
Comparison of molecular profiles of upper tract urothelial carcinoma vs. urinary bladder cancer in the era of targeted therapy: a narrative review
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare malignancy of the renal pelvis or ureter, accounting for approximately 5–10% of urothelial carcinoma (UC), with an estimated annual incidence of 1–2 cases per 100,000 inhabitants (1). While UTUC has a similar histologic appearance to urinary bladder cancer (UBC), it shows distinct characteristics from UBC (2). For instance, UTUC develops in the mesoderm-derived epithelium (3) and is more often invasive than UBC at surgery (1). In addition, UTUC is a Lynch syndrome-associated malignancy and can be induced by aristolochic acid (AA), whereas UBC is rarely associated with Lynch syndrome or AA exposure (4-6). These differences suggest that UTUC and UBC represent two distinct disease entities. Therefore, different clinical management strategies from UBC are required for the treatment of UTUC, but the existing treatment approaches for UTUC are virtually extrapolated from the evidence on UBC; there is a lack of evidence on UTUC, which can be attributed to its low incidence and lower number of cases included in clinical trials compare to UBC.
For decades, systemic treatment for locally advanced or metastatic UC (including UTUC) was limited to platinum-containing chemotherapy (7). However, the treatment landscape has changed significantly with the recent approvals of targeted drugs such as immune-checkpoint inhibitors [programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) inhibitors (pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab)] (8), fibroblast growth factor receptor (FGFR) inhibitors (erdafitinib), and antibody-drug conjugates (enfortumab vedotin and sacituzumab govitecan). As these drugs target specific molecules, unlike conventional platinum-containing chemotherapy, a thorough understanding of the target-molecule profiles of each drug is essential to developing optimal treatment strategies for UTUC. Knowledge of expression profiles of these therapeutic target molecules is expected to yield precision oncology approaches matched to UTUC.
In the review, we aimed to explore the differential effects of each targeted drug when administered for UTUC vs. when administered for UBC. In addition, we explored the current insights on the molecular landscape of UTUC compared to that of UBC to discuss its relationship with the efficacy of each targeted drug. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-457/rc).
Methods
We assessed the main clinical trials of targeted drugs for UC (PD-1/PD-L1 inhibitors: pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab; FGFR inhibitors: erdafitinib; antibody-drug conjugates: enfortumab vedotin and sacituzumab govitecan) on which Food and Drug Administration (FDA)-approved (or withdrawn) indications for each drug are based. In addition, we summarized published subgroup analyses of each trial (including supplementary data) to explore the differential effects of each drug when administered for UTUC vs. when administered for UBC.
Next, we reviewed studies which analyzed the mutational landscape of UTUC tissues by next-generation sequencing (NGS) and summarized alteration frequencies of representative genes between UTUC (divided into low-grade and high-grade) and UBC tissues (high-grade) in the studies. In addition, we summarized analyses of RNA expression subtypes of UTUC performed in these studies. Studies without available information on tumor grade or studies focusing on UTUC associated with Lynch syndrome or AA exposure only were excluded from the summary.
Finally, we covered and summarized immunohistochemical studies which analyzed the expressions of therapeutic target proteins (specifically PD-L1, Nectin-4, and Trop-2) between UTUC and UBC. The search strategy is summarized in Table 1. The literature search was limited to original English-language studies, and case reports, systematic reviews, editorials, commentaries, and meeting abstracts were excluded.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1st July 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “upper tract urothelial carcinoma” AND (“genomic” OR “next-generation sequencing” OR “whole-exome sequencing”) |
| Timeframe | 2004–2022 |
| Inclusion and exclusion criteria | Included studies: studies which analyzed the mutational landscape of upper tract urothelial carcinoma tissues by next-generation sequencing with available information on mutational frequency and tumor grade; the literature search was limited to original English-language studies |
| Excluded Studies: studies focusing on upper tract urothelial carcinoma associated with Lynch syndrome or aristolochic acid exposure only; case reports, systematic reviews, editorials, commentaries, and meeting abstracts were excluded | |
| Selection process | ET conducted the selection |
Comparison of targeted drug efficacy between UBC and UTUC in clinical trials
Although UTUC is a part of UC in cohorts of clinical trials for targeted drugs, no previous trials have specifically examined the efficacy of drugs against UTUC. Here, we focused on subgroup analyses of clinical trials of each drug to explore the differential effects of individual drugs administered for UTUC vs. those administered for UBC. Figure 1 summarizes subgroup analyses by primary tumor site in clinical trials on which FDA-approved indications of targeted drugs for UC are based. However, these studies were not designed to compare the treatment efficacy of each drug for UTUC and UBC; UTUC cases are inappropriately fewer than UBC cases, and there is a bias in the backgrounds between the two subgroups. Therefore, the interpretations of subgroup analyses should be considered only as a reference.

Immune-checkpoint inhibitors
Pembrolizumab (anti-PD-1)
Keynote 045 study (second-line setting)
Pembrolizumab was approved by the FDA in May 2017 as a second-line treatment after progression during platinum-based chemotherapy based on the results of the phase 3 Keynote 045 study (9). The Keynote 045 study enrolled 542 cases of advanced UC after progression from platinum-based chemotherapy, of which 76 (14%) cases had UTUC. The subgroup analyses for overall survival (OS) showed that the hazard ratio (HR) was 0.77 [95% confidence interval (CI): 0.60–0.97] for UBC and 0.53 (95% CI: 0.28–1.01) for UTUC (Figure 1).
Keynote 052 study (first-line setting)
Pembrolizumab was granted accelerated FDA approval in May 2017 as a first-line treatment for locally advanced or metastatic urothelial carcinoma in patients who are not eligible for any platinum-containing chemotherapy based on results from the phase 2 Keynote 052 study (10,11). The FDA converted this indication to a regular approval in August 2021 based on the results from the phase 3 Keynote 361 study (12).
Keynote 052 study enrolled 369 cases of cisplatin-ineligible patients with advanced UC who had not been previously treated with systemic chemotherapy, of which 69 (19%) cases had UTUC. The subgroup analyses for objective response rate (ORR %) showed that ORR was 28% (95% CI: 23–34%) for UBC and 22% (95% CI: 12–35%) for UTUC (Figure 1).
Nivolumab (anti-PD-1)
CheckMate 275 (second-line setting)
Nivolumab received accelerated FDA approval in February 2017 as a second-line treatment after progression on platinum-based chemotherapy based on the results from the phase 2 CheckMate 275 study (13). CheckMate 275 enrolled 270 cases of advanced UC after progression on platinum-based chemotherapy, but no information about the number of UTUC cases was available, and no subgroup analysis was performed.
CheckMate 274 (adjuvant treatment for resected high-risk UC)
Nivolumab was approved by the FDA in August 2021 for adjuvant treatment of patients with resected high-risk UC based on results from the phase 3 CheckMate 274 study (14). The CheckMate 274 trial enrolled 709 patients with resected high-risk UC, of which 149 (21%) had UTUC (renal pelvis or ureter). The subgroup analyses for disease-free survival showed that the HR was 0.62 (95% CI: 0.49–0.78) for UBC and 1.23 (95% CI: 0.67–2.23) and 1.56 (95% CI: 0.70–3.48) for renal pelvis carcinoma and ureter carcinoma, respectively (Figure 1).
Atezolizumab (anti‑PD‑L1)
IMvigor210 trial (Cohort 2, second-line setting)
Atezolizumab received accelerated FDA approval in May 2016 as second-line treatment after progression on platinum-based chemotherapy based on the results from the phase 2 IMvigor210 trial (15). However, FDA withdrew the indication as second-line treatment in March 2021, based on the results of the phase 3 IMvigor211 trial (16). The IMvigor210 trial (Cohort2) enrolled 310 cases of advanced UC after progression on platinum-based chemotherapy, of which 65 (21%) had UTUC. ORR was 17% (39/230) for UBC and 8% (5/65) for UTUC.
IMvigor210 trial (Cohort 1, first-line setting)
Atezolizumab received accelerated FDA approval in April 2017 as the first-line treatment for patients with advanced UC who are not eligible for cisplatin-containing chemotherapy (later restricted to patients whose tumors expressed high levels of PD-L1 (17) or who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status, based on the results from the phase 2 IMvigor210 trial (18). The IMvigor210 trial (Cohort1) enrolled 119 cases of advanced UC that were ineligible for cisplatin chemotherapy, of which 33 (28%) had UTUC, but no subgroup analysis was performed in this cohort.
IMvigor211 trial (second-line setting)
The IMvigor211 trial, a phase 3 trial in a second-line setting, did not meet the primary endpoint of improved OS by atezolizumab for patients with high PD-L1 expression l (16).
The IMvigor211 trial enrolled 931 cases of advanced UC after progression on platinum-based chemotherapy, of which 236 (25.3%) patients had UTUC. The subgroup analyses for OS showed that the HR was 0.80 (95% CI: 0.67–0.96) for UBC and 1.12 (95% CI: 0.74–1.70) and 0.86 (95% CI: 0.56–1.32) for renal pelvis carcinoma and ureter carcinoma, respectively. Based on the results of this study, the FDA withdrew the indication as a second-line treatment in March 2021.
Durvalumab (anti‑PD‑L1)
Study 1108 (second-line setting)
Durvalumab received accelerated FDA approval in May 2017 as a second-line treatment after progression on platinum-based chemotherapy. This approval was based on the updated results from the phase 1 and 2 Study 1108 (19), but the FDA withdrew the indication as second-line treatment in February 2021 based on the results of the DANUBE study (20). Study 1108 enrolled 182 cases of advanced UC after progression on platinum-based chemotherapy, but no information about the number of UTUC cases was available, and no subgroup analysis was performed.
DANUBE study (first-line setting: durvalumab with or without tremelimumab)
The DANUBE study, phase 3 trial, evaluated durvalumab monotherapy compared to chemotherapy as a first-line treatment for advanced UC patients whose tumors express high levels of PD-L1 (20). However, the trial showed that durvalumab monotherapy did not prolong OS compared to chemotherapy in advanced UC with high PD-L1 expression in a first-line setting. The DANUBE study enrolled 690 cases (without tremelimumab), 147 (21.3%) had UTUC, but no subgroup analysis was performed. In addition, the trial also demonstrated that durvalumab plus the CTLA-4 inhibitor tremelimumab did not improve OS compared with chemotherapy in a first-line setting. Based on the results of the DANUBE study, the FDA withdrew the indication as a second-line treatment in February 2021.
Avelumab (anti‑PD‑L1)
JAVELIN Solid Tumor study (second-line setting)
Avelumab received accelerated FDA approval in May 2017 as second-line treatment after progression on platinum-based chemotherapy based on results from the phase 1b JAVELIN Solid Tumor study (21). The JAVELIN Solid Tumor study enrolled 249 cases of advanced UC after progression on platinum-based chemotherapy, of which 161 cases underwent at least 6 months of follow-up, of which 36 (22%) cases had UTUC. The subgroup analyses for ORR showed that ORR was 18% (95% CI: 12–26%) for UBC and 11% (95% CI: 3–26%) for UTUC (Figure 1).
JAVELIN Bladder 100 study (first-line maintenance treatment after chemotherapy)
Avelumab was approved by FDA in June 2020 as a first-line maintenance treatment for patients with locally advanced or metastatic UC that had not progressed with first-line platinum-based chemotherapy based on results from the phase 3 JAVELIN Bladder 100 study (22-24). The JAVELIN Bladder 100 study enrolled 700 cases of advanced UC after progression on platinum-based chemotherapy, of which 187 (26.7%) had UTUC. The subgroup analyses (unpublished data) showed that the HR for OS was 0.62 (95% CI: 0.48–0.80) for UBC and 0.89 (95% CI: 0.58–1.37) for UTUC.
FGFR inhibitors
Erdafitinib
BLC2001 study (second-line setting)
Erdafitinib, a pan-FGFR inhibitor, was approved by the FDA in March 2018 as a second-line treatment for patients with FGFR3-altered advanced UC after platinum-based chemotherapy based on the results from the phase 2 BLC2001 study (25,26). The BLC2001 study enrolled 101 cases of previously treated patients who had advanced UC with FGFR alterations, of which 25 (25%) had UTUC. The subgroup analyses showed that the median OS was 13.8 months (95% CI: 9.8–15.8) for UBC and 10.3 months (95% CI: 6.8–15.3) for UTUC (Figure 1).
Antibody-drug conjugates
Enfortumab vedotin
EV201 (cohort1, third-line setting)
Enfortumab vedotin, a nectin cell adhesion molecule 4 (Nectin-4)-directed antibody and microtubule inhibitor conjugate was granted accelerated FDA approval in December 2019 as a third-line treatment for patients with locally advanced or metastatic UC who previously received platinum-containing chemotherapy and a PD-1/PD-L1 inhibitor based on the results of the phase 2 EV-201 trial (cohort1) (27). The EV-201 trial (cohort1) enrolled 125 patients with advanced UC previously treated with platinum-containing chemotherapy and a PD-1/PD-L1 inhibitor, of which 44 patients (35%) had UTUC. The subgroup analyses showed that ORR was 47% (95% CI: 35.7–58.3%) for UBC and 39% (95% CI: 24.4–54.5%) for UTUC (Figure 1).
EV201 (cohort2, second-line setting)
Enfortumab vedotin was approved by the FDA in July 2021 as a second-line treatment for cisplatin-ineligible patients who had previously received one or more prior lines of therapy based on the results of the phase 2 EV-201 trial (cohort2) (28).
The EV-201 trial (cohort2) enrolled 89 cisplatin-ineligible patients with advanced UC who were previously treated with PD-1 or PD-L1 inhibitors, of which 38 (43%) had UTUC. The subgroup analyses showed that ORR was 45% (95% CI: 31.1–59.7%) for UBC and 61% (95% CI: 43.4–76.0%) for UTUC (Figure 1).
EV301 (third-line setting)
Enfortumab vedotin was converted from accelerated FDA approval in 2019 to regular approval in July 2021 as a third-line treatment for patients with locally advanced or metastatic UC who previously received platinum-containing chemotherapy and a PD-1/PD-L1 inhibitor based on results from the phase 3 EV-301 trial (29). The EV-301 trial enrolled 608 patients with advanced UC who received a prior PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, of which 205 patients (33.7%) had UTUC. The subgroup analyses for OS showed that HR was 0.67 (95% CI: 0.51–0.88) for UBC and 0.85 (95% CI: 0.57–1.27) for UTUC (Figure 1).
Sacituzumab govitecan
TROPHY-U-01 study (third-line setting)
Sacituzumab govitecan, an anti-Trop-2 monoclonal antibody conjugated to SN-38—an active metabolite of irinotecan—was granted accelerated FDA approval in April 2021 as a third-line treatment for patients with advanced UC who previously received platinum-containing chemotherapy and a PD-1/PD-L1 inhibitor based on results from the phase 2 TROPHY-U-01 trial (30). The TROPHY-U-01 trial enrolled 113 cases of patients with advanced UC who received prior treatment with platinum-containing chemotherapy and either a PD-1 or PD-L1 inhibitor, but no information about the number of UTUC cases was available, and no subgroup analysis was performed.
In summary, several subgroup analyses of clinical trials suggested possible differential effects of targeted agents (especially nivolumab and enfortumab vedotin) between UTUC and UBC. For instance, CheckMate 274 implied that nivolumab might be less efficacious in UTUC than in UBC in an adjuvant setting (14); however, subgroup analysis of other clinical trials, such as the Keynote045 trial, implied that the efficaciousness of pembrolizumab for UTUC appears to be nearly equal or greater than that of UBC (9) (Figure 1). In addition, clinical trials of enfortumab vedotin [EV201 and EV301 both in a third-line setting (27,29)] also implied that enfortumab vedotin might be less efficacious in UTUC than in UBC (Figure 1).
Comparison between genomic characteristics in UTUC and UBC
The advances in NGS technologies over the past few years have provided a better understanding of the mutational landscape of UTUC.
To date, several studies have analyzed the mutational frequency of UTUC tissues by NGS, and most of them compared mutation frequencies between UTUC and UBC (31-39). It should be noted that mutation frequencies of the UTUC cohort in each study are affected by the proportion of low-grade UTUC cases included because genetic mutation profiles of low-grade and high-grade UC are different. For example, the FGFR3 mutation frequency has been noted to be higher in low-grade tumors, and the PT53 mutation frequency is higher in high-grade tumors. Therefore, the gene mutation profile in UTUC needs to be stratified by tumor grade if compared with high-grade UBC.
Sfakianos et al. analyzed UTUC tissues (low-grade; n=23, high-grade; n=59) and high-grade UBC tissues (n=102) using the MSK-IMPACT platform and compared mutation frequencies in high-grade UTUC and high-grade UBC (31). They reported that FGFR3 (36% vs. 22%), HRAS (14% vs. 1%), and CDKN2B (15% vs. 4%) were more frequently altered in high-grade UTUC, whereas TP53 (25% vs. 58%), RB1 (0% vs. 19%), and ARID1A (13.6% vs. 27.5%) were more frequently altered in UBC. They also showed that FGFR3 alterations in high-grade UTUC were mutually exclusive with mutations in TP53. Using the same UTUC tissues, Bagrodia et al. reported that TP53/MDM2 alterations were associated with adverse clinicopathological outcomes, whereas FGFR3 mutations were associated with favorable outcomes (40).
Audenet et al. also compared the mutational landscapes of UTUC tissues (low-grade; n=30, high-grade; n=165) to UBC tissues (low-grade; n=26, high-grade; n=428) using the MSK-IMPACT platform and revealed that FGFR3 (40% vs. 26%) and HRAS (12% vs. 4%) were more significantly altered in UTUC, whereas TP53 (26% vs. 46%), RB1 (3% vs. 20%), and ERBB2 (8% vs. 19%) were more often altered in UBC (32). Furthermore, even after adjusting for the tumor grade, high-grade UTUC tissues harbored more frequent FGFR3 alterations than high-grade UBC tissues (31% vs. 21%).
Moss et al. conducted whole-exome sequencing (WES) in UTUC tissues (low-grade; n=12, high-grade; n=15) (33). They confirmed higher rates of FGFR3 mutations UTUC (low-grade; 92%, high-grade; 60%) compared to the mutation rate in high-grade UBC in TCGA data (n=404) (13%). They also demonstrated a higher frequency of TP53 mutations in high-grade UTUC (33%) than in low-grade UTUC (8%).
Lee et al. performed targeted sequencing (the Ion Torrent Ampliseq cancer panel v2) to detect frequent somatic mutations and compare the genetic alterations between UTUC (n=31) and UBC (n=61) (34). Although the number of high-grade UTUC and low-grade UTUC in this study is not available, the overall frequency of FGFR3 mutations in UTUC (13%) was relatively lower than the values reported by Sfakianos et al. (54%) (31), Audenet et al. (40%) (32), and Moss et al. (74%) (33). Lee et al. (34) analyzed the Korean cohort, whereas the others analyzed Western patients. This difference in the mutation frequency of FGFR3 might be attributed to the difference in the race/region profile of the cohorts since FGFR3 mutation frequencies were reported to be relatively lower in UTUC in Asian patients, particularly Han Chinese patients (3–9%), than in Western patients (36–60%) (41,42). The TP53 mutation frequency in UTUC (71%) by Lee et al. (34) was also markedly higher than in other reports, which might also be due to racial/region differences, including AA exposure. Similarly, Yang et al. performed targeted sequencing in UTUC (n=45) and UBC (n=73) tissues from Chinese patients (35). In this study, 21% of patients with UTUC were supposed to harbor AA exposure. Although the number of high-grade UTUC and low-grade UTUC is also not available, the frequency of FGFR3 mutations in UTUC (20%) was relatively low, as in the Korean cohort by Lee et al. (34).
Robinson et al. performed WES in high-grade UTUC tissues (n=37) and compared the mutational frequency of UTUC to that of UBC tissues from the TCGA cohort (n=124) (36). They demonstrated that FGFR3 was more frequently altered in high-grade UTUC than UBC (30% vs. 14%).
Nassar et al. performed targeted sequencing and compared mutation frequencies in UTUC tissues (n=65, low-grade; n=10, high-grade; n=55) and UBC tissues (n=407, low-grade; n=82, high-grade; n=325) (37). They indicated that HRAS mutations were enriched in UTUC (low-grade; 10%, high-grade13%) in comparison with UBC. In addition, FGFR3 (80% vs. 16%) and KDM6A alterations (50% vs. 20%) were enriched in low-grade UTUC than in high-grade UTUC, whereas the converse applied to TP53 (0% vs. 47%).
Grahn et al. performed targeted exome-sequencing in UTUC tissues (low-grade; n=7, high-grade; n=29) (43). They investigated the association between mutations and survival in groups of various grades and stages and found that HRAS and TP53 mutation might be linked to poor prognosis, and FGFR3 mutations might be linked to a favorable prognosis.
In addition to the mutations mentioned in the above studies, TERT promoter mutations are one of the most frequent mutations in both UTUC and UBC (44-46). Fujii et al. performed TERT promoter sequencing in addition to WES in high-grade UTUC tissues (n=199) (38). They demonstrated that the most frequently affected genes included the TERT promoter (49%), KMT2D (46%), CDKN2A (45%), FGFR3 (45%), and TP53 (35%). They also compared the mutational frequency of invasive UTUC (n=81) to that of invasive UBC tissues from the TCGA cohort (n=375). As a result, they reported that FGFR3, CDKN2A, and KMT2D were more preferentially altered in invasive UTUC than in invasive UBC, while ERBB2 was more frequently mutated in invasive UBC.
Necchi et al. analyzed high-grade UTUC tissues (n=479) and high-grade UBC tissues (n=1,984) using the Foundation One platform (39). This is the largest dataset analyzing the mutational landscapes of UTUC vs. UBC. They demonstrated that FGFR3 (26% vs. 19%) and HRAS (7% vs. 3%), and CDKN2A (40% vs. 35%) was more common in high-grade UTUC than in high-grade UBC, while TERT promoter mutations (47% vs. 68%) and TP53 (49% vs. 58%) and RB1 (8% vs. 21%) were more common in high-grade UBC.
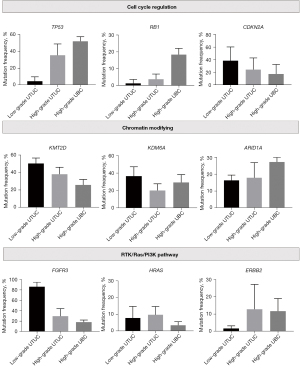
To summarize these genomic studies in UTUC [except for the reports without tumor-grade information (34,35)], similar mutations were seen in both UTUC and UBC, but the two tumors showed differences in the prevalence of mutations——FGFR3, HRAS, CDKN2A, and KMT2D were more frequently altered in high UTUC, whereas TP53, RB1, KDM6A, and ARID1A were more frequently altered in high-grade UBC (Figure 2). Notably, FGFR3 mutations are present in a significant proportion of high-grade UTUC tumors as well as low-grade UTUC in comparison with high-grade UBC. Thus, the high frequency of FGFR3 mutations is a hallmark of sporadic UTUC. Contrastingly, TP53 mutations are predominantly found in high-grade UTUC and UBC.
In addition to sporadic mutations, UTUC is also caused by mutations associated with Lynch syndrome and AA exposure (4-6). Patients with Lynch syndrome who have germline mutations in the five MMR genes MLH1, MSH2, MSH6, PMS2, or EPCAM have an increased risk of UTUC than that of UBC. Lynch syndrome-associated UTUC has a higher mutation frequency than in the sporadic UTUC samples but has a similar mutational landscape, and FGFR3 mutation frequencies are similar to those in sporadic UTUC (4).
AA exposure causes a unique mutational pattern, which differs from that in sporadic UTUC: A>T transversions are significantly enriched at splice sites, with a higher mutational load than in sporadic UTUC (5,6,47). In AA exposure-associated UTUC, TP53 was frequently mutated (58%), and TP53 mutation is dominated by A>T transversions. In contrast, FGFR mutations were rare (8%) in AA exposure-associated UTUC and did not possess A>T transversions (6).
RNA expression subtypes of UTUC
Several studies performed RNA sequencing in addition to WES of DNA in UTUC and analyzed the RNA expression subtypes of UTUC using unsupervised consensus clustering (33,36,38).
Robinson et al. reported that sporadic UTUC shows a lower mutational burden than UBC and predominantly shows the luminal papillary subtype (20/32, 63%), in contrast to the proportion of luminal papillary UBC tumors in the TCGA UBC cohort (35/128; 27%). The luminal papillary subtype is characterized by FGFR3 gene expression signatures and low immune cell infiltration (36).
Moss et al. segregated UTUC samples into four subtypes with unique molecular and clinical features. They demonstrated that clusters characterized by FGFR3 gene alteration (cluster2 and cluster3, which have 100% FGFR3 gene alteration) had lower mRNA expression levels of CD274 (PD-L1) (33).
Fujii et al. also conducted gene expression analysis of UTUC through unbiased clustering analysis using RNA sequencing data from 158 UTUC tissues and identified five specific expression subtypes (C1–C5). The C1 subtype showed the highest expression of FGFR3-associated markers and was dominant in the luminal papillary subtype, accounting for most UTUC samples (72%). However, the C3 subtypes, many of which belong to the TP53/MDM2-mutated subtype, were classified into the basal-squamous subtype and exhibited high expression of immune-checkpoint molecules, such as PD-L1 (38).
In conclusion, several studies have shown that UTUC predominantly shows the luminal papillary subtype more than UBC (33,36,38). The luminal papillary subtype is characterized by T-cell depletion and may be an immunologically “cold” tumor. Although FGFR3 mutation itself was reportedly not associated with a response rate to PD-1/PD-L1 inhibitors (but FGFR expression was) (48), the predominance of the luminal papillary subtype in UTUC may afford a possible lower response rate to immunotherapy in sporadic UTUC than that in UBC. On the contrary, UTUC classified into the basal-squamous subtype may express high levels of immune-checkpoint molecules, which might benefit from immunotherapy. However, we previously reported that PD-L1 expression is independent of subtype classification in UTUC based on immunostaining analysis (49), and further studies are required to reveal the PD-L1 expression among molecular subtypes of UTUC.
Comparison of the expression of therapeutic target proteins between UTUC and UBC
As discussed in the section titled “Comparison of targeted drug efficacy between UBC and UTUC in clinical trials”, subgroup analyses of clinical trials of targeted therapies have indicated the possibility of differential effects of individual drugs when administered for UTUC and when administered for UBC. These differences may be attributed to the expression status of therapeutic target proteins in UTUC and UBC. Here, we have focused on the differences in the expression status of therapeutic target proteins (specifically PD-L1, Nectin-4, and Trop-2) between UTUC and UBC and discussed the efficaciousness of each targeted drug in UTUC. Table 2 summarizes the expression status of therapeutic target proteins in UTUC and UBC primary tissues analyzed by immunohistochemical analysis (50-54). We also analyzed the positive rates of each protein in different molecular subtypes of UTUC: luminal (GATA3+/CK5/6−), basal (GATA3−/CK5/6+), and double-negative [considered as p53-like or neuroendocrine-like subtype (GATA3−/CK5/6−)] subtypes, since immunohistochemical staining for GATA3 and cytokeratin 5/6 (CK5/6) is reportedly sufficient to determine molecular subtypes (49,55-57).
Table 2
| Target proteins | UTUC | UBC total | Reference | |||
|---|---|---|---|---|---|---|
| Total | Luminal subtype (GATA3+/CK5/6–) | Basal subtype (GATA3-/CK5/6+) | Double-negative subtype (GATA3-/CK5/6–) | |||
| PD-L1 | 10% | 8% | 13% | 12% | 18% | (50,51) |
| Nectin-4 | 66% | 69% | 69% | 64% | 82% | (50,52) |
| Trop-2 | 94% | 100% | 95% | 88% | 83% | (53,54) |
CK5/6, cytokeratin 5/6; GATA3+, GATA binding protein 3; UBC, urinary bladder cancer; UTUC, upper tract urothelial carcinoma; PD-L1, programmed cell death ligand-1.
PD-L1
Although the value of PD-L1 expression in predicting the effect of PD-1/PD-L1 inhibitors in urothelial carcinoma is still inconclusive, some studies have suggested differences in response according to PD-L1 expression in tumor or immune cells (11,15,23). Therefore, we compared the PD-L1-positive rate (defined as positive when more than 5% of the tumor cell membrane was stained) between UTUC tissues (n=99) (50) and UBC tissues (n=56) (51) with the expectation that it would be a predictive marker of response to PD-1/PD-L1 inhibitors. Notably, the PD-L1-positive rates in the two studies are good comparisons since both UTUC and UBC samples were stained with the same antibody and under the same conditions in the same laboratory, though there is a difference in the race of patients (50,51). Thus, we found that the PD-L1-positive rate was lower in UTUC than that in UBC in immunohistochemical analysis (UTUC, 10%; UBC, 18%) (Table 2), but the difference was not statistically significant (P=0.21, Fisher’s exact test). If PD-L1 expression on tumor cells is a predictive marker of response to PD-1/PD-L1 inhibitors, the lower PD-L1 positive rate in UTUC than UBC in our analysis is consistent with the results of a subgroup analysis of CheckMate 274 (nivolumab), in which PD-1/PD-L1 inhibitors appear to be less efficacious for UTUC than for UBC. However, this contradicts the subgroup analysis in the Keynote045 trial, in which the efficaciousness of PD-1/PD-L1 inhibitors for UTUC appears to be nearly equal to or greater than that of UBC (9).
In the IMvigor210 trial, higher levels of PD-L1 immunohistochemistry expression on tumor-infiltrating immune cells were associated with a higher response rate to atezolizumab in advanced UC and longer OS, but the PD-L1 expression rate on tumor cells did not show an association with an objective response (15). Thus, the clinical usefulness of PD-L1 as a biomarker in advanced urothelial carcinoma is an area of uncertainty, and it may be difficult to explain the difference in response rates to PD-1/PD-L1 inhibitors between UTUC and UBC solely based on PD-L1 expression on tumor cells. The only FDA-approved indication for PD-L1 expression testing is limited to determining the suitability of atezolizumab as first-line monotherapy in patients with advanced UC who were unfit for cisplatin-containing chemotherapy and have not received prior therapy. In this situation, tumor mutation burden (TMB) is reported to be superior to PD-L1 expression as a biomarker for PD-1/PD-L1 inhibitors (58). In addition to TMB, molecular subtypes were also associated with response to PD-1/PD-L1 inhibitors, suggesting that molecular subtypes differed in their underlying immune biology (15). As mentioned in the previous section about RNA expression subtypes of UTUC, UTUC more predominantly shows the luminal papillary subtype, which is immunologically cold with low T-cell infiltration. This finding may afford a possible lower response rate to immunotherapy in UTUC than that in UBC. Further studies are expected to identify biomarkers for predicting the efficacy of immunotherapy in advanced UC.
Nectin-4
Nectin-4 (encoded by PVRL4) is the target protein of enfortumab vedotin, an antibody-drug conjugate for locally advanced or metastatic urothelial carcinoma. Since enfortumab vedotin targets Nectin-4, the expression of Nectin-4 in cancer cells is necessary for its cytotoxic effect, and a correlation between Nectin-4 expression and response to enfortumab vedotin has been demonstrated in vitro and in vivo (59). Nectin-4 expression was reported in 83% of UBC cases (434/524) (52), while we demonstrated that Nectin-4 expression positivity was found in 68% (67/99) of UTUC cases in our previous study (50). These findings indicated that UTUC might have a slightly lower rate of Nectin-4 positivity than UBC, which supports the results of a subgroup analysis in the EV-201 (cohort 1) and EV301 trials (27,29) and indicates that enfortumab vedotin was less efficacious in UTUC than in UBC. In contrast, the EV-201 (cohort 2) trial indicated that enfortumab vedotin is more efficacious in UTUC than in UBC (28). Several studies have reported an association between molecular subtype and Nectin4 expression (38,59). Chu et al. reported that Nectin-4 expression is significantly enriched in luminal subtypes in muscle-invasive UBC (59). For UTUC, Fujii et al. showed that the expression level of PVRL4, which encodes Nectin-4, was the lowest in the basal/squamous subtype (38). Tumor mutation burden (TMB) is reported to be superior to PD-L1 expression as a biomarker for PD-1/PD-L1 inhibitors. However, in our analysis, Nectin-4 positivity rates did not differ significantly among the UTUC subtypes (Table 2).
Thus, the Nectin4-positive rate in UTUC and the relationship between molecular subtype and Nectin4 expression are topics of debate. The Nectin4-positive rate in UTUC and its association with molecular subtype and actual response rate of EV in clinical practice are expected to be analyzed in future studies.
Trop-2
The trophoblast cell surface antigen 2 (Trop-2, encoded by TACSTD2) is the target protein of sacituzumab govitecan, an antibody-drug conjugate for locally advanced or metastatic urothelial carcinoma. A Previous study have indicated that cells overexpressing Trop-2 are highly sensitive to sacituzumab govitecan (60), and a pilot study suggested that high Trop-2 expression in UC was positively correlated with treatment response (53). Therefore, evaluation of the expression of Trop-2 in solid tumors is clinically important to predict the efficacy of sacituzumab govitecan. The Trop-2-positive rate in UTUC was found in 94% (94/99) of UTUC cases in our previous study (54), while it was reported to be 83% in UBC (53). This finding suggests that UTUC also has a high Trop-2-positivity rate and is a treatment option for advanced UTUC and UBC.
Regarding the molecular subtype of UC and Trop-2 expression, Chou et al. reported that Trop-2 is highly expressed in most subtypes except in the neuroendocrine subtype (60). Similarly, in our data, Trop-2 positivity rates did not differ significantly among the UTUC subtypes (Table 2). In our previous study (54), high Trop-2 expression was associated with a good prognosis in UTUC, and the findings also confirmed that the high TASCSTD2 expression group still showed a favorable prognosis in gene expression analysis using RNA sequencing data. These findings were inconsistent with those reported for UBC; high Trop-2 expression was associated with increased tumor aggressiveness and poor prognosis. This association between high Trop-2 expression and favorable prognosis in UTUC may be a unique feature of UTUC that differs from UBC; therefore, further studies are expected to improve our knowledge of the differences between UTUC and UBC.
Discussion
In this review, we summarized subgroup analyses of clinical trials of FDA-approved targeted drugs to explore the differential effects of each drug when administered for UTUC vs. when administered for UBC. Besides, we summarized the differences in mutation frequency, RNA expression subtype, and therapeutic target protein expressions, specifically PD-L1, Nectin-4, and Trop-2.
Some subgroup analyses of clinical trials suggested possible differential effects of targeted agents; in particular, CheckMate 274 implied that immune-checkpoint inhibitors might be less efficacious in UTUC than in UBC in adjuvant settings (14). Several studies have recently compared mutation frequencies between UTUC and UBC by NGS. Our summary suggested that FGFR3, HRAS, CDKN2A, and KMT2D were more frequently altered in high UTUC, whereas TP53, RB1, KDM6A, and ARID1A were more frequently altered in high-grade UBC (31-33,36-39). Thus, UTUC has a high frequency of FGFR3 mutations and is reported to predominantly show the luminal papillary subtype, which is immunologically cold with low T-cell infiltration (36). The feature of UTUC is consistent with a possible lower response rate to immunotherapy in UTUC than that in UBC.
In addition, clinical trials of enfortumab vedotin in a third-line setting (EV201 and EV301) implied that enfortumab vedotin might be less efficacious in UTUC than in UBC (27,29). Previous immunohistochemical analyses suggest that UTUC might have a slightly lower rate of Nectin-4 positivity than UBC (50,52), which supports the results of a subgroup analysis indicating that enfortumab vedotin was less efficacious in UTUC than in UBC.
Thus, clinical differences in the effects of targeted drugs between UTUC and UBC shed light on the potential for molecular differences between the two diseases, such as molecular subtypes and the status of the target molecule of each drug.
The limitation of this review is the absence of clinical data on the efficacy of individual targeted drugs administered for UTUC compared with those administered for UBC. Needless to say, the results of subgroup analysis should be limited to only a reference because of some factors, such as inappropriately fewer cases of UTUC compared with that of UBC and potential bias in background factors between the two subgroups caused by subgroups not being randomly assigned. Further study is needed to investigate the differences in the efficacy of each drug between UTUC and UBC and its relationship to molecular characteristics in real-world clinical practice.
Summary
This narrative review facilitates a deeper understanding of the clinical differences in the effects of targeted drugs between those administered for UTUC and those for UBC and explores the possibility of existing molecular differences between these two diseases, such as the status of the target molecule of each drug.
Optimizing the treatment strategy based on further investigation of the molecular characteristics of UTUC will gain prominence in the future and can help improve treatment outcomes. Future clinical trials are required to contribute data on the efficacy of targeted drugs for UTUC to drive the treatment decision-making process.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-457/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-457/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-457/coif). KF serves as an unpaid editorial board member of Translational Andrology and Urology from October 2021 to September 2023. KF receives grants from Pfizer; receives honoraria for lectures from Pfizer, Janssen Pharma, Astrazeneca, Astellas, ONO Pharmaceutical, and Bristol Myers Squibbs; and participates advisory board of Astellas. HU receives grants from Ono pharma, Astrazeneca, and Astellas; receives payment or honoraria for lectures from Pfizer, Janssen Pharma, Merck BioPharma, MSD, ONO Pharmaceutical, and Bristol Myers Squibbs. NN receives payment or honoraria for lectures from Pfizer, Janssen Pharma, Merck BioPharma, AstraZeneca, MSD, ONO Pharmaceutical, and Bristol Myers Squibb; and participates advisory board of Pfizer and Bristol Myers Squibb. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013;189:1214-21. [Crossref] [PubMed]
- Leow JJ, Chong KT, Chang SL, et al. Upper tract urothelial carcinoma: a different disease entity in terms of management. ESMO Open 2017;1:e000126. [Crossref] [PubMed]
- Carlo MI, Ravichandran V, Srinavasan P, et al. Cancer Susceptibility Mutations in Patients With Urothelial Malignancies. J Clin Oncol 2020;38:406-14. [Crossref] [PubMed]
- Chen CH, Dickman KG, Moriya M, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci U S A 2012;109:8241-6. [Crossref] [PubMed]
- Hoang ML, Chen CH, Sidorenko VS, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci Transl Med 2013;5:197ra102. [Crossref] [PubMed]
- Ozen H. Bladder cancer. Curr Opin Oncol 1999;11:207-12. [Crossref] [PubMed]
- Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol 2018;15:112-24. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483-92. [Crossref] [PubMed]
- Vuky J, Balar AV, Castellano D, et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 2020;38:2658-66. [Crossref] [PubMed]
- Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:931-45. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med 2021;384:2102-14. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748-57. [Crossref] [PubMed]
- Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547-57. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Powles T, O'Donnell PH, Massard C, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol 2017;3:e172411. [Crossref] [PubMed]
- Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2020;21:1574-88. [Crossref] [PubMed]
- Apolo AB, Ellerton JA, Infante JR, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer 2020;8:e001246. [Crossref] [PubMed]
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020;383:1218-30. [Crossref] [PubMed]
- Powles T, Sridhar SS, Loriot Y, et al. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med 2021;27:2200-11. [Crossref] [PubMed]
- Powles T, Petrylak DP, Park SH, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Analysis of clinical and genomic subgroups from the JAVELIN Bladder 100 trial. J Clin Oncol 2021;39:4520. [Crossref]
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019;381:338-48. [Crossref] [PubMed]
- Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol 2022;23:248-58. [Crossref] [PubMed]
- Rosenberg JE, O'Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019;37:2592-600. [Crossref] [PubMed]
- Yu EY, Petrylak DP, O'Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV 201): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2021;22:872-82. [Crossref] [PubMed]
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021;384:1125-35. [Crossref] [PubMed]
- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol 2021;39:2474-85. [Crossref] [PubMed]
- Sfakianos JP, Cha EK, Iyer G, et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2015;68:970-7. [Crossref] [PubMed]
- Audenet F, Isharwal S, Cha EK, et al. Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma. Clin Cancer Res 2019;25:967-76. [Crossref] [PubMed]
- Moss TJ, Qi Y, Xi L, et al. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2017;72:641-9. [Crossref] [PubMed]
- Lee JY, Kim K, Sung HH, et al. Molecular Characterization of Urothelial Carcinoma of the Bladder and Upper Urinary Tract. Transl Oncol 2018;11:37-42. [Crossref] [PubMed]
- Yang K, Yu W, Liu H, et al. Comparison of Genomic Characterization in Upper Tract Urothelial Carcinoma and Urothelial Carcinoma of the Bladder. Oncologist 2021;26:e1395-405. [Crossref] [PubMed]
- Robinson BD, Vlachostergios PJ, Bhinder B, et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun 2019;10:2977. [Crossref] [PubMed]
- Nassar AH, Umeton R, Kim J, et al. Mutational Analysis of 472 Urothelial Carcinoma Across Grades and Anatomic Sites. Clin Cancer Res 2019;25:2458-70. [Crossref] [PubMed]
- Fujii Y, Sato Y, Suzuki H, et al. Molecular classification and diagnostics of upper urinary tract urothelial carcinoma. Cancer Cell 2021;39:793-809.e8. [Crossref] [PubMed]
- Necchi A, Madison R, Pal SK, et al. Comprehensive Genomic Profiling of Upper-tract and Bladder Urothelial Carcinoma. Eur Urol Focus 2021;7:1339-46. [Crossref] [PubMed]
- Bagrodia A, Cha EK, Sfakianos JP, et al. Genomic Biomarkers for the Prediction of Stage and Prognosis of Upper Tract Urothelial Carcinoma. J Urol 2016;195:1684-9. [Crossref] [PubMed]
- Yuan X, Liu C, Wang K, et al. The genetic difference between Western and Chinese urothelial cell carcinomas: infrequent FGFR3 mutation in Han Chinese patients. Oncotarget 2016;7:25826-35. [Crossref] [PubMed]
- Springer SU, Chen CH, Rodriguez Pena MDC, et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018;7:e32143. [Crossref] [PubMed]
- Grahn A, Eisfeldt J, Malm C, et al. Genomic profile - a possible diagnostic and prognostic marker in upper tract urothelial carcinoma. BJU Int 2022;130:92-101. [Crossref] [PubMed]
- Eich ML, Rodriguez Pena MDC, Springer SU, et al. Incidence and distribution of UroSEEK gene panel in a multi-institutional cohort of bladder urothelial carcinoma. Mod Pathol 2019;32:1544-50. [Crossref] [PubMed]
- Hayashi Y, Fujita K, Matsuzaki K, et al. Diagnostic potential of TERT promoter and FGFR3 mutations in urinary cell-free DNA in upper tract urothelial carcinoma. Cancer Sci 2019;110:1771-9. [Crossref] [PubMed]
- Hayashi Y, Fujita K, Matsuzaki K, et al. Clinical Significance of Hotspot Mutation Analysis of Urinary Cell-Free DNA in Urothelial Bladder Cancer. Front Oncol 2020;10:755. [Crossref] [PubMed]
- Poon SL, Pang ST, McPherson JR, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci Transl Med 2013;5:197ra101. [Crossref] [PubMed]
- Necchi A, Raggi D, Giannatempo P, et al. Can Patients with Muscle-invasive Bladder Cancer and Fibroblast Growth Factor Receptor-3 Alterations Still Be Considered for Neoadjuvant Pembrolizumab? A Comprehensive Assessment from the Updated Results of the PURE-01 Study. Eur Urol Oncol 2021;4:1001-5. [Crossref] [PubMed]
- Tomiyama E, Fujita K, Hashimoto M, et al. Programmed cell death-ligand 1 expression in different molecular subtypes of upper tract urothelial carcinoma. Int J Urol 2022;29:89-90. [Crossref] [PubMed]
- Tomiyama E, Fujita K, Rodriguez Pena MDC, et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int J Mol Sci 2020;21:5390. [Crossref] [PubMed]
- Faraj SF, Munari E, Guner G, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015;85:703.e1-6. [Crossref] [PubMed]
- Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res 2016;76:3003-13. [Crossref] [PubMed]
- Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab Govitecan, a Novel Antibody-Drug Conjugate, in Patients with Metastatic Platinum-Resistant Urothelial Carcinoma. Clin Genitourin Cancer 2016;14:e75-e79. [Crossref] [PubMed]
- Tomiyama E, Fujita K, Nakano K, et al. Trop-2 in Upper Tract Urothelial Carcinoma. Curr Oncol 2022;29:3911-21. [Crossref] [PubMed]
- Dadhania V, Zhang M, Zhang L, et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016;12:105-17. [Crossref] [PubMed]
- Hodgson A, Liu SK, Vesprini D, et al. Basal-subtype bladder tumours show a ‘hot’ immunophenotype. Histopathology 2018;73:748-57. [Crossref] [PubMed]
- Inoue S, Mizushima T, Fujita K, et al. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum Pathol 2017;64:83-90. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Chu CE, Sjöström M, Egusa EA, et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin Cancer Res 2021;27:5123-30. [Crossref] [PubMed]
- Chou J, Trepka K, Sjöström M, et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur Urol Oncol 2022;S2588-9311(21)00215-7. Epub ahead of print. [Crossref] [PubMed]


