
Can 18F-PSMA-7Q PET/CT replace prostate biopsy for the diagnosis of prostate cancer?—A single-center retrospective study
Highlight box
Key findings
• For patients with a high miPSMA score, particularly those with a miPSMA score of 3 on 18F-PSMA-7Q PET/CT, prostate biopsy can be omitted and prostate cancer-related treatment can be considered.
What is known and what is new?
• PSMA PET/CT plays an important role in the diagnosis and staging of prostate cancer. The detection rate of prostate cancer for standard prostate biopsy is low, and some patients have to undergo repeated biopsies until prostate cancer is found.
• The 18F-PSMA-7Q we used is a novel quinoline-containing PSMA PET tracer, which has the advantage of being excreted mainly through the liver and almost no excretion through urine. We used miPSMA score to evaluate the suspected lesions in the prostate found by 18F-PSMA-7Q PET/CT.
What is the implication, and what should change now?
• Further prospective multicenter studies are warranted to validate these findings.
Introduction
Prostate cancer is one of the most common malignancies in older men (1). The current gold standard for diagnosing prostate cancer relies on a transrectal ultrasound-guided, systematic twelve-core random biopsy that is blind to the cancer location, and thus, can lead to false-negative prostate cancer diagnoses (2). Moreover, prostate biopsies often underestimate the final prostate cancer Gleason score compared to histologic examination after radical prostatectomy (3). Repeated biopsy is permitted for patients whose initial biopsy is negative but is still highly suspicious for prostate cancer. However, repeated biopsies are challenging for patients.
In contrast to needle biopsy, imaging is non-invasive. Therefore, accurately identifying men with prostate cancer using imaging rather than (repeat) systematic prostate biopsies is appealing. However, the imaging tools must be accurate (4). Presently, the primary reason that conventional imaging examinations [such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI)] have not replaced needle biopsy is that their diagnostic accuracy is too low.
Prostate-specific membrane antigen (PSMA) is a cell-surface glycoprotein with an increased expression in prostate cancer cells (5). Due to this unique characteristic, PSMA is an excellent target for binding radiolabeled ligands. As a result, PSMA positron emission tomography (PET) imaging is superior to conventional imaging methods and shows high accuracy about 91% in the diagnosis and staging of prostate cancer (6-8). Of the currently available PSMA PET tracers, only 68Ga-PSMA-11 and 18F-DCFPyL are approved by US Food and Drug Administration (FDA) for PET imaging of prostate cancer. However, both tracers are excreted rapidly through the urinary tract, resulting in strong accumulation in the bladder and blurring the prostate.18F-PSMA-7Q is a novel quinoline-containing PSMA PET tracer developed by our team, which is mainly excreted through the liver (9). Since 18F-PMA-7Q is rarely excreted in the urine, the incidence of false positives in the prostate may be reduced. However, it remains unknown whether 18F-PMA-7Q PET can replace prostate biopsies in the diagnosis of prostate cancer. This study is a retrospective analysis to determine whether 18F-PSMA-7Q PET/CT can replace prostate biopsy for the diagnosis of prostate cancer, and under which circumstances this can be used. We present the following article in accordance with the STARD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-813/rc).
Methods
Study design and patient population
A total of 125 patients, with a median age of 68 years (range, 48–88 years), were included in this retrospective study. The Ethics Committee of the Chinese PLA General Hospital approved this study (No. S2020-324–01) and written informed consent was obtained from all patients. The serum prostate-specific antigen (PSA) levels at the time of the PET/CT scan were available for all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All patients who performed an 18F-PSMA-7Q PET/CT scan for newly diagnosed and suspected prostate cancer between June 2020 and December 2021 were identified from the databases. Only the newly diagnosed prostate cancer patients and the suspected prostate cancer patients with biopsy-naïve who underwent prostate biopsy (at least with transrectal ultrasound-guided systematic twelve-core biopsy and a combination of 18F-PSMA-7Q PET/CT-ultrasound cognitive fusion-guided 2–4 core targeted biopsy for PET-positive lesions) or radical prostatectomy within three months after the PET/CT scan were selected. Patients with androgen deprivation therapy, chemotherapy, or radionuclide therapy were excluded from the analysis.
18F-PSMA-7Q PET/CT scan and image analysis
18F-PSMA-7Q was synthesized as described previously (10). The PET/CT (Siemens Biograph 64) scans were performed one hour after the 18F-PSMA-7Q (5.55 MBq per kilogram of body weight) injection. The 18F-PSMA-7Q PET/CT images were evaluated by two nuclear medicine physicians with more than five years of experience in PSMA PET/CT and PET/MR interpretation, and the lesions were assigned molecular imaging PSMA (miPSMA) scores according to the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria (11). Finally, pathological diagnosis with prostate biopsy or radical prostatectomy was used as the gold standard to evaluate the diagnostic efficacy of 18F-PSMA-7Q PET/CT. And the prostate cancer detection rate in lesions with different miPSMA scores on 18F-PSMA-7Q PET/CT were analyzed.
Clinically significant prostate cancer was defined as International Society of Urological Pathology (ISUP) grade ≥3 and/or cancer core length ≥6 mm (12). For patients who underwent both prostate biopsy and radical prostatectomy, the final pathological result used was the one with a higher ISUP grade.
Statistical analysis
SPSS26.0 statistical software was used to process the data, the measurement data were described by median or average (range), and the counting data were described by examples or percentage. Receiver operating characteristic curve (ROC) was plotted and area under (AUC) curve was calculated to obtain the optimal diagnostic threshold of miPSMA score. The nonparametric McNemar test was used to compare the prostate cancer detection rate of different miPSMA score lesions.
Results
Histopathological diagnoses
Of the 125 enrolled patients, 101 had prostate cancer (Gleason Score 3+3: n=8; Gleason Score 3+4: n=17; Gleason Score 3+5: n=1; Gleason Score 4+3: n=36; Gleason Score 4+4: n=14; Gleason Score 4+5: n=18; Gleason Score 5+3: n=1; Gleason Score 5+4: n=4; and Gleason Score 5+5: n=2) and 24 had prostatic hyperplasia or prostatitis.
Diagnostic efficiency of 18F-PSMA-7Q PET/CT
miPSMA ≥2 was the optimal diagnostic threshold, and AUC was 0.948. Under this threshold, the sensitivity and specificity were 91.1% and 83.0%. ROC curve is shown in Figure 1.
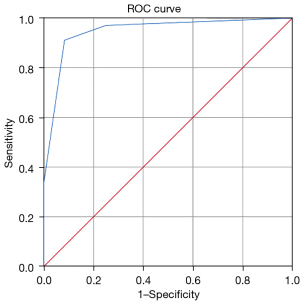
The prostate cancer detection rate for different miPSMA scores on 18F-PSMA-7Q PET/CT
Among the 125 patients, there are 21 with a miPSMA score of 0, 10 with a miPSMA score of 1, 60 with a miPSMA score of 2, and 34 with a miPSMA score of 3.
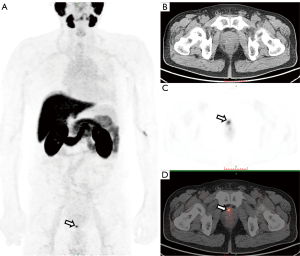
Two patients with a miPSMA score of 2 on 18F-PSMA-7Q PET/CT were diagnosed with prostatic hyperplasia or prostatitis after the first prostate biopsy. However, both were found to have prostate cancer after the second prostate biopsy (1 of the cases is shown in Figure 2).
The detection rates of prostate cancer and clinically significant prostate cancer for different miPSMA scores are shown in Table 1.
Table 1
| miPSMA | Total (n) | Prostate cancer rate | Clinically significant prostate cancer rate |
|---|---|---|---|
| 0 | 21 | 14.3% (3/21) | 4.8% (1/21) |
| 1 | 10 | 60.0% (6/10) | 0.0% (0/10) |
| 2 | 60 | 96.7% (58/60) | 76.7% (46/60) |
| 3 | 34 | 100% (34/34) | 85.3% (29/34) |
miPSMA, molecular imaging prostate-specific membrane antigen.
Comparison of prostate cancer rate among the different miPSMA score lesions
There was no significant difference in the prostate cancer detection rate between groups with a miPSMA score of 2 and 3, but there were significant differences between any other 2 groups (Table 2).
Table 2
| miPSMA score group | miPSMA score group | P value |
|---|---|---|
| 0 | 1 | 0.004 |
| 0 | 2 | <0.001 |
| 0 | 3 | <0.001 |
| 1 | 2 | 0.019 |
| 1 | 3 | 0.009 |
| 2 | 3 | 0.533 |
miPSMA, molecular imaging prostate-specific membrane antigen.
Discussion
This retrospective study explored whether 18F-PSMA-7Q PET/CT could replace prostate biopsy for the diagnosis of prostate cancer. The results showed that using a pathological diagnosis as the gold standard, the prostate cancer detection rates for miPSMA scores of 2 and 3 on 18F-PSMA-7Q PET/CT were as high as 96.7% and 100%, respectively. Notably, the detection rates were underestimated because there is a specific false-negative rate for prostate biopsies. Therefore, for these cases, particularly patients with a miPSMA score of 3, clinicians could forego the prostate biopsy and directly commence prostate cancer treatment.
It remains unclear whether patients with highly suspected prostate cancer can forego needle biopsy and go directly to radical prostatectomy. This decision primarily depends on the accuracy of the imaging diagnosis. Although MRI is increasingly used to diagnose or monitor prostate cancer, it cannot replace prostate biopsy because of its relatively low specificity (13,14). PSMA PET imaging has high accuracy in detecting prostate cancer. Of the currently available PSMA PET tracers, 68Ga-PSMA-11 and 18F-DCFPyL are approved by the FDA (15,16). 68Ga-PSMA PET/MRI improves specificity for clinically significant prostate cancer compared with multiparametric MRI (mpMRI), particularly in Prostate Imaging Reporting and Data System grade 3 lesions (17,18). In 1 study, 42 patients underwent 68Ga-PSMA-11 PET/MRI-guided biopsy, and the sensitivity, specificity, negative and positive predictive value, and accuracy for clinically significant prostate cancer were 96%, 81%, 93%, 89%, and 90%, respectively (12).
In our previous study (19), we performed 18F-DCFPyL PET/CT ultrasound (PET/CT-US) or PET/MRI ultrasound (PET/MRI-US) software fusion-targeted biopsy for intra-prostatic PET-positive lesions (2–4 cores/lesion), and 92.7% patients were pathologically confirmed as having prostate cancer. However, since 68Ga-PSMA-11 and 18F-DCFPyL are primarily excreted through the urinary system, the retention of radioactive urine in the prostate may interfere with the accurate diagnosis of prostate lesions (20).
In contrast to 68Ga-PSMA-11 and 18F-DCFPyL, 18F-PSMA-7Q used in this study is a novel quinoline-containing PSMA PET tracer that is mainly excreted through the liver. The diagnostic performance of 18F-PSMA-7Q PET/CT in patients with newly diagnosed prostate cancer is not inferior to that of 18F-DCFPyL PET/CT (9). Furthermore, since 18F-PMA-7Q is rarely excreted through the urine, it may reduce the incidence of false-positives in the prostate to some extent.
The miPSMA scoring system enables standardized reporting of PSMA PET imaging based on visual scores of PSMA expression (11). In our study, the miPSMA score system was used to classify the intra-prostatic lesions. The prostate cancer detection rate was high for patients with high miPSMA scores of 2 and 3. Repeated prostate biopsies often burden patients who have suspicious (particularly highly suspicious) prostate cancer, but a negative initial biopsy.
In our study, 2 patients with a miPSMA score of 2 on 18F-PSMA-7Q PET/CT were found to have prostatic hyperplasia or prostatitis on the first prostate biopsy. However, both were diagnosed with prostate cancer on the second prostate biopsy.
It remains unclear whether to recommend a prostate biopsy for patients with low miPSMA scores (miPSMA scores of 0 and 1). In our study, 14.3% and 60.0% of patients with miPSMA scores of 0 and 1, respectively, had prostate cancer. Therefore, prostate biopsy should not be omitted for these patients. However, the clinical significance of prostate cancer was only 4.8% and 0.0% for the patients with miPSMA scores of 0 and 1, respectively. Clinically insignificant prostate cancer is associated with a high rate of overdiagnosis and overtreatment. Therefore, it is not easy to judge whether a prostate biopsy should be recommended by relying solely on low miPSMA scores (miPSMA score 0 or 1).
There are some limitations to this investigation. First, this was a retrospective, single-center study in which the number of patients is relatively small, so it is statistically insufficient and therefore was statistically underpowered, for example, the proportion of patients with prostate cancer is higher in this study. Further prospective multicenter studies are warranted to validate these results. Second, the reference standard for prostate cancer in some patients is based on biopsy specimens, which underestimates the clinical value of PSMA PET imaging to some extent because of inevitable missed diagnoses through biopsy. Finally, the object of our study was PSMA PET/CT rather than PSMA PET/MRI. The use of PSMA PET improves the management of prostate cancer patients as it outperforms mpMRI (21). In addition, PSMA PET/MRI was found to have a higher clinical diagnostic accuracy in prostate cancer than PSMA PET or mpMRI (22-24).
Conclusions
In this study, the prostate cancer detection rate of 18F-PSMA-7Q PET/CT was elevated for lesions with high miPSMA scores of 2 and 3. Therefore, the diagnosis of prostate cancer in patients with a high miPSMA score, particularly those with a miPSMA score of 3, could involve foregoing prostate biopsy and directly commencing treatment. Further research is warranted to verify these findings.
Acknowledgments
Funding: This work was financially supported by the National Natural Science Foundation of China project (No. 81571715), National Natural Science Foundation of China (No. 81970594), the Achievement Transformation Project of Chinese PLA General Hospital (No. 2018-TM-07), and the Special Scientific Research Topic of Health Care of Chinese PLA General Hospital (No. 19BJZ19).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-813/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-813/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-813/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Chinese PLA General Hospital approved this study (No. S2020-324–01) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Culp MB, Soerjomataram I, Efstathiou JA, et al. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020;77:38-52. [Crossref] [PubMed]
- Ahdoot M, Wilbur AR, Reese SE, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020;382:917-28. [Crossref] [PubMed]
- Qin C, Gai Y, Liu Q, et al. Optimized Application of (68)Ga-Prostate-Specific Membrane Antigen-617 Whole-Body PET/CT and Pelvic PET/MR in Prostate Cancer Initial Diagnosis and Staging. Front Med (Lausanne) 2021;8:657619. [Crossref] [PubMed]
- Scheltema MJ, Chang JI, Stricker PD, et al. Diagnostic accuracy of (68) Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: impact of the addition of (68) Ga-PSMA PET to mpMRI. BJU Int 2019;124:42-9. [Crossref] [PubMed]
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395:1208-16. [Crossref] [PubMed]
- Liu Y, Dong Y, Liu J, et al. Comparison between (18) F-DCFPyL PET and MRI for the detection of transition zone prostate cancer. Prostate 2021;81:1329-36. [Crossref] [PubMed]
- Farolfi A, Calderoni L, Mattana F, et al. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J Nucl Med 2021;62:596-604. [Crossref] [PubMed]
- Satapathy S, Singh H, Kumar R, et al. Diagnostic Accuracy of (68)Ga-PSMA PET/CT for Initial Detection in Patients With Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2021;216:599-607. [Crossref] [PubMed]
- Liu Y, Zhang X, Liu J, et al. Prospective intraindividual comparison of 18F-PSMA-7Q and 18F-DCFPyL PET/CT in patients with newly diagnosed prostate cancer. Nucl Med Commun 2022;43:725-30. [Crossref] [PubMed]
- Zhang X, Wu Y, Zeng Q, et al. Synthesis, Preclinical Evaluation, and First-in-Human PET Study of Quinoline-Containing PSMA Tracers with Decreased Renal Excretion. J Med Chem 2021;64:4179-95. [Crossref] [PubMed]
- Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J Nucl Med 2018;59:469-78. [Crossref] [PubMed]
- Ferraro DA, Becker AS, Kranzbühler B, et al. Diagnostic performance of (68)Ga-PSMA-11 PET/MRI-guided biopsy in patients with suspected prostate cancer: a prospective single-center study. Eur J Nucl Med Mol Imaging 2021;48:3315-24. [Crossref] [PubMed]
- Falagario UG, Martini A, Wajswol E, et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-specific Antigen Density, 4Kscore and Risk Calculators. Eur Urol Oncol 2020;3:700-4. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Song H, Iagaru A, Rowe SP. 18F-DCFPyL PET Acquisition, Interpretation, and Reporting: Suggestions After Food and Drug Administration Approval. J Nucl Med 2022;63:855-9. [Crossref] [PubMed]
- Masters SC, Hofling AA, Gorovets A, et al. FDA Approves Ga 68 PSMA-11 for Prostate Cancer Imaging. Int J Radiat Oncol Biol Phys 2021;111:27-8. [Crossref] [PubMed]
- Margel D, Bernstine H, Groshar D, et al. Diagnostic Performance of (68)Ga Prostate-specific Membrane Antigen PET/MRI Compared with Multiparametric MRI for Detecting Clinically Significant Prostate Cancer. Radiology 2021;301:379-86. [Crossref] [PubMed]
- Metser U, Ortega C, Perlis N, et al. Detection of clinically significant prostate cancer with (18)F-DCFPyL PET/multiparametric MR. Eur J Nucl Med Mol Imaging 2021;48:3702-11. [Crossref] [PubMed]
- Liu Y, Yu H, Liu J, et al. A Pilot Study of (18)F-DCFPyL PET/CT or PET/MRI and Ultrasound Fusion Targeted Prostate Biopsy for Intra-Prostatic PET-Positive Lesions. Front Oncol 2021;11:612157. [Crossref] [PubMed]
- Sharma P, Watts A, Singh H. Comparison of Internal Dosimetry of 18 F-PSMA-1007 and 68 Ga-PSMA-11-HBED-CC. Clin Nucl Med 2022;47:948-53. [Crossref] [PubMed]
- Chen M, Zhang Q, Zhang C, et al. Comparison of (68)Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and multi-parametric magnetic resonance imaging (MRI) in the evaluation of tumor extension of primary prostate cancer. Transl Androl Urol 2020;9:382-90. [Crossref] [PubMed]
- Coşar U, Şen İ, Aydos U, et al. Diagnostic accuracy of (68) Ga-PSMA PET/MRI and multiparametric MRI in detecting index tumours in radical prostatectomy specimen. Int J Clin Pract 2021;75:e14287. [Crossref] [PubMed]
- Arslan A, Karaarslan E, Güner AL, et al. Comparison of MRI, PSMA PET/CT, and Fusion PSMA PET/MRI for Detection of Clinically Significant Prostate Cancer. J Comput Assist Tomogr 2021;45:210-7. [Crossref] [PubMed]
- Eiber M, Weirich G, Holzapfel K, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol 2016;70:829-36. [Crossref] [PubMed]


