
Treatment-related neuroendocrine prostate cancer managed with partial stereotactic ablative radiotherapy (P-SABR) for long-term survival: a case series
Highlight box
Key findings
• P-SABR radiotherapy is safe and effective in the treatment of patients with prostate bulky tumor and may prolong the survival of these patients.
What is known and what is new?
• It is known that the amount of treatment-related neuroendocrine carcinoma of the prostate (t-NEPC) increases after hormonal therapy, especially novel androgen receptor pathway inhibitors (ARPIs);
• P-SABR is a radiotherapy regimen that is used in a SABR boost (such as 6 Gy × 4 f, 8 Gy × 3 f) prior to conventional radiotherapy to enhance the tumor biological effective dose (BED) without increasing the dose to organs at risk.
What is the implication, and what should change now?
• Prostate cancer with a large mass and neuroendocrine differentiation should be treated with radiotherapy as early as possible, even in patients with high-volume metastases. Radiotherapy can improve patients’ lower urinary tract symptoms, improve local control, and prolong the effective time of systemic therapy.
Introduction
Primary neuroendocrine prostate cancer (NEPC) rarely arises de novo. However, treatment-related neuroendocrine prostate cancer (t-NEPC) is relatively common after hormonal therapy, especially novel androgen receptor pathway inhibitors (ARPIs; abiraterone, enzalutamide, apalutamide, etc.), and has an incidence of 17–30% (1,2).
T-NEPC has the morphological characteristics of small cell carcinoma with low or no expression of androgen receptor (AR), so it is prone to extensive visceral metastasis, osteolytic metastasis, and large mass even with a very low prostate-specific antigen (PSA) level. Moreover, the effective time of hormonal therapy is short, and the prognosis is extremely poor. At present, there is no standard treatment model for t-NEPC. Although tumors are initially responsive to platinum-based chemotherapy, the drugs are only effective for a short time. Immunotherapy has been added in recent years, but the improvement is limited, and thus whether local treatment can prolong survival is of great concern.
With the development of radiotherapy (RT), stereotactic ablative radiotherapy (SABR) has improved the hope for achieving local control (3). To increase the efficacy and safety of RT, a mode of partial SABR (P-SABR) can be used. P-SABR is a RT regimen that is used for bulky tumors in a SABR boost [such as 6 Gy × 4 fractions (f), 8 Gy × 3 f] prior to conventional RT to enhance the tumor biological effective dose (BED) without increasing the dose to organs at risk. The study (4) has found that SABR can release more tumor-associated antigens (TAAs) after killing tumor cells, produce in situ vaccine effects, stimulate immune T-cell activation, and synergize with immunotherapy to kill tumor cells. Here, we report a single-center retrospective case series of patients with t-NEPC undergoing primary tumor P-SABR combined with systemic therapy. We present the following article in accordance with the AME Case Series reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-867/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of Peking University First Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Diagnosis stage
The patient was 74 years old at the time of diagnosis (August 2018). He visited a doctor due to increased nocturia, and PSA was 1,302 ng/mL. Abdomen and pelvis computed tomography (CT) showed a cauliflower-like mass of prostate 4.8 cm × 5.9 cm in size, with invasion into the bladder, seminal vesicles, and the end of right ureter; hydrops in the right kidney and right ureteral; and multiple pelvic and retroperitoneal lymph node metastases. Prostate biopsy showed Gleason score 4+4 acinar adenocarcinoma was seen in 12/12 cores. Bone scan showed metastases located at the thoracic 11 to the lumbar 1 vertebra and the right side of the lumbar 5 vertebra, the right 4th and 6th ribs, the left 5th rib, the left ilium, and the middle and upper segments of the left femur. The initial diagnosis was metastatic prostate cancer [cT4N1M1b, The American Joint Committee on Cancer (AJCC) eighth edition].
First-line treatment: hormonal therapy
The patient started androgen deprivation therapy (ADT) combined with AR antagonist bicalutamide in September 2018. The symptoms of nocturia and dysuria improved. Re-examination by CT and bone scans showed the prostate and metastatic lesions were significantly reduced, with a minimum prostate size of 3.1 cm × 4.1 cm. After 6 months of medication, bicalutamide was switched to abiraterone. The PSA was 0.19 ng/mL after 3 months of abiraterone (June 2019). Zoledronic acid was initiated to prevent bone adverse events.
Clinical progression and repeat biopsy: small cell neuroendocrine (NE) carcinoma
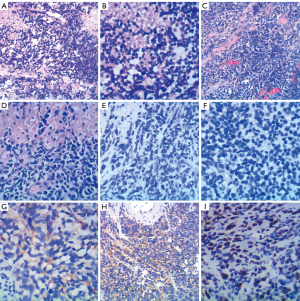
After 1 year of ADT, the patient began to experience aggravated local symptoms, such as urgency, frequent urination, and dysuria in October 2019. CT showed that the prostate tumor had increased from 3.1 cm × 4.1 cm (maximum dimensions) (June 2019) to 9.5 cm × 6.6 cm (December 2019) and invaded the entire bladder. Whole body osseous metastases had improved significantly. However, the PSA remained low (0.09 ng/mL). As the prostate tumor progressed rapidly under the low PSA, the pathological type of the primary tumor might have undergone transformation, and the patient received a repeat prostate biopsy. The repeat biopsy showed that the pathological morphology combined with immunohistochemistry (IHC) was consistent with small-cell NE carcinoma (Figure 1A,1B). The IHC result were the following: PSA (−), AR (−), ETS-related gene (ERG) (−), chromogranin A (CgA) (+), synaptophysin (Syn) (+), cluster of differentiation 56 (CD56) (+), and Ki-67 80%. The genetic testing showed 18 key gene mutations including STAG2, PIK3C2G, SPOP, TP53, RB1, HSP90AA1, AKT1, ERBB2, BRCA1, CDKN1B, DICE1, NFKBIA, CD79B, BRIP1, PRKARIA, NKX2-1, NF1, and SOX9. The tumor mutational burden (TMB) was 4 mutations/megabase, and the tumor was microsatellite stable (MSS) [the library was constructed with the target capture method, 520 genes were detected by next-generation sequencing (NGS), and they were sequenced by using an Illumina NextSeq 500 platform].
Second-line treatment: chemotherapy
The patient continued to receive ADT without abiraterone. Six cycles of chemotherapy (docetaxel + cisplatin) were performed from December 2019 to May 2020. After the first 4 cycles of chemotherapy, the patient’s symptoms of frequent urination and dysuria improved. During the last 2 cycles of chemotherapy, there was gradual worsening of symptoms, accompanied by edema. The most obvious symptom was frequent urination at a frequency of approximately once an hour. PSA remained stable (0.09 ng/mL) during chemotherapy.
Third-line treatment: RT + immunotherapy
The imaging examination after 6 cycles of chemotherapy showed the progression of prostate and bladder lesions, and the evaluation was progressive disease (PD). In July 2020, transurethral resection of bladder tumors and prostate tumors (TURBT and TURP, respectively) was performed. Cystoscopy showed a solid tumor extending from the prostate to the bladder neck and the right wall. The tumor was cotton wool-shaped and had a wide range. It was partially excised, revealing that the tumor reached the deep muscle layer. Pathological examination revealed small-cell NE carcinoma. IHC results were as follows: P504 (−), P63 (−), PSA (−), CgA (partial +), Syn (+), CD56 (+), Ki-67 (80%), P53 (partial+), vimentin (−), leukocyte common antigen (LCA) (−), S-100 (−), prostate-specific membrane antigen (PSMA) (−), AR (−), and programmed cell death 1 ligand 1 (PD-L1) (−) (22C3) (Figure 1C-1I). The PSA level was 0.354 ng/mL. There was no significant increase in NE-related tumor markers [neuron-specific enolase (NSE): 17.17 ng/mL; progastrin-releasing peptide (proGRP): 53.66 pg/mL].
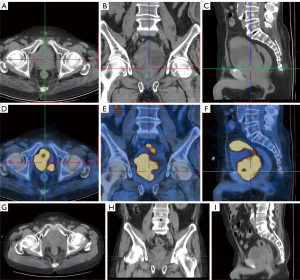
To alleviate the symptoms, RT was delivered. Positron emission tomography (PET)-CT was performed before RT. Because the pathology was small-cell NE carcinoma, 18F-fluorodeoxyglucose (18F-FDG) tracers was used. PET-CT showed that the hypermetabolic lesions were located in the bladder and prostate, no hypermetabolism was present in the lymph nodes or the whole-body bone, and a slight increase in the metabolism of the left ilium and thoracic 11th vertebral body, which was considered a posttreatment change (Figure 2A-2F).
RT was performed from August to September 2020. Treatment was delivered using daily image-guided radiation therapy-volumetric modulated arc therapy (IGRT-VMAT), with 6-MV X-ray. The target volumes included the planning gross tumor volume (PGTV) and the planning target volume (PTV).
The PGTV included the whole bladder and prostate, with the GTV expanded by 5 mm to form the PGTV; the PGTV margin near the intestine and rectum was repaired to the edge of the normal organ; a total dose of 60 Gy/3 Gy/20 f was given.
For the PTV, the clinical target volume (CTV) included the lymph node drainage area on the same plane of the GTV, with the CTV being expanded by 5 mm to form the PTV. A total dose of 46 Gy/2.3 Gy/20 f was given.
The GTV_boost was based on the high metabolic range of PET-CT; in delineating the GTV_boost, the target area was required to be 1 cm away from the rectum, urethra, and intestine, and the first 4 fractions were given at a dose of 24 Gy/6 Gy/4 f with a cumulative BED10 of 100.8 Gy.
The RT plan consisted of 2 courses: 4 f for the first course and 16 fractions for the second course.
The first course was as follows: GTV_boost 24 Gy/6 Gy/4 f, PGTV 12 Gy/3 Gy/4 f, and PTV 9.2 Gy/2.3 Gy/4f; second course was as follows: PGTV 48 Gy/3 Gy/16 f and PTV 36.8 Gy/2.3 Gy/16 f; the overlay plan was as follows: GTV_boost 72 Gy/20 f, PGTV 60 Gy/20 f, and PTV 46 Gy/20 f.
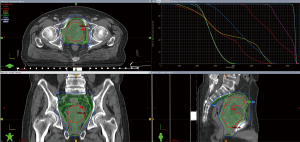
Figure 2G-2I shows the prostate tumor 2 years after RT. Figure 3 shows the radiation isodose line of the 2 courses added.
Concurrent chemotherapy and PD-L1 immunotherapy were used during RT. The chemotherapy regimen was cisplatin (60 mg Qw) during RT, and the immunotherapy regimen was durvalumab (1,500 mg Q3w). A total of 4 cycles of durvalumab were performed. The changes of T lymphocyte subsets before and during immunotherapy are shown in Table S1.
The patient completed RT safely, with grade 1 fatigue and grade 1 urinary frequency [Common Terminology Criteria for Adverse Events (CTCAE) 5.0]. After 6 months of RT, the frequency, urgency and pain during urination were relieved.
The pathological evaluation after RT was as follows: on the last day of RT, cystoscopy showed floccules in the bladder, diffuse thickening of the bladder wall, and deep biopsy of the anterior wall tissue. No tumor cells were found in pathology.
Fourth-line treatment: chemotherapy
CT was performed 2 months after the end of RT. It was found that the primary lesion was reduced and that the enhancement was lower than before. However, there were multiple metastatic lymph nodes out of the range of RT. The patient was thus started on chemotherapy in November 2020, with a regimen of etoposide + carboplatin + programmed cell death 1 (PD-1) for a total of 6 cycles. After re-examination, the metastatic lymph nodes in the abdomen and pelvis were smaller than before, and there were no new lesions. After 6 cycles of chemotherapy, oral etoposide (100 mg Qd; D1–14, Q3w) and PD-1 maintenance therapy were continued until January 2022, imaging examinations were performed regularly, and there were no new lesions.
Fifth-line treatment: rechallenge with novel ARPI
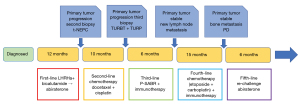
In February 2022, the PSA increased to 18 ng/mL, and the bone scan showed that the left sacroiliac joint metastases had progressed compared with 6 months ago. The patient began receiving abiraterone acetate. The last examination was taken in September 2022. CT showed that the primary tumor was still stable (Figure 2G-2I). Bone scan showed metastatic lesions in the left iliac bone and lumbar 2 vertebral body were larger than 4 months ago, and no new lesions were found. The patient was prepared for reradiation of the oligo-progressive site (left iliac bone and lumbar 5 vertebral body metastasis) but continued on abiraterone administration. As of this writing, the patient has been in remission for 3 years after the initial diagnosis of t-NEPC. Figure 4 shows the patient’s treatment flowchart. Table S2 shows treatments and changes in tumour markers.
Discussion
The incidence of small cell/NE carcinoma at initial diagnosis is less than 2% (1). However, with the increasing use of novel ARPIs, a notable phenomenon is that the development of t-NEPC rate has increased (2). The origin of t-NEPC cells is still controversial. The normal prostate gland contains NE cells randomly distributed among the basal cells and liminal cells (5). These cells secrete various peptide hormones, including CgA, enolase 2 (NSE), and calcitonin, and affect surrounding epithelial cells in a paracrine manner. However, in the normal prostate gland, these NE cells are in a quiescent state. Whether t-NEPC arises from the formation and clonal selection of existing NE cells or from the transformation of adenocarcinoma cells has been controversial (6,7). However, an increasing amount of evidence supports the notion that t-NEPC transdifferentiates from adenocarcinoma as a consequence of epithelial plasticity. Cancer cells transform from AR-driven adenocarcinoma to t-NEPC and escape from the AR pathway (2,8-11). In addition to hormonal therapy, t-NEPC can be induced by RT (12,13) and chemotherapy (14). T-NEPC usually exhibits NE differentiation-related genes, such as Syn, CgA, and NSE. Several gene mutations have been discovered in the past 20 years, including RB1 deletion, TP53 mutation, TMPRSS2-ERG fusion, and MYCN amplification (15-20). Table S3 lists the common NEPC biomarkers.
The characteristics of t-NEPC are as follows: (I) the morphological manifestations congruent with the characteristics of small cell carcinoma and (II) the molecular markers characterized by low or no expression of AR (21) and positive NE markers such as CgA, Syn, and CD56; (III) the clinical manifestations are prone to extensive visceral metastases, osteolytic metastases, large masses, low PSA levels relative to the tumor burden, a short effective time for hormonal therapy, and extremely poor prognosis (22,23). In one study, the time from diagnosis of prostate cancer to t-NEPC in 123 patients was 20 months, and the median survival after t-NEPC was only 7 months (24).
Docetaxel is the preferred chemotherapy regimen for prostate cancer (25). However, in small cell carcinoma or t-NEPC, the chemotherapy regimen is similar to that in small cell lung cancer, and the commonly used regimens are cisplatin or carboplatin combined with etoposide.
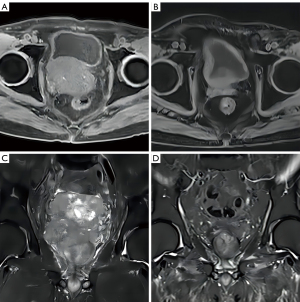
The patient described in this article (patient 1) had a large prostate mass at the time of the initial diagnosis. After 1 year of hormonal therapy, the PSA was low. After the primary tumor progressed, a secondary biopsy showed the transformation to small-cell NE carcinoma. IHC was negative for AR and positive for NE-related protein. Genetic testing results were consistent with typical NE features. The patient received a second-line chemotherapy regimen of docetaxel + cisplatin after the progression of t-NEPC. Local symptoms were aggravated in the 5th to 6th cycles. Chemotherapy controlled the local lesions for only 3 months, and after chemotherapy, the tumor had invaded to the entire bladder and prostate. The patient was treated with P-SABR RT regimen for large masses (26). Pathological complete remission (pCR) was achieved after RT. Table 1 shows 3 other t-NEPC patients who received P-SABR and obtained ideal tumor control. Figure 5 shows patient 3’s magnetic resonance imaging (MRI) before and after RT.
Table 1
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age at diagnosis | 75 years | 70 years | 80 years | 66 years |
| TNM stage | T4N1M1b | T3bN0M1b | T4N1M1b | T4N0M1b |
| Pathology | Acinar adenocarcinoma | Acinar adenocarcinoma | Acinar adenocarcinoma | Acinar adenocarcinoma |
| GS score | 4+5 | 4+5 | 5+4 | 5+4 |
| PSAmax at diagnosis (ng/mL) | >100 | 74.58 | 177 | >100 |
| Time to CRPC (months) | 12 | 8 | 18 | 3 |
| Time to RT (months) | 22 | 10 | 50 | 3 |
| Repeat biopsy | Small-cell carcinoma | Adenocarcinoma with neuroendocrine differentiation and ductal adenocarcinoma | Adenocarcinoma with neuroendocrine differentiation | Adenocarcinoma with neuroendocrine differentiation |
| Genetic testing | STAG2, PIK3C2G, SPOP, TP53, RB1, HSP90AA1, AKT1, ERBB2, BRCA1, CDKN1B, DICE1, NFKBIA, CD79B, BRIP1, PRKARIA, NKX2-1, NF1, and SOX9 | TP53 (c.782+2T>A, 36.90%), FOXA1 (p.F254_N256delinsY, 29.50%), BRCA2 del (exon2–exon24) | CDK12 (exon5 c.2275A>T p.R759, 13.86%), VHL (exon3 c.473T>G p.L158R, 1.92%), FOXA1 (c.763_774delGAGAACGGCTGC p.E255_C258del, 17.98%) | Not done |
| Tumor biomarker before RT | PSA 0.354 ng/mL, NSE 17.17 ng/mL, proGRP 53.66 pg/mL, CEA 0.87 ng/mL | PSA 0.01 ng/mL, NSE 91.27 ng/mL, CEA 1,288 ng/mL | PSA 35.8 ng/mL, NSE 20.26 ng/mL, CEA 3.56 ng/mL | PSA 0.128 ng/mL, NSE 15.64 ng/mL, CEA 117.5 ng/mL |
| Tumor size (LR × AP × SI) (cm3) | 6.2×8.1×6.2 | 9.7×11.2×11.7 | 7.3×6.2×9.7 | 4.8×5.1×5.1 |
| ADT before RT | LHRHa + Bicalutamide → abiraterone | LHRHa + abiraterone | LHRHa + Bicalutamide → abiraterone | LHRHa + apalutamide |
| Chemotherapy | Yes, docetaxel + cisplatin 6 cycles | Yes, docetaxel + cisplatin 6 cycles | No | No |
| RT schedule | Prostate tumor: GTV_boost 24 Gy/4 f, PTV 60 Gy/20 f, pelvic lymph node (PTV level) 40 Gy/20 f | Right iliac bone metastases: GTV_boost 27 Gy/3 f, GTV 60 Gy/20 f | Prostate tumor: GTV_boost 24 Gy/4 f, PTV 60 Gy/25 f, GTV 70 Gy/25 f, pelvic lymph node (PTV level) 47.5 Gy/25 f | Prostate tumor: GTV_boost 24 Gy/4 f, PTV 70 Gy/25 f, pelvic lymph node (PTV level) 47.5 Gy/25 f |
| RT side effect | Grade 1 fatigue, grade 1 urinary frequency | Grade 2 skin side effect | Grade 1 urinary frequency | Grade 2 urinary frequency |
| Time since RT (months) | 39 | 17 | 9 | 6 |
| Local control | Yes. pCR and stable until now | Yes. No tumor signal on MR 12 months after RT | Yes. No tumor signal on MR 6 months after RT | Yes. No tumor signal on MR 6 months after RT |
| Treatment after RT | LHRHa + etoposide + PD-1 → abiraterone | LHRHa + docetaxel + cisplatin → olaparib | LHRHa + olaparib + abiraterone (6 months), discontinued due to side effects | LHRHa + apalutamide |
ADT, androgen deprivation therapy; AP, anterior-posterior; CEA, carcinoembryonic antigen; CRPC, castration-resistant prostate cancer; f, fractions; GS, Gleason score; GTV, gross tumor volume; LHRHa, luteinizing hormone-releasing hormone agonist; LR, left-right; MR, magnetic resonance; NSE, neuron-specific enolase; pCR, pathological complete remission; PD-1, programmed cell death 1; PSA, prostate-specific antigen; PTV, planning target volume; RT, radiotherapy; SI, superior-inferior; TNM, tumor, node, metastasis.
The P-SABR regimen is primarily used for 3 main reasons:
- The radiation dose needs to be increased as much as possible. According to the recommendations of the National Comprehensive Cancer Network (NCCN) guidelines, the treatment should follow the principles for that of small cell lung cancer. In small cell lung cancer, large masses that progress after developing resistance to chemotherapy are also less sensitive to RT. Consequently, the dose of local RT needs to be increased.
- The safety of organs at risk (e.g., the rectum, bladder, urethra) should be protected. If traditional SABR is used, it will cause serious and irreversible late damage to the rectum, bladder and urethra. However, if a simultaneous integrated boost (SIB) is adopted, the BED cannot reach the level of P-SABR, and the local control rate may be low.
- SABR stimulates tumors to release neoantigens, stimulates T-cell activation, and cooperates with immunotherapy to make activated immune cells kill systemic tumor cells. Some studies have found that the killing of tumor cells by RT can activate an in-situ vaccine effect, upregulate major histocompatibility complex (MHC) molecules, promote T-cell epitope differentiation, and enhance the T-cell recognition of tumor cells to kill distant tumor cells and control distant metastasis. Therefore, SABR can have the dual effects of tumor reduction and immunity.
Therefore, patients exhibited good local control after RT. For patient 1, under the combined effect of local therapy and systemic therapy, the patient was still in a stable state more than 36 months after the diagnosis of t-NEPC. The overall RT doses of whole tumor reached 60 Gy/20 f and BED3 reached 120 Gy, which reached the radical dose of prostate cancer. For the boost area, higher doses are required due to bulky tumor of t-NEPC. At the same time, it is necessary to reduce the dose of normal bladder wall in order to protect the bladder function and reduce the toxicity. So based on our center’s previous experience with P-SABR, a SABR boost (away from normal bladder wall) is given at a dose of 24 Gy/6 Gy/4 f as the first RT course. Compared to conventional RT, P-SABR can significantly enhance the bioequivalent dose of bulky tumor while ensuring the tolerance of normal organs, especially to protect the functional organs. Therefore, P-SABR is recommended for both the primary tumor and metastasis of t-NEPC patients with large masses (especially larger than 5 cm).
From the perspective of the treatment experience of small cell lung cancer, NE tumors can be considered radiation-sensitive tumors. Therefore, prostate cancer with a large mass and NE differentiation should be treated with RT as early as possible, even in patients with high-volume metastases. RT can improve patients’ lower urinary tract symptoms, improve local control, and prolong the effective time of systemic therapy. In this case series, it was precisely because the primary lesion achieved a good effect through RT that subsequent systemic therapy could be performed.
Conclusions
This t-NEPC case and the other 3 patients listed in Table 1 achieved good results after P-SABR. These results indicate that P-SABR is safe and effective in the treatment of a large prostate mass and may prolong the survival of these patients.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 82271771), the National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital; grant No. 2022CR29), and the Beijing Xisike Clinical Oncology Research Foundation (grant No. Y-2019AZQN-0003).
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-867/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-867/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of Peking University First Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487-95. [Crossref] [PubMed]
- Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 2014;20:2846-50. [Crossref] [PubMed]
- Li GJ, Arifin AJ, Al-Shafa F, et al. A review of ongoing trials of stereotactic ablative radiotherapy for oligometastatic disease in the context of new consensus definitions. Ann Palliat Med 2021;10:6045-51. [Crossref] [PubMed]
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67:65-85. [Crossref] [PubMed]
- Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38:756-67. [Crossref] [PubMed]
- Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol 2014;4:60. [Crossref] [PubMed]
- Hu Y, Ippolito JE, Garabedian EM, et al. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem 2002;277:44462-74. [Crossref] [PubMed]
- Akamatsu S, Wyatt AW, Lin D, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep 2015;12:922-36. [Crossref] [PubMed]
- Lin D, Wyatt AW, Xue H, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res 2014;74:1272-83. [Crossref] [PubMed]
- Zou M, Toivanen R, Mitrofanova A, et al. Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov 2017;7:736-49. [Crossref] [PubMed]
- Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 2016;22:298-305. [Crossref] [PubMed]
- Deng X, Liu H, Huang J, et al. Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression. Cancer Res 2008;68:9663-70. [Crossref] [PubMed]
- Bonkhoff H. Factors implicated in radiation therapy failure and radiosensitization of prostate cancer. Prostate Cancer 2012;2012:593241. [Crossref] [PubMed]
- Berruti A, Dogliotti L, Mosca A, et al. Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer 2000;88:2590-7. [Crossref] [PubMed]
- Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 2009;15:67-78. [Crossref] [PubMed]
- Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 2013;15:1-10. [Crossref] [PubMed]
- Olivier M, Eeles R, Hollstein M, et al. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat 2002;19:607-14. [Crossref] [PubMed]
- Kaye FJ. RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene 2002;21:6908-14. [Crossref] [PubMed]
- Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 2014;20:890-903. [Crossref] [PubMed]
- Tsai H, Morais CL, Alshalalfa M, et al. Cyclin D1 Loss Distinguishes Prostatic Small-Cell Carcinoma from Most Prostatic Adenocarcinomas. Clin Cancer Res 2015;21:5619-29. [Crossref] [PubMed]
- Sargos P, Ferretti L, Gross-Goupil M, et al. Characterization of prostate neuroendocrine cancers and therapeutic management: a literature review. Prostate Cancer Prostatic Dis 2014;17:220-6. [Crossref] [PubMed]
- Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013;19:3621-30. [Crossref] [PubMed]
- Merkens L, Sailer V, Lessel D, et al. Aggressive variants of prostate cancer: underlying mechanisms of neuroendocrine transdifferentiation. J Exp Clin Cancer Res 2022;41:46. [Crossref] [PubMed]
- Wang HT, Yao YH, Li BG, et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol 2014;32:3383-90. [Crossref] [PubMed]
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20. [Crossref] [PubMed]
- Bai Y, Gao XS, Qin SB, et al. Partial stereotactic ablative boost radiotherapy in bulky non-small cell lung cancer: a retrospective study. Onco Targets Ther 2018;11:2571-9. [Crossref] [PubMed]
(English Language Editor: J. Gray)


