
Influencing factors for mortality in prostate cancer patients with T1 and T2 stage: a retrospective cohort study
Highlight box
Key findings
• Age of diagnosis, race, marital status, therapeutic method, socioeconomic status, American Joint Committee on Cancer stage, level of prostate-specific antigen, and Gleason score were associated with the risk of localized prostate cancer mortality.
What is known and what is new?
• The Cox model was used to identify the influencing factors.
• A competitive risk model was adopted to identify the influencing factors.
What is the implication, and what should change now?
• The results may remind urologists to pay attention to patients with the above characteristics, and perform early interventions and develop personalized diagnostic and treatment programs for these patients.
Introduction
Prostate cancer (PCa) is the second most common cancer worldwide. It is the fifth leading cause of cancer-related deaths among men globally (1,2), and the second leading cause of cancer-related deaths among men in the United States (3). It is one of the most common malignant tumors in the male genitourinary system, with most patients diagnosed with localized tumor (4). Globally, the mortality rate associated with localized PCa has shown an uptrend in the past decade (5). Since early symptoms are often mild and the age at diagnosis is older, the possibility of disease development leading to poor prognosis increases (6). Therefore, it is essential to focus on the risk factors that affect the mortality of patients diagnosed with localized PCa, which could help clinicians develop personalized diagnostic and treatment programs.
Previous studies have mainly investigated the risk factors of death among PCa patients and its prevention (7,8). In the study by Perdana et al., they pointed out that older men were associated with a high risk PCa and had lower overall survival (9). Additionally, race and clinical stages (10) were also regarded as risk factors for PCa death. However, to date, there have been few studies exploring the influencing factors of death for localized PCa patients. In recent years, Cox model and competitive risk models have been gradually applied in the prediction of mortality for different cancers (11,12). Furthermore, it was reported that compared with the Cox model, the Fine-Gray proportional model for competing risks provides a better estimation for the risk of the main outcome of benefit when one or more competing risks exist (13). In other words, compared with the traditional survival analysis method, using a competitive risk model to assess the risk factors affect the prognosis of localized PCa patients is more helpful in discovering the true influencing variables and more accurately identifies the relevant risk factors (13). In the study of Zhou et al., they only reported that tumor sizes were associated with localized PCa by Cox regression analysis (14). To our knowledge, there is a paucity of reports to predict the influencing factors of localized PCa-specific mortality (14).
Herein, we developed a competitive risk model to identify the influencing factors of localized PCa mortality based on the Surveillance, Epidemiology, and End Results (SEER) database. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-818/rc).
Methods
Study population
The data for analysis were obtained from the SEER database, which covered approximately 27.8% of the United States population from 18 regions (including Los Angeles, New Mexico, Greater Georgia, etc.) (15). In this cohort study, male patients with localized PCa whose age of pathological diagnosis was between 25–80 years old were selected from the SEER database between 2004 and 2016. Patients with T3 stage or T4 stage cancer; had unclear T, or N, or M stage; had unknown prostate-specific antigen (PSA) or Gleason score; were loss to follow-up or who did not survival for more than one month; did not undergo surgery nor radiotherapy treatment; had unknown marital status or residence status; or had chemotherapy and developed cancer metastasis were excluded from the study. After screening, a total of 135,310 patients with localized PCa were eligible for this study (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
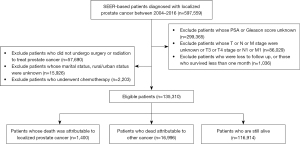
Outcomes and follow-up
Localized PCa was defined as clinical or pathological tumor stages T1 and T2 by the American Cancer Society (16). All included patients were divided into the following 3 groups according to the survival status of patients as of November 2018: died of localized PCa, died of other causes, and survived.
Data extraction
Patient demographic information, including age of diagnosis (<55 years, 55–70 years, >70 years), race (Caucasian, African American, American India/Alaska Native, Asian/Pacific Islander), socioeconomic status (SES), American Joint Committee on Cancer (AJCC) stage (T1, T2), marital status (married, divorced, separated, single or never married, unmarried or domestic partner, widowed), population-scale (≥1,000,000 people, 250,000 to 999,999 people, <250,000 people, 20,000 to 249,999 people, 2,500 to 19,999 people, <2,500 people), therapeutic method (surgery, radiotherapy), PSA, and Gleason score were collated from the SEER database. SES was considered as the county-level socioeconomics which was composed of the patient’s education level, family income level, and poverty level (17). The above three socioeconomic variables were equally weighted and added together to create the composite SES score, with scores ≤3 considered low SES, scores ranging from 4–10 considered middle SES, and scores ≥11 considered high SES (8).
Statistical analysis
Descriptive statistics was used to present basic demographic details of the included patients, and no-normal variables were showed by median and interquartile. The univariate Gray’s proportional model for competing risk was built to analyze the cumulative incidence of interest events and compare the differences among groups. Subsequently, the multivariate Fine-Gray proportional model for competing risk was used to analyze the statistically significant variables to screen out the competing bias to predict risk factors related to PCa mortality. The results are shown by hazard ratio (HR) and 95% confidence interval (CI). All statistical tests were two-sided, with statistical significance evaluated at the 0.05 alpha level and CI presented at the 95% level. Baseline information was analyzed using SPSS 20.0 software (version 20.0). A competing risk model was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline information of patients with localized PCa
A total of 135,310 patients were enrolled in this study, including 1,400 patients who died of localized PCa, 16,996 patients who died from other causes, and 116,914 patients who survived. The diagnostic age of 119,899 (88.61%) patients was ≥55 years. A total of 103,894 (76.78%) patients were married. Approximately 9.89% of patients had low SES, 67.26% had middle SES, and 22.84% had high SES. There were 54,598 (40.35%) patients with AJCC stage T1, and 80,712 (59.65%) patients with T2 stage. Moreover, 68,194 (50.40%) localized PCa patients chose surgery as their primary treatment, and 69,822 (51.60%) patients received radiotherapy. The detailed information is presented in Table 1.
Table 1
| Description | Values |
|---|---|
| Age of prostate cancer diagnosis, years, n (%) | |
| <55 | 15,411 (11.39) |
| 55–70 | 85,701 (63.34) |
| >70 | 34,198 (25.27) |
| Race/ethnicity, n (%) | |
| Caucasian | 106,632 (78.81) |
| African American | 22,026 (16.28) |
| Other (American India/Alaska Native, Asian/Pacific Islander) | 6,652 (4.92) |
| Marital status, n (%) | |
| Married | 103,894 (76.78) |
| Divorced | 10,129 (7.49) |
| Separated | 1,207 (0.89) |
| Single (never married) | 14,666 (10.84) |
| Unmarried or domestic partner | 295 (0.22) |
| Widowed | 5,119 (3.78) |
| Urban/rural residence status, n (%) | |
| Countries in metropolitan, ≥1,000,000 people | 81,575 (60.29) |
| Countries in metropolitan, 250,000 to 999,999 people | 10,328 (7.63) |
| Countries in metropolitan, <250,000 people | 28,347 (20.95) |
| Urban, 20,000 to 249,999 people | 5,438 (4.02) |
| Urban, 2,500 to 19,999 people | 7,869 (5.82) |
| Rural, <2,500 people | 1,753 (1.30) |
| SES | |
| ≤3 | 13,388 (9.89) |
| 4–10 | 91,011 (67.26) |
| ≥11 | 30,911 (22.84) |
| AJCC, n (%) | |
| T1 | 54,598 (40.35) |
| T2 | 80,712 (59.65) |
| Surgery, n (%) | |
| Yes | 68,194 (50.40) |
| No | 67,116 (49.60) |
| Radiotherapy, n (%) | |
| Yes | 69,822 (51.60) |
| No | 65,488 (48.40) |
| PSA, M (Q1, Q3) | 61.00 (46.00, 90.00) |
| Gleason score, Mean ± SD | 6.80±0.84 |
| Outcome, n (%) | |
| Died from other causes | 16,996 (12.56) |
| Died from localized PCa | 1,400 (1.03) |
| Alive | 116,914 (86.40) |
| Survival months, M (Q1, Q3) | 51.00 (31.00, 70.00) |
SES, socioeconomic status; AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen; PCa, prostate cancer.
An analysis of differences based on survival status
An analysis of the survival status showed statistical differences in the patient’s age (χ2=4,391.730, P<0.001), race (χ2=181.793, P<0.001), marital status (χ2=481.909, P<0.001), distribution of urban and rural residents (χ2=56.732, P<0.001), SES (χ2=29.498, P<0.001), AJCC tumor staging (χ2=946.193, P<0.001), surgery (χ2=1,233.536, P<0.001), radiotherapy (χ2=1,377.268, P<0.001), PSA (χ2=745.791, P<0.001), and Gleason score (F=1,129.662, P<0.001) among the 3 survival groups (Table 2).
Table 2
| Variances | Description (n=135,310) | ||||
|---|---|---|---|---|---|
| Died from other causes (n=16,996) | Died from localized PCa (n=1,400) | Alive (n=116,914) | Statistic | P | |
| Age of prostate cancer diagnoses (years), N (%) | χ2=4,391.730 | <0.001 | |||
| <55 | 760 (4.47) | 59 (4.21) | 14,592 (12.48) | ||
| 55–70 | 8,837 (51.99) | 630 (45.00) | 76,234 (65.21) | ||
| >70 | 7,399 (43.53) | 711 (50.79) | 26,088 (22.31) | ||
| Race/ethnicity, N (%) | χ2=181.793 | <0.001 | |||
| Caucasian | 14,003 (82.39) | 1,093 (78.07) | 91,536 (78.29) | ||
| African American | 2,409 (14.17) | 258 (18.43) | 19,359 (16.56) | ||
| Other (American India/Alaska Native, Asian/Pacific Islander) | 584 (3.44) | 49 (3.50) | 6,019 (5.15) | ||
| Marital status, N (%) | χ2=481.909 | <0.001 | |||
| Married | 12,610 (74.19) | 936 (66.86) | 90,348 (77.28) | ||
| Divorced | 1,431 (8.42) | 140 (10.00) | 8,558 (7.32) | ||
| Separated | 174 (1.02) | 16 (1.14) | 1,017 (0.87) | ||
| Single (never married) | 1,693 (9.96) | 184 (13.14) | 12,789 (10.94) | ||
| Unmarried or domestic partner | 14 (0.08) | 2 (0.14) | 279 (0.24) | ||
| Widowed | 1,074 (6.32) | 122 (8.71) | 3,923 (3.36) | ||
| Urban/rural residence status, N (%) | c2=56.732 | <0.001 | |||
| Countries in metropolitan, ≥1,000,000 people | 9,977 (58.70) | 816 (58.29) | 70,782 (60.54) | ||
| Countries in metropolitan, 250,000 to 999,999 people | 1,356 (7.98) | 118 (8.43) | 8,854 (7.57) | ||
| Countries in metropolitan, <250,000 people | 3,553 (20.90) | 281 (20.07) | 24,513 (20.97) | ||
| Urban, 20,000 to 249,999 people | 737 (4.34) | 69 (4.93) | 4,632 (3.96) | ||
| Urban, 2,500 to 19,999 people | 1,105 (6.50) | 88 (6.29) | 6,676 (5.71) | ||
| Rural, <2,500 people | 268 (1.58) | 28 (2.00) | 1,457 (1.25) | ||
| SES, N (%) | χ2=29.498 | <0.001 | |||
| ≤3 | 1,785 (10.50) | 172 (12.29) | 11,431 (9.78) | ||
| 4–10 | 11,441 (67.32) | 961 (68.64) | 78,609 (67.24) | ||
| ≥11 | 3,770 (22.18) | 267 (19.07) | 26,874 (22.99) | ||
| AJCC, N (%) | c2=946.193 | <0.001 | |||
| T1 | 8,586 (50.52) | 737 (52.64) | 45,275 (38.73) | ||
| T2 | 8,410 (49.48) | 663 (47.36) | 71,639 (61.27) | ||
| Surgery, N (%) | χ2=1,233.536 | <0.001 | |||
| Yes | 6,637 (39.05) | 445 (31.79) | 61,112 (52.27) | ||
| No | 10,359 (60.95) | 955 (68.21) | 55,802 (47.73) | ||
| Radiotherapy, N (%) | χ2=1,377.268 | <0.001 | |||
| Yes | 10,776 (63.40) | 1,007 (71.93) | 58,029 (49.63) | ||
| No | 6,220 (36.60) | 393 (28.07) | 58,885 (50.37) | ||
| PSA, M (Q1, Q3) | 66.00 (48.00,101.00) | 88.50 (57.00,175.50) | 60.00 (46.00,88.00) | χ2=745.791 | <0.001 |
| Gleason score, Mean ± SD | 6.93±0.92 | 7.71±1.18 | 6.77±0.81 | F=1,129.662 | <0.001 |
PCa, prostate cancer; SES, socioeconomic status; AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen.
Univariate Fine-Gray test
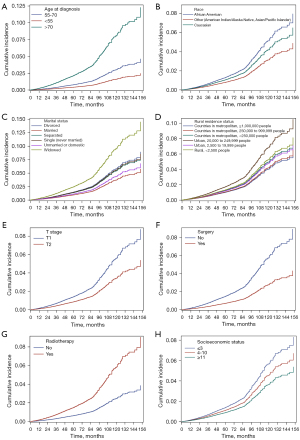
The risk factors that had a significant effect on the mortality of localized PCa in the Fine-Gray model were age at diagnosis, race, marital status, residence status, SES, therapeutic method, PSA, and Gleason score (P<0.05). It clearly showed that the HR of localized PCa increased with the higher older diagnostic age and higher Gleason score. The detailed information is listed in Table 3. The cumulative incidence of different age at diagnosis, different race, different marital status, different residence status, SES, different T stage, surgery and radiotherapy method are shown in Figure 2A-2H.
Table 3
| Variances | Univariable Fine-Gray test | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Age of prostate cancer diagnoses (years) | |||
| <55 | Ref | ||
| 55–70 | 1.919 | (1.470, 2.505) | <0.001 |
| >70 | 5.270 | (4.040, 6.873) | <0.001 |
| Race/ethnicity | |||
| Caucasian | Ref | ||
| African American | 1.228 | (1.072, 1.406) | 0.003 |
| Other (American India/Alaska Native, Asian/Pacific Islander) | 0.782 | (0.587, 1.041) | 0.092 |
| Marital status | |||
| Married | Ref | ||
| Divorced | 1.559 | (1.305, 1.862) | <0.001 |
| Separated | 1.465 | (0.893, 2.404) | 0.131 |
| Single (never married) | 1.496 | (1.277, 1.752) | <0.001 |
| Unmarried or domestic partner | 1.191 | (0.297, 4.785) | 0.805 |
| Widowed | 2.629 | (2.177, 3.175) | <0.001 |
| Urban/rural residence status | |||
| Countries in metropolitan, ≥1,000,000 people | Ref | ||
| Countries in metropolitan, 250,000 to 999,999 people | 0.953 | (0.832, 1.091) | 0.483 |
| Countries in metropolitan, <250,000 people | 1.178 | (0.971, 1.429) | 0.096 |
| Urban, 20,000 to 249,999 people | 1.136 | (0.912, 1.416) | 0.254 |
| Urban, 2,500 to 19,999 people | 1.255 | (0.982, 1.604) | 0.070 |
| Rural, <2,500 people | 1.643 | (1.128, 2.392) | 0.010 |
| SES | |||
| ≤3 | Ref | ||
| 4–10 | 0.800 | (0.680, 0.940) | 0.007 |
| ≥11 | 0.636 | (0.525, 0.770) | <0.001 |
| AJCC | |||
| T1 | Ref | ||
| T2 | 0.603 | (0.543, 0.670) | <0.001 |
| Surgery | |||
| No | Ref | ||
| Yes | 0.468 | (0.418, 0.524) | <0.001 |
| Radiotherapy | |||
| No | Ref | ||
| Yes | 2.435 | (2.165, 2.738) | <0.001 |
| PSA | 1.004 | (1.003, 1.004) | <0.001 |
| Gleason score | 2.601 | (2.480, 2.728) | <0.001 |
HR, hazard ratio; CI, confidence interval; SES, socioeconomic status; AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen.
Multivariate Fine-Gray test
The results from the Fine-Gray model showed that patients whose diagnostic age were 55–70 years (HR: 1.473, 95% CI: 1.124 to 1.930) or >70 years (HR: 2.528, 95% CI: 1.901 to 3.362) had a significantly higher risk of mortality compared to patients whose age at diagnosis was less 55 years. Taking “Caucasian” race as a reference, the “African American” race was considered a risk factor for localized PCa death (HR: 1.137, 95% CI: 0.985 to 1.312), and people with other races were associated with a decreased risk of death (HR: 0.653, 95% CI: 0.490 to 0.870). The risk of death for localized PCa patients who were divorced, single, or widowed increased by 0.433 times (HR: 1.433, 95% CI: 1.197 to 1.717), 0.463 times (HR: 1.463, 95% CI: 1.244 to 1.719), and 0.485 times (HR: 1.485, 95% CI: 1.222 to 1.804), respectively, compared to married people. The risk of death for patients who had undergone radiotherapy was increased by 0.500 times compared to those who had not radiotherapy (HR: 1.500, 95% CI: 1.119 to 2.011). Using the low SES (score ≤3) as a reference, localized PCa patients who had middle SES (score 4–10; HR: 0.799, 95% CI: 0.664 to 0.961) and those with high SES (score ≥11; HR: 0.670, 95% CI: 0.534 to 0.839) were associated with a decreased risk of death, which indicated that patients with higher SES scores had a better outcome. Furthermore, the results also indicated that the higher levels of PSA (HR: 1.002, 95% CI: 1.002 to 1.002) and higher Gleason score (HR: 2.226, 95% CI: 2.108 to 2.350) were associated with higher specific-death risk for localized PCa patients. The detailed data is listed in Table 4.
Table 4
| Variances | Multivariable Gray-test | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Age of prostate cancer diagnoses (years) | |||
| <55 | Ref | ||
| 55–70 | 1.473 | (1.124, 1.930) | 0.005 |
| >70 | 2.528 | (1.901, 3.362) | <0.0001 |
| Race/ethnicity | |||
| Caucasian | Ref | ||
| African American | 1.137 | (0.985, 1.312) | 0.079 |
| Other (American India/Alaska Native, Asian/Pacific Islander) | 0.653 | (0.490, 0.870) | 0.004 |
| Marital status | |||
| Married | Ref | ||
| Divorced | 1.433 | (1.197, 1.717) | <0.0001 |
| Separated | 1.317 | (0.796, 2.179) | 0.284 |
| Single (never married) | 1.463 | (1.244, 1.719) | <0.0001 |
| Unmarried or domestic partner | 1.461 | (0.360, 5.929) | 0.596 |
| Widowed | 1.485 | (1.222, 1.804) | <0.0001 |
| Urban/rural residence status | |||
| Countries in metropolitan, ≥1,000,000 people | Ref | ||
| Countries in metropolitan, 250,000 to 999,999 people | 0.899 | (0.782, 1.033) | 0.134 |
| Countries in metropolitan, <250,000 people | 0.976 | (0.793, 1.201) | 0.819 |
| Urban, 20,000 to 249,999 people | 1.051 | (0.816, 1.352) | 0.702 |
| Urban, 2,500 to 19,999 people | 0.887 | (0.697, 1.129) | 0.331 |
| Rural, <2,500 people | 1.163 | (0.785, 1.724) | 0.451 |
| SES | |||
| ≤3 | Ref | ||
| 4–10 | 0.799 | (0.664, 0.961) | 0.017 |
| ≥11 | 0.670 | (0.534, 0.839) | 0.001 |
| AJCC | |||
| T1 | Ref | ||
| T2 | 0.820 | (0.715, 0.940) | 0.004 |
| Surgery | |||
| No | Ref | ||
| Yes | 1.297 | (0.996, 1.690) | 0.054 |
| Radiotherapy | |||
| No | Ref | ||
| Yes | 1.500 | (1.119, 2.011) | 0.007 |
| PSA | 1.002 | (1.002, 1.002) | <0.0001 |
| Gleason score | 2.226 | (2.108, 2.350) | <0.0001 |
HR, hazard ratio; CI, confidence interval; SES, socioeconomic status; AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen.
Discussion
In this study, we developed a Fine-Gray proportional model to assess the influencing factors of localized PCa mortality based on a large sample size. The results of competitive risk modeling showed that age of diagnosis, race, marital status, SES, AJCC stage, therapeutic method, PSA, and Gleason score were influencing factors for death in localized PCa patients.
It is well known that Cox model commonly considers only a single endpoint. Nevertheless, when there are competitive risk events, the endpoint analysis method might cause bias for the estimated probabilities of endpoint events (18). In the present study, we adopted a competitive risk model which considered not only deaths from localized PCa, but also deaths from other causes, to determine the risk factors that influenced mortality in localized PCa patients. We speculate that the Cox model also may misestimate the direction between risk factors and outcome correlation. The competitive risk model was more beneficial to accurately determine the risk factors that influence death in localized PCa patients.
Our results showed that age at diagnosis was an influencing factor for mortality in patients with localized PCa. A similar result was reported in a previous competing risk regression analyze on mortality data (19,20). An increased risk of it with an elevated age at the time of diagnosis for older adults. The higher risk of mortality might be influenced by poor health status and less early detection. Moon et al. investigated the influence of marital intimacy on localized PCa patients and reached a conclusion that mortality was higher for patients with marriage issues than those with a happy marriage (21), because patients in a good marriage received higher quality life-care and less mental pressure, which could encourage them to receive treatment positively and effectively (21,22). This latter finding was consistent with our research showing that the risk of death among divorced, single, and widowed patients with localized PCa was increased compared with married people.
The results herein also suggested that African Americans had a poorer prognosis than Caucasians. Some studies have noted that the racial disparities in accessing health insurance and health care may be an important factor for survival in the United States (23,24). Whites tend to have greater access to health insurance and treatment, and more frequent early screening can help improve outcomes (23). As to therapeutic method, the risk of death for people who underwent radiotherapy was significantly increased than those without radiotherapy, which maybe because these patients were at higher risk of dying from cancer-specific mortality (worst stage in PCa) or other-cause mortality (localized PCa patients who cannot undergo surgery because they are too old or too frail, or have too many comorbidities) (25,26). This was also consistent with research by Antonelli et al. showing that localized PCa patients who were younger, married, working, and had better physical and sexual function were more likely to undergo surgery than radiotherapy (27). More comprehensive and in-depth research related to this are warranted in the future. A previous study indicated that the PSA levels and Gleason scores are powerful predictors of PCa prognosis (20), and its prognostic role in localized PCa should not be overlooked. Indeed, our study demonstrated that higher PSA levels and Gleason scores in localized PCa patients were associated with an increased risk of death. Additionally, T2 stage patients had better prognosis compared to T1 patients, and this may be due to the heterogeneity of PCa, with some T1 patients showing a worse outcome than those with T2 stage (28). In the present study, patients with higher SES scores were associated with a better outcome, which was consistent with the result of previous studies (20,29). A higher SES score suggests a better socio-economic status. In general, localized PCa patients with lower SES have a higher comorbidity burden and poorer lifestyle (such as smoking, lack of exercise, obesity), which might affect the outcome of the patients (20). Furthermore, high SES may provide timely and high-quality cancer care (30).
In summary, this study identified some risk factors related localized PCa mortality. PCa patients who were aged above 55 years at the time of diagnosis, were African American or other (American India/Alaska Native, Asian/Pacific Islander), were divorced, single, and widowed, had lower SES, had T2 stage, underwent radiotherapy, and had a higher PSA level and Gleason score, were associated with an increased risk of mortality. These results may remind urologists to pay attention to patients with the above characteristics, and conduct early interventions and develop personalized diagnostic and treatment programs for such patients.
This investigation used a large sample size and applied the Fine-Gray proportional model for competing risks to predict the influencing factors of mortality in patients with localized PCa. However, there were some limitations. First, this study was conducted based on the SEER database, which might contain information bias, such as potential coding errors. Second, the SEER database did not provide detailed information linked to mortality, such as family cancer history, life-style, causes of death, and comorbidities. Moreover, detailed cancer-related parameters, such as PSA or Gleason score, have only been available from 2004 onwards. Finally, we also were unable to account for selection biases associated with primary treatment assignment. Stricter selection criteria are needed in future studies.
Conclusions
The present study was based on a large sample size in the SEER database, and through competitive risk modeling, the factors influencing mortality in patients with localized PCa were identified. These findings may provide a reference for early interventions of localized PCa patients and help clinicians to develop personalized diagnostic and treatment programs for these patients.
Acknowledgments
Funding: This study was supported by the Guangdong Province Science and Technology Special Fund Project (No. 210713116901740).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-818/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-818/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Tsujino T, Komura K, Inamoto T, et al. CRISPR Screen Contributes to Novel Target Discovery in Prostate Cancer. Int J Mol Sci 2021;22:12777. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Xu H, Zhu Y, Dai B, et al. National Comprehensive Cancer Network (NCCN) risk classification in predicting biochemical recurrence after radical prostatectomy: a retrospective cohort study in Chinese prostate cancer patients. Asian J Androl 2018;20:551-4. [Crossref] [PubMed]
- Achard V, Panje CM, Engeler D, et al. Localized and Locally Advanced Prostate Cancer: Treatment Options. Oncology 2021;99:413-21. [Crossref] [PubMed]
- Beebe-Dimmer JL, Ruterbusch JJ, Cooney KA, et al. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: A SEER-Medicare investigation. Cancer Med 2019;8:3325-35. [Crossref] [PubMed]
- Gandaglia G, Leni R, Bray F, et al. Epidemiology and Prevention of Prostate Cancer. Eur Urol Oncol 2021;4:877-92. [Crossref] [PubMed]
- Liu D, Kuai Y, Zhu R, et al. Prognosis of prostate cancer and bone metastasis pattern of patients: a SEER-based study and a local hospital based study from China. Sci Rep 2020;10:9104. [Crossref] [PubMed]
- Perdana NR, Mochtar CA, Umbas R, et al. The Risk Factors of Prostate Cancer and Its Prevention: A Literature Review. Acta Med Indones 2016;48:228-38. [PubMed]
- Siegel DA, O'Neil ME, Richards TB, et al. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep 2020;69:1473-80. [Crossref] [PubMed]
- Song Y, Tian S, Zhang P, et al. Construction and Validation of a Novel Ferroptosis-Related Prognostic Model for Acute Myeloid Leukemia. Front Genet 2022;12:708699. [Crossref] [PubMed]
- Wang J, Zhanghuang C, Tan X, et al. Development and Validation of a Competitive Risk Model in Elderly Patients With Chromophobe Cell Renal Carcinoma: A Population-Based Study. Front Public Health 2022;10:840525. [Crossref] [PubMed]
- Nolan EK, Chen HY. A comparison of the Cox model to the Fine-Gray model for survival analyses of re-fracture rates. Arch Osteoporos 2020;15:86. [Crossref] [PubMed]
- Zhou Z, Yue F, Jin L, et al. Characteristics and risk differences of different tumor size on localized prostate cancer: A retrospective cohort study in the SEER database. Cancer Med 2021;10:2763-73. [Crossref] [PubMed]
- Zhang AC, Rasul R, Golden A, et al. Incidence and mortality trends of metastatic prostate cancer: Surveillance, Epidemiology, and End Results database analysis. Can Urol Assoc J 2021;15:E637-43. [Crossref] [PubMed]
- Borley N, Feneley MR. Prostate cancer: diagnosis and staging. Asian J Androl 2009;11:74-80. [Crossref] [PubMed]
- Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer 2006;106:1276-85. [Crossref] [PubMed]
- Wu W, Yang J, Li D, et al. Competitive Risk Analysis of Prognosis in Patients With Cecum Cancer: A Population-Based Study. Cancer Control 2021;28:1073274821989316. [Crossref] [PubMed]
- Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol 2011;29:235-41. [Crossref] [PubMed]
- He H, Han D, Xu F, et al. How socioeconomic and clinical factors impact prostate-cancer-specific and other-cause mortality in prostate cancer stratified by clinical stage: Competing-risk analysis. Prostate 2022;82:415-24. [Crossref] [PubMed]
- Moon S, Jin J, Cheon SH, et al. The influence of marital intimacy on urinary and sexual symptom experience among patients with prostate cancer: a cross-sectional study. Contemp Nurse 2018;54:171-81. [Crossref] [PubMed]
- Gidron Y, De Couck M, Reynders T, et al. Stronger Correlations between Neurophysiological and Peripheral Disease Biomarkers Predict Better Prognosis in Two Severe Diseases. J Clin Med 2019;9:26. [Crossref] [PubMed]
- Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. Eur Urol 2017;71:195-201. [Crossref] [PubMed]
- Bernstein AN, Talwar R, Handorf E, et al. Assessment of Prostate Cancer Treatment Among Black and White Patients During the COVID-19 Pandemic. JAMA Oncol 2021;7:1467-73. [Crossref] [PubMed]
- Sooriakumaran P, Nyberg T, Akre O, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ 2014;348:g1502. [Crossref] [PubMed]
- Robinson D, Garmo H, Lissbrant IF, et al. Prostate Cancer Death After Radiotherapy or Radical Prostatectomy: A Nationwide Population-based Observational Study. Eur Urol 2018;73:502-11. [Crossref] [PubMed]
- Antonelli A, Palumbo C, Noale M, et al. Overview of potential determinants of radical prostatectomy versus radiation therapy in management of clinically localized prostate cancer: results from an Italian, prospective, observational study (the Pros-IT CNR study). Minerva Urol Nefrol 2020;72:595-604. [Crossref] [PubMed]
- Tolkach Y, Kristiansen G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018;85:108-16. [Crossref] [PubMed]
- Khadhra HB, Saint F, Trecherel E, et al. Relationship between socioeconomic status and prostate cancer (incidence, aggressiveness, treatment with curative intent, and mortality): a spatial analysis using population-based cancer registry data. Rev Epidemiol Sante Publique 2021;69:329-36. [Crossref] [PubMed]
- Seikkula HA, Kaipia AJ, Ryynänen H, et al. The impact of socioeconomic status on stage specific prostate cancer survival and mortality before and after introduction of PSA test in Finland. Int J Cancer 2018;142:891-8. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


