
Long-term prognosis and prognostic factors of brachytherapy and propensity score matched comparisons of the outcomes between brachytherapy and radical prostatectomy: a retrospective cohort study
Highlight box
Key findings
• In matched-pair analyses, BT and RP offered similar bPFS and CSS in the localized prostate cancer.
What is known and what is new?
• Comparisons of the prognosis between BT and RP without PSM have been reported, and the follow-up time is not long.
• This study provides long-term outcomes of older prostate cancer patients undergoing BT. To our knowledge, we are the first cohort study of Chinese patients to compare the survival outcomes of RT and RP by using PSM.
What is the implication, and what should change now?
• This means that both BT can also well control the tumor progression of localized prostate cancer. In the clinic, physicians and patients can have more individual treatment options for localized prostate cancer patients such as BT, than surgery is recommended first.
Introduction
Prostate cancer is one of the most common malignancies and a leading cause of cancer-related death in men worldwide (1). Patients with localized prostate cancer typically have a favorable prognosis (2). Thus, it is important to select an appropriate treatment method that can reduce the mortality and recurrence risk of patients while improving their quality of life. At present, localized prostate cancer is often treated using curative methods, including radical proctectomy (RP) and radical radiotherapy, which includes external radiotherapy and brachytherapy (BT) (3-5).
Several studies have shown that BT has good long-term efficacy in patients with low-, intermediate-, and high-risk prostate cancer (4,6-9). However, the utilization of brachytherapy is declining, and this trend may lead to fewer physicians with the requisite expertise to perform quality brachytherapy and thereby limit access to this effective therapy for men with prostate cancer. This may be related to the fact that patients are not fully informed about the comparative information of these treatments and their effects, Therefore, it is very important to understand the prognosis and prognostic factors of BT, which can provide patients with more individualized treatment options to help patients make wise choices. At present, there is no conclusive conclusion about the prognostic factors of BT, and there are few analyses on prognostic factors of BT alone in China.
RP is the standard treatment method for localized prostate cancer (10); Thus, its efficacy can be used as the benchmark when investigating additional treatment methods. Treatment options are primarily influenced by risk stratification and physician and patient preference. Patients undergoing BT are generally older and have higher comorbidity scores and more aggressive cancer characteristics, such as initial prostate-specific antigen (PSA) levels, Gleason scores, and clinical staging, than patients undergoing RP (11-13). RP is recommended for patients who have favorable clinical characteristics, such as good cardiopulmonary function. Such differences in patient cohorts make it difficult to compare BT and RP in randomized controlled trials. No large prospective trials comparing the 2 treatment methods have been reported, and the number of retrospective studies regarding the efficacies of BT and RP in China is small.
Thus, the clinical data of 87 patients with prostate cancer who underwent BT at a single hospital were retrospectively analyzed in this study. In addition, propensity score matching (PSM) was performed to identify 42 patient pairs to compare the efficacy and prognosis of BT and RP. The goal of the study was to find the prognosis of BT and evaluate the effect of BT on the long-term survival of patients with prostate cancer in China. The findings of this study will be useful for clinical decision making. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-755/rc).
Methods
Data source
In order to evaluate the prognosis and prognostic factors of patients with localized prostate cancer, we enrolled 87 patients with localized prostate cancer who underwent BT (125I seed implant for prostate cancer) at Huadong Hospital affiliated to Fudan University between 2009 and 2016. The clinical data of 142 patients with localized prostate cancer who underwent RP (including robot-assisted laparoscopy and open radical prostatectomy) during the same period were also collected to be compared with BT. All the patients were diagnosed with prostate adenocarcinoma via prostate biopsy. Both treatment methods were performed by the same team of urologists. Endocrine therapy (6 months for patients with low-risk prostate cancer and 2 years for patients with intermediate- or high-risk prostate cancer) was used in some patients.
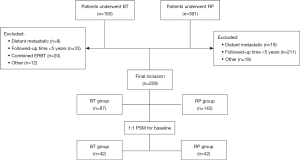
Patients with a clinical T stage of T1a–T3, who were followed-up for at least 5 years, did not undergo external radiotherapy, had no distant metastasis, and did not develop postoperative biochemical recurrence during endocrine therapy were included in the study. The choice of treatment was determined by the doctor and/or patients. Details of the patient selection process are given in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Huadong Hospital (No: 2021K095) and informed consent was taken from all the patients.
Data collection
Data on patients’ age, prostate volume, body mass index (BMI), America Society of Anesthesiologists (ASA) physical status classification, PSA, Gleason score, tumor clinical stage, risk stratification, comorbidities, platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) were collected. Data on the number of seeds implanted and the biochemical recurrence rate were also collected. Risk stratification was performed by dividing the patients into low-, intermediate-, and high-risk groups based on clinical stage, Gleason score, and initial PSA(3). Patients in the low-risk group had a clinical stage of T1–T2a and a Gleason score ≤6 and an initial PSA <10 ng/mL. Patients in the intermediate-risk group had a clinical stage of T2b or a Gleason score of 7 or an initial PSA level =10–20 ng/mL. Patients in the high-risk group had a clinical stage of T2c–T3 or a Gleason score >7 or an initial PSA level ≥20 ng/mL. The cut-off values for the NLR and PLR were calculated using receiver operating characteristic curves and the Youden index.
Treatments
BT was performed by a 125I seed implant for prostate cancer. A transrectal ultrasound to determine the treatment location was performed 3–5 days before the implantation for preplanning and intraoperative planning. According to the dose distribution curve, 125I seeds were accurately introduced into pre-planned positions. The intraoperative prescribed dose was 144 Gy. Postimplant dosimetry was performed with computed tomography imaging at Day 7 after implantation and each patient could be obtained D90 (the minimum dose covering 90% of the prostate). RP was performed by robot-assisted laparoscopy and open radical prostatectomy. The risk stratification of patients determined the extent of pelvic lymph node dissection.
Follow-up and study endpoints
Patients were monitored by physical examination and regular outpatient follow-up, including serum PSA determination which were monitored every 3 months for 2 years after treatment, every 6 months between 2 and 5 years after treatment, and every year after 5 years of treatment. In cases with a rise in PSA level or patient presenting with bone pain, a CT scan of the chest/abdomen/pelvis along with bone scintigraphy should be performed. All the patients in this study were followed-up regularly. The primary outcome was biochemical progression-free survival (bPFS), and the secondary outcomes were overall survival (OS) and cause-specific survival (CSS). Biochemical recurrence among patients who underwent BT was determined using the Phoenix definition (i.e., nadir + 2 ng/mL after seed implantation) (14,15). In patients who underwent RP, biochemical recurrence was defined as PSA >0.2 ng/mL for 2 consecutive measurements after surgery (16). Cause of death was obtained from death certificates and determined for each deceased patient. Patients with metastatic prostate cancer or castration-resistant disease without obvious metastases who died of any cause were classified as dead of prostate cancer. All other deaths were attributed to the immediate cause of death.
Statistical analysis
All the statistical analyses were conducted using SPSS v.26.0 statistical software (IBM SPSS Inc., Armonk, USA). Statistical significance was set at a 2-sided P<0.05. The survival rate was estimated using the Kaplan-Meier method and a Cox regression survival analysis, while univariate and multivariate analyses were performed using the Cox proportional hazards model. The normally distributed continuous data are expressed as the mean and standard deviation and were compared using 2-sample t-tests. The non-normally distributed continuous data are expressed as the median and interquartile range and were compared using the Mann-Whitney U test. The categorical data are expressed as the number and percentage. Pairwise comparisons of the categorical data were conducted using the chi-squared test or Fisher’s exact test.
The prognoses of the 87 patients who underwent BT and the associated factors were analyzed before PSM was conducted to match patients undergoing BT with those undergoing RP at a ratio of 1:1. Under a set tolerance of 0.02, 42 patients undergoing BT (the BT group) and 42 patients undergoing RP (the RP group) were matched. The preoperative indicators of the 2 groups, including age, BMI, prostate volume, preoperative PSA, preoperative clinical stage, preoperative Gleason score, and preoperative ASA physical status classification, were compared.
Results
Prognosis after BT and associated factors
The mean follow-up period of the 87 patients undergoing BT was 101 months (range, 64–144 months), and the median patient age was 78 years (range, 55–86 years). Among the patients, 31 had no preoperative comorbidities, 5 had other tumors, 53 had cardiovascular diseases, and 7 had both other tumors and cardiovascular diseases. Surgery was successfully completed in all the patients. The median number of seeds implanted was 74.5 (range, 44–129 seeds). The clinical indicators of patients in the BT group are listed in Table 1.
Table 1
| Clinical indicator | No. of cases, n (%) |
|---|---|
| iPSA | |
| <10 ng/mL | 26 (29.9) |
| ≥10–20 ng/mL | 26 (29.9) |
| 20 ng/mL | 35 (40.2) |
| Gleason score | |
| ≤6 | 17 (19.5) |
| 7 | 49 (56.3) |
| ≥8 | 21 (24.1) |
| Clinical stage | |
| T1a–T2a | 29 (33.3) |
| T2b–c | 49 (56.3) |
| T3 | 9 (10.3) |
| Risk stratification | |
| Low | 5 (5.7) |
| Intermediate | 37 (42.5) |
| High | 55 (51.7) |
| PLR | |
| ≤124 | 58 (66.7) |
| >124 | 29 (33.3) |
| NLR | |
| ≤2.3 | 59 (67.8) |
| >2.3 | 28 (32.2) |
iPSA, initial prostate-specific antigen; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
In the BT group, 11 (12.6%) patients developed biochemical recurrence, 3 (3.4%) developed bone metastases, and 27 (31.0%) died. The cause of death was prostate cancer in 3 patients, and cardiovascular disease, cerebrovascular disease, or other in the remaining patients. The median survival time of all patients was 91 months (range, 12–144 months). The 5- and 10-year OS rates were 82.8% (72/87) and 64.0% (16/25), respectively. The 5- and 10-year bPFS rates were 97.2% (70/72) and 87.5% (14/16), respectively. The 5-year bPFS rates of the low-, intermediate-, and high-risk groups were 100%, 96.3%, and 97.6%, respectively. The 5-year OS rates of the low-, intermediate-, and high-risk groups were 80%, 59.3%, and 97.6%, respectively. The 10-year bPFS rates of the intermediate- and high-risk patients were 100% and 88.9%, respectively. The 10-year OS rates of the intermediate- and high-risk patients were 66.7% and 80%, respectively. No patients in the low-risk group were followed-up for 10 years. The 5-year CSS rate was 96.6%.
A clinical stage of T3 was associated with decreased bPFS (P<0.05). The initial PSA, Gleason score, and risk stratification were not associated with decreased bPFS (P>0.05) (Table 2).
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (years) | |||||||
| ≤75 | 1 | 1 | |||||
| >75 | 3.212 | 0.937–11.008 | 0.063 | 1.978 | 0.454–8.619 | 0.364 | |
| Clinical T stage | |||||||
| T1a–T2a | 1 | 1 | |||||
| T2b–T2c | 0.301 | 0.075–1.204 | 0.090 | 0.092 | 0.007–1.200 | 0.069 | |
| T3 | 0.156 | 0.035–0.701 | 0.015 | 0.097 | 0.009–1.077 | 0.049 | |
| iPSA (ng/mL) | |||||||
| <10 | 1 | 1 | |||||
| 10–20 | 1.259 | 0.338–4.693 | 0.731 | 4.175 | 0.415–41.961 | 0.225 | |
| ≥20 | 0.604 | 0.117–3.120 | 0.547 | 1.892 | 0.160–20.975 | 0.627 | |
| Gleason score | |||||||
| ≤6 | 1 | 1 | |||||
| 7 | 2.092 | 0.349–12.552 | 0.420 | 3.231 | 0.449–23.266 | 0.244 | |
| ≥8 | 1.089 | 0.219–5.401 | 0.917 | 1.260 | 0.250–6.364 | 0.779 | |
| Risk stratification | |||||||
| Low | 1 | ||||||
| Intermediate | 2.496 | 0.294–21.214 | 0.402 | ||||
| High | 0.895 | 0.252–3.179 | 0.864 | ||||
| NLR | |||||||
| ≤2.3 | 1 | ||||||
| >2.3 | 1.463 | 0.379–5.643 | 0.580 | ||||
| PLR | |||||||
| ≤124 | 1 | ||||||
| >124 | 2.089 | 0.450–9.780 | 0.345 | ||||
| Prostate volume (mL) | |||||||
| ≤35 | 1 | ||||||
| >35 | 2.600 | 0.561–12.049 | 0.222 | ||||
Significant at P<0.05. iPSA, initial prostate-specific antigen; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
Comparison of the efficacies of BT and RP
Age, BMI, prostate volume, initial PSA, preoperative clinical stage, preoperative Gleason score, and preoperative ASA physical status classification did not differ significantly between the BT and RP groups (P>0.05). The proportion of patients receiving endocrine therapy in the BT group was significantly higher than that in the RP group (P<0.05); however, a previous study reported that this is not an independent risk factor for the prognosis of prostate cancer (8). No patients developed biochemical recurrence during endocrine therapy. In total, 42 BT patients were matched with 42 RP cases. The patient demographics after propensity score adjustment are summarized in Table 3.
Table 3
| Item | BT group (n=42) | RP group (n=42) | P value |
|---|---|---|---|
| Age (years) | 72.95±6.09 | 72.71±4.63 | 0.841 |
| BMI (kg/m2) | 23.90±3.42 | 23.69±2.16 | 0.744 |
| Prostate volume (mL) | 33.28 (25.08, 42.96) | 32.29 (24.08, 44.56) | 0.872 |
| iPSA | 0.924 | ||
| <10 ng/mL | 18 (42.9) | 17 (40.5) | |
| 10–20 ng/mL | 10 (23.8) | 12 (28.6) | |
| ≥20 ng/mL | 14 (33.3) | 13 (31.0) | |
| Clinical stage | 0.210 | ||
| T1a–T2a | 16 (38.1) | 11 (26.2) | |
| T2b–T2c | 20 (47.6) | 28 (66.7) | |
| T3 | 6 (14.3) | 3 (7.1) | |
| Gleason score | 0.655 | ||
| ≤6 | 12 (28.6) | 17 (40.5) | |
| 7 | 21 (50.0) | 19 (45.2) | |
| ≥8 | 9 (21.4) | 6 (14.2) | |
| ASA | 0.458 | ||
| Grade I | 4 (9.5) | 5 (26.2) | |
| Grade II | 34 (81.0) | 36 (85.7) | |
| Grade III | 4 (9.5) | 1 (2.4) | |
| 5-year biochemical recurrence | |||
| No | 31 | 32 | <0.05 |
| Yes | 2 | 9 | |
| 10-year biochemical recurrence | 0.279 | ||
| No | 8 | 9 | |
| Yes | 1 | 4 | |
| Survival status | |||
| Survival | 28 | 37 | <0.05 |
| Cause-specific death | 2 | 1 | 0.538 |
| Death from other causes | 12 | 4 | |
| Endocrine therapy | <0.05 | ||
| No | 0 | 12 | |
| Yes | 42 | 30 |
Data are presented as mean ± standard deviation, number (percentage), or median (interquartile range), or median (range). Significant at P<0.05. iPSA, initial prostate-specific antigen; BMI, body mass index; ASA, America Society of Anesthesiologists; BT, brachytherapy; RP, radical prostatectomy.
The mean follow-up period was 90.28±30.66 months in the BT group and 94.27±26.15 months in the RP group. In the BT group, 7 (16.7%) patients developed biochemical recurrence and 14 (33.3%) died, 2 (4.8%) of whom died of prostate cancer (Table 3). In the RP group, 11 (26.2%) patients developed biochemical recurrence and 5 (11.9%) died, 1 (2.4%) of whom died of prostate cancer.
bPFS, OS, and CSS between BT and RP
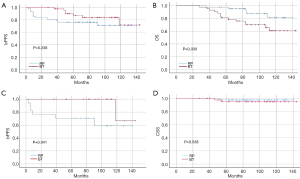
The propensity-adjusted 5- and 10-year bPFS rates were 93.9% and 88.9%, respectively, in the BT group, and 78.0% and 69.2%, respectively, in the RP group. Based on the Kaplan-Meier curve, the propensity-adjusted bPFS rate of the RP group was lower than that of the BT group, but the difference was not significant (Figure 2, P=0.238). The 5- and 10-year propensity-adjusted OS rates were 81.0% and 100%, respectively, in the BT group, and 97.6% and 100%, respectively, in the RP group. The propensity-adjusted OS of the RP group was significantly higher than that of the BT group (Figure 2, P=0.030). The propensity-adjusted CSS did not differ significantly between the groups (Figure 2, P=0.538).
bPFS curves between LDR and RP
Based on the differences of the preoperative indicators between the adjusted groups, different variable settings of the log-rank test were used to compare the bPFS curves of the BT and RP groups (Table 4). Among the patients with a prostate volume >35 mL in the matched cohorts, the bPFS rate of the BT group was significantly higher than that of the RP group (Figure 2, P=0.041).
Table 4
| Variable | P value |
|---|---|
| Age (year) | |
| ≤75 | 0.406 |
| >75 | 0.362 |
| Gleason score | |
| ≤6 | 0.494 |
| 7 | 0.279 |
| ≥8 | 0.059 |
| iPSA (ng/mL) | |
| <10 | 0.572 |
| 10–20 | 0.093 |
| ≥20 | 0.798 |
| Prostate volume (mL) | |
| ≤35 | 0.992 |
| >35 | 0.041 |
| Risk stratification | |
| Low | 0.371 |
| Intermediate | 0.413 |
| High | 0.318 |
| Clinical stage | |
| T1a–T2a | 0.847 |
| T2b–T2c | 0.220 |
| T3 | 0.081 |
Significant at P<0.05. iPSA, initial prostate-specific antigen; BT, brachytherapy; RP, radical prostatectomy.
Discussion
125I seed implantation, which has a significant curative effect, has been used to treat patients with prostate cancer in recent years. 125I seed implantation has several advantages over other treatment methods, including a simple procedure, reduced damage to surrounding tissues, a fast recovery, and fewer complications (14). However, BT is not as popular with physicians and patients as RP. Several studies have shown that BT has good long-term efficacy in patients with low-, intermediate-, and high-risk prostate cancer (4,6-9); however, the efficacy of BT in elderly patients with prostate cancer remains unclear. This study analyzed the clinicopathological data of 87 elderly patients with prostate cancer (median age: 78 years) and further investigated the efficacy of BT via PSM of 42 patients who underwent BT with 42 patients who underwent RP to provide additional guidance for clinical decision-making in the treatment of localized prostate cancer.
Yorozu et al. (6) reported that the 7-year bPFS rates of the patients with low-, intermediate-, and high-risk prostate cancer were 98%, 93%, and 81%, respectively, and the 7-year OS rate was 92.8%. However, in Yorozu et al.’s study, 48% of the patients received external radiotherapy as an adjuvant to BT, and the median age of the patients was 68 years. These differences may explain the inconsistencies in the results of the previous study and the current study. Taira et al. (7) reported that the 12-year bPFS rates of patients with low-, intermediate-, and high-risk prostate cancer were 98.6%, 96.5%, and 90.5%, respectively, while the 12-year OS rate was 72.6%. However, Taira et al.’s study adopted a stricter definition of biochemical recurrence (a postoperative PSA level >0.04 ng/mL), and 49.8% of the patients underwent external radiotherapy as an adjuvant to BT. The mean follow-up period of the 87 patients in the current study was 101.5 months. The OS of patients in the present study was lower than that of patients in previous studies (6,7); however, the biochemical recurrence rate was similar, which may be due to the fact that the patients included in this study were older and had a shorter life expectancy. Differences in the baseline characteristics of patients and the BT techniques used in each study and the heterogeneity across the studies may also account for the differences in the reported results.
Age, the proportion of positive biopsies, Gleason score, initial PSA, clinical stage, and risk stratification have been reported as predictors of recurrence and metastasis in patients with prostate cancer (6,7,17-19). Previous retrospective studies have also shown that the serum NLR and PLR could predict the prognosis for patients with localized and advanced prostate cancer (20-23). In this study, a Cox proportional hazards model was used to conduct univariate and multivariate analyses. Clinical stage T3 was identified as a predictor of poor prognosis (biochemical recurrence) in patients with localized prostate cancer who underwent BT. Conversely, the initial PSA, Gleason score, risk stratification, NLR, PLR, and prostate volume were not associated with biochemical recurrence.
BT and RP are 2 contemporary methods for the radical treatment of localized prostate cancer. However, previous studies comparing the efficacy of BT and RP have been predominantly retrospective, and patients who undergo BT are typically older and have more comorbidities than those who undergo RP. Pairwise differences in patients’ baseline characteristics render the findings of previous retrospective studies less conclusive. In the absence of randomized trials, retrospective comparisons of the two treatments are essential to provide patients and physicians with more rational treatment options. To our knowledge, furthermore, this study is the first paired-matched study between BT and RP in China. In a meta-analysis of 23 retrospective studies conducted in the last 2 decades, the average patient age was consistently <70 years and most studies did not consider differences in baseline characteristics (8). Conversely, the average patient age was >72 years in both groups in this study. Thus, this is the first study to compare the efficacy of BT and RP in elderly patients. In addition, PSM was performed to reduce the effect of differences in patients’ baseline characteristics.
PSM was used to match 42 patients with localized prostate cancer who underwent BT with 42 patients who underwent RP. There were no significant differences in the bPFS and the CSS between the adjusted groups. The propensity-adjusted OS of the RP group was significantly better than that of the BT group. Several studies have reported no significant differences between the bPFS, CSS, and OS of patients undergoing BT and RP (19,24,25). Several studies have used PSM to compare the prognosis between BT and RP. Hayashi et al. (26) compared the outcomes of RP, EBRT, and BT using PSM analysis in localized prostate cancer. In patients at intermediate risk, bPFS was better for BT than for RP (P=0.003), and there was no significant difference in OS between the two groups (P=0.429). Urabe et al. (27) conducted a retrospective analysis applying PSM in 1241 intermediate-risk prostate cancer patients. The propensity-adjusted 10‐year bPFS was 65.2% for RP versus 81.7% for BT (P<0.001). There was no significant difference in OS between the adjusted treatment groups. The adjusted 10-year OS for BT versus RP was 95.3% and 97.8% (P=0.15). Goy et al. (28) used PSM to retrospectively analysis the prognostic data in 1,503 intermediate-risk prostate cancer patients. The median follow-up was relatively long (10.0 years for RP and
9.8 years for BT). The adjusted 10-year bPFS was 80.2% for BT and 57.1% for RP (P=0.0003). Zhou et al. (25) reported no differences in the bPFS, OS, or CSS of patients with prostate cancer who underwent BT or RP. The 5- and 8- year OS rates were 97.8% and 93.6%, respectively, in the BT group, and 99.4% and 99.4%, respectively, in the RP group. However, the previous study had a short mean follow-up time (63 months) and a small number of deaths, which may have made it difficult to find a statistical difference in OS. Another study (8) reported no difference in CSS between the BT group and the RP group (99.5% vs. 98.5%, P>0.05), but the OS and bPFS were not compared between the groups. OS is the most intuitive indicator of patient survival; however, CSS remains the most important indicator of the success of prostate treatment (7). bPFS reflects the efficacy of delaying disease progression. In this study, the OS of the BT group was lower than that of the RP group. However, after accidental deaths were excluded, 85.7% of the deaths in the BT group were due to cardiovascular and cerebrovascular disease. In addition, while PSM was used to reduce differences in the baseline characteristics and risk stratification between the 2 groups in this study, it was not possible to compensate for the fact that the severity of comorbidities in the BT group was higher than that in the RP group, which was likely due to physician and patient preferences.
In the adjusted cohort, patients with prostate cancer with a prostate volume >35 mL had a significantly higher bPFS than patients in the RP group. Hayashi et al. (26) identified RP as an independent factor for bPFS (P<0.001) and salvage therapy (P<0.001) by Multivariate Cox regression analysis after PSM. Taussky et al. (19) reported that the treatment method cannot be used to predict biochemical recurrence among patients with low- and intermediate-risk prostate cancer who undergo BT and RP. Conversely, Zhou et al. (25) reported a prostate volume ≤35 mL, an initial PSA <10 ng/mL, a clinical stage of T2b–T2c, a Gleason score of 6 or 7, and an intermediate-risk stratification were predictors of better bPFS in the BT group comparing to the RP group. However, in the previous study PSM was not used. Thus, while age is an independent predictor of postoperative biochemical recurrence (19), there was a significant difference in the age between the BT group and the RP group in the previous study.
The present study had some limitations. First, the patients were recruited from a single hospital; thus, information bias cannot be ruled out. Second, due to limitations in data collection, the sample size of this study was small, and the proportion of patients with low-risk prostate cancer was extremely low (5.2%), which may compromise the validity of the data. Third, patients with low- and intermediate-risk prostate cancer also underwent endocrine therapy. According to modern guidelines, patients with low-risk prostate cancer should be treated solely with BT and patients with intermediate- and high-risk prostate cancer should be treated with combined therapies, such as external radiotherapy and endocrine therapy (3,13). Thus, prospective, large-sample, randomized controlled studies with active surveillance need to be conducted to clarify the clinical efficacy of BT for patients with localized prostate cancer and its associated factors.
Conclusions
In conclusion, in matched-pair analyses, BT and RP offered similar bPFS and CSS in the localized prostate cancer. Stage T3 prostate cancer who undergo BT was associated with worse biochemical failure and was the only variable significantly predictive of biochemical recurrence. All these statements above need to be tested and verified in a well-designed, prospective randomized controlled studies.
Acknowledgments
Funding: This research was supported by Shanghai Municipal Key Clinical Specialty (No. shslczdzk02801).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-755/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-755/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-755/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Wilt TJ, Ullman KE, Linskens EJ, et al. Therapies for Clinically Localized Prostate Cancer: A Comparative Effectiveness Review. J Urol 2021;205:967-76. [Crossref] [PubMed]
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021;79:243-62. [Crossref] [PubMed]
- Ito K, Saito S, Yorozu A, et al. Nationwide Japanese Prostate Cancer Outcome Study of Permanent Iodine-125 Seed Implantation (J-POPS): first analysis on survival. Int J Clin Oncol 2018;23:1148-59. [Crossref] [PubMed]
- Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol 2018;41:409-15. [Crossref] [PubMed]
- Yorozu A, Kuroiwa N, Takahashi A, et al. Permanent prostate brachytherapy with or without supplemental external beam radiotherapy as practiced in Japan: outcomes of 1300 patients. Brachytherapy 2015;14:111-7. [Crossref] [PubMed]
- Taira AV, Merrick GS, Butler WM, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:1336-42. [Crossref] [PubMed]
- Zhang P, Qian B, Shi J, et al. Radical prostatectomy versus brachytherapy for clinically localized prostate cancer on oncological and functional outcomes: a meta-analysis. Transl Androl Urol 2020;9:332-43. [Crossref] [PubMed]
- Ohashi T, Yorozu A, Saito S, et al. Urinary and Rectal Toxicity Profiles After Permanent Iodine-125 Implant Brachytherapy in Japanese Men: Nationwide J-POPS Multi-institutional Prospective Cohort Study. Int J Radiat Oncol Biol Phys 2015;93:141-9. [Crossref] [PubMed]
- Costello AJ. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat Rev Urol 2020;17:177-88. [Crossref] [PubMed]
- Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol 2012;187:1259-65. [Crossref] [PubMed]
- Cooperberg MR, Vickers AJ, Broering JM, et al. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer 2010;116:5226-34. [Crossref] [PubMed]
- Hoffman KE, Penson DF, Zhao Z, et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020;323:149-63. [Crossref] [PubMed]
- Tanaka N, Asakawa I, Hasegawa M, et al. Low-dose-rate brachytherapy for prostate cancer: A 15-year experience in Japan. Int J Urol 2020;27:17-23. [Crossref] [PubMed]
- Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. [Crossref] [PubMed]
- Reese AC, Pierorazio PM, Han M, et al. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology 2012;80:1075-9. [Crossref] [PubMed]
- Dariane C, Taussky D, Delouya G, et al. Validation of the new STAR-CAP prognostic group staging system in prostate cancer patients treated with radiation therapy. World J Urol 2021;39:4127-33. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Taussky D, Ouellet V, Delouya G, et al. A comparative study of radical prostatectomy and permanent seed brachytherapy for low- and intermediate-risk prostate cancer. Can Urol Assoc J 2016;10:246-50. [Crossref] [PubMed]
- Guan Y, Xiong H, Feng Y, et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis 2020;23:220-31. [Crossref] [PubMed]
- Pisano C, Tucci M, DI, Stefano RF, et al. Prognostic role of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with metastatic castration resistant prostate cancer treated with abiraterone or enzalutamide. Minerva Urol Nephrol 2021;73:803-14. [PubMed]
- Guo J, Fang J, Huang X, et al. Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: A meta-analysis of results from multivariate analysis. Int J Surg 2018;60:216-23. [Crossref] [PubMed]
- Tang L, Li X, Wang B, et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Localized and Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0153981. [Crossref] [PubMed]
- Cozzi G, Musi G, Bianchi R, et al. Meta-analysis of studies comparing oncologic outcomes of radical prostatectomy and brachytherapy for localized prostate cancer. Ther Adv Urol 2017;9:241-50. [Crossref] [PubMed]
- Zhou Z, Yan W, Zhou Y, et al. (125)I low-dose-rate prostate brachytherapy and radical prostatectomy in patients with prostate cancer. Oncol Lett 2019;18:72-80. [PubMed]
- Hayashi N, Osaka K, Muraoka K, et al. Outcomes of treatment for localized prostate cancer in a single institution: comparison of radical prostatectomy and radiation therapy by propensity score matching analysis. World J Urol 2020;38:2477-84. [Crossref] [PubMed]
- Urabe F, Miki K, Kimura T, et al. Long-term outcomes of radical prostatectomy versus low-dose-rate brachytherapy in patients with intermediate-risk prostate cancer: Propensity score matched comparison. Prostate 2023;83:135-41. [Crossref] [PubMed]
- Goy BW, Burchette R, Soper MS, et al. Ten-Year Treatment Outcomes of Radical Prostatectomy Vs External Beam Radiation Therapy Vs Brachytherapy for 1503 Patients With Intermediate-risk Prostate Cancer. Urology 2020;136:180-9. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


