassburg C, et al. Ileus in Adults. Dtsch Arztebl Int 2017;114:508-18. [PubMed]
- Lacy BE, Cangemi D, Vazquez-Roque M. Management of Chronic Abdominal Distension and Bloating. Clin Gastroenterol Hepatol 2021;19:219-231.e1. [Crossref] [PubMed]
- Matsutani T, Nagayoshi M, Tamaru M, et al. Changes in the levels of neural cell specific proteins in the developing rat brain. Neurochem Res 1985;10:1155-72. [Crossref] [PubMed]
- Cohen SA, Mirheydar HS, Parsons JK, et al. Minimally invasive cystectomy is associated with improved perioperative patient safety outcomes compared with open cystectomy in a national cohort. Urology 2014;84:314-9. [Crossref] [PubMed]
- Zehnder P, Gill IS. Cost-effectiveness of open versus laparoscopic versus robotic-assisted laparoscopic cystectomy and urinary diversion. Curr Opin Urol 2011;21:415-9. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Froehner M, Brausi MA, Herr HW, et al. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol 2009;56:443-54. [Crossref] [PubMed]
- Koch KE, Hahn A, Hart A, et al. Male sex, ostomy, infection, and intravenous fluids are associated with increased risk of postoperative ileus in elective colorectal surgery. Surgery 2021;170:1325-30. [Crossref] [PubMed]
- Vather R, O’Grady G, Bissett IP, et al. Postoperative ileus: mechanisms and future directions for research. Clin Exp Pharmacol Physiol 2014;41:358-70. [Crossref] [PubMed]
- Cheatham ML, Chapman WC, Key SP, et al. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg 1995;221:469-76; discussion 476-8. [Crossref] [PubMed]
- Inman BA, Harel F, Tiguert R, et al. Routine nasogastric tubes are not required following cystectomy with urinary diversion: a comparative analysis of 430 patients. J Urol 2003;170:1888-91. [Crossref] [PubMed]
- Rybakov EG, Shelygin YA, Khomyakov EA, et al. Risk factors for postoperative ileus after colorectal cancer surgery. Colorectal Dis 2017; Epub ahead of print. [Crossref] [PubMed]
- Lee SY, Kim CH, Kim YJ, et al. Laparoscopic surgery for colorectal cancer patients who underwent previous abdominal surgery. Surg Endosc 2016;30:5472-80. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Melnyk M, Casey RG, Black P, et al. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urol Assoc J 2011;5:342-8. [Crossref] [PubMed]


