
Prostate cancer risk assessment by the primary care physician and urologist: transabdominal- versus transrectal ultrasound prostate volume-based use of the Rotterdam Prostate Cancer Risk Calculator
Highlight box
Key findings
• Rotterdam Prostate Cancer Risk Calculator (RPCRC)-based risk stratification in primary care using transabdominal ultrasound (TAUS) measured prostate volume is feasible and may prevent unnecessary referrals to the urologist and reduce costs.
What is known and what is new?
• Risk stratification by the urologist using the RPCRC can prevent unnecessary prostate biopsies and/or MRI.
• This study shows that even though prostate volume measured by general practitioner with TAUS differs from transrectal ultrasound measured prostate volume by the urologist, risk stratification in the primary care using the RPCRC could prevent unnecessary referrals at the expense of several non-referred men who had a biopsy indication according to the urologist.
What is the implication, and what should change now?
• Due to the learning curve, the accuracy of the risk assessment with TAUS is likely to be improved by centralization among general practitioner’s practices. Additionally, TAUS prostate volume measurement with RPCRC-based risk stratification in the primary care needs to be studied further in a larger cohort including longer follow-up.
Introduction
Opportunistic prostate cancer (PCa) screening using digital rectal examination (DRE) and prostate specific-antigen (PSA) measurement is widespread in the primary care setting (1). Men with an increased risk of having PCa based on an elevated PSA and/or abnormal DRE are referred to the urologist by their primary care physician for diagnostic work-up with transrectal ultrasound (TRUS) and if applicable multiparametric MRI and prostate biopsy (2-4). Multivariable risk stratification by the urologist using a risk calculator can prevent unnecessary prostate biopsies (5). The Rotterdam Prostate Cancer Risk Calculators (RPCRCs) (www.prostatecancer-riskcalculator.org) have been shown to prevent one-third of unnecessary TRUS-biopsies and pre-biopsy MRI scans when used by the urologist (5-12). Due to increasing health care costs and workload in secondary care, governments stimulate diagnostics in the primary care setting to reduce unnecessary referrals to secondary care (13-15). Risk-stratification using the RPCRCs in the general practitioners’ office, instead of solely performing a DRE and PSA measurement, could cause a reduction in unnecessary referrals to the urologist. Prostate volume measurement significantly improves the accuracy of multivariable risk-stratification for PCa (7,16). Since TRUS is impractical in the primary care setting, prostate volume measurement could be performed with either DRE or transabdominal ultrasound (TAUS). TAUS prostate volume measurement is non-invasive and relatively simple, with an acceptable correlation with TRUS-measured prostate volume when performed by the urologist (17-21). The present study is a feasibility study of PCa risk-stratification in the general practitioner’s office, using TAUS-measured prostate volume. The objective is to assess the correlation of TAUS prostate volume measured by the general practitioner with the TRUS prostate volume measured by the urologist. Furthermore, the potential reduction of referrals to the urologist by risk-stratification in primary care is assessed. We present the following article in accordance with the STARD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-640/rc).
Methods
Study population
Our prospective cohort study was performed in two primary care facilities (primary care centers ‘Hillegersberg’ and ‘Bergse Bos’) in Rotterdam in the Netherlands between September 2014 and November 2018. All consecutive men who visited these two primary care centers with an opportunistic PCa screening request were offered to participate in this study after the process of shared decision making: men received oral and written information on the advantages and disadvantages of PCa screening as well as information about participation within the present study. Men with a prior PCa diagnosis were not eligible for inclusion. All men provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by both primary care centers and the Ethics Committee of the Erasmus University Medical Center, the Netherlands (No. MEC-2014-069).
Study procedures
Two (EH and WB) general practitioners of the primary care centers performed the screening consultations. These general practitioners received two one-hour training sessions in TAUS prostate volume measurement by an experienced urologist (CB). All participating men were screened by one of the two general practitioners with DRE, PSA measurement, and TAUS prostate volume measurement using a handheld ultrasound device (Sonosite MicroMaxx, USA). The general practitioner then calculated the risk of (clinically significant) PCa using the RPCRC and referred the patient to the urologist at a specialized outpatient clinic (Prostate Center, Franciscus Gasthuis, Rotterdam). Here, all included men were examined by a single urologist (CB) with DRE and TRUS with prostate volume measurement, again followed by PCa risk assessment using the RPCRC. During the assessment, the urologist was blinded to previous findings by the general practitioner. A TRUS-guided systematic prostate biopsy was performed in men with an elevated risk of PCa as calculated by the urologist. A pre-biopsy MRI was not yet standard clinical practice during the study period. Men without an elevated PCa risk received information on follow-up with repeated PSA-screens in the primary care setting if wanted. Follow-up data were collected prospectively.
Rotterdam prostate cancer risk calculator
The RPCRC consists of several risk calculators, which are developed using data of 3,624 men of the first screening round of the Rotterdam section of the ERSPC study and are freely available on the web (www.prostatecancer-riskcalculator.org) or as an app for iOS and Android (6). The risk calculators calculate the risk of any PCa and the risk of clinically significant PCa, defined as Gleason ≥3 + 4 and/or locally advanced (T-stage ≥2C) PCa. In the present study two of these risk calculators, the RPCRC-3 and the RPCRC-3+DRE, are utilized. The urologist assessed the risk of PCa using the RPCRC-3, which includes PSA, DRE (suspicious: no/yes), TRUS (suspicious: no/yes), and TRUS-measured prostate volume (continuous). The urologist used a ≥4% risk threshold for clinically significant PCa as an indication for prostate biopsy. As the two general practitioners did not perform a TRUS and therefore could not screen for hypoechoic lesions, they used the RPCRC-3+DRE model, which is a simplified risk model using PSA, DRE (suspicious: no/yes), and DRE-measured prostate volume (categorized into volume classes of 25, 40, or 60 mL). Instead of the DRE-measured prostate volume, the TAUS-measured prostate volume was used for risk-stratification, categorized as 25 mL (<30 mL), 40 mL (30–50 mL), or 60 mL (>50 mL) [since the volume in RPCRC-3+DRE statistical model is also categorized with these cut-offs (16) and has shown to hardly affect predictive capability (22)]. The general practitioners used a ≥4% risk threshold for clinically significant PCa as calculated by the RPCRC-3+DRE as an indication for referral to the urologist. Furthermore, the indication for biopsy as assessed by the urologist was compared with the indication for referral as assessed by the general practitioner.
Statistical analysis
Our study’s primary objective was to compare TAUS prostate volume measurement by the general practitioner with TRUS volume measurement by the urologist. As no superiority of TAUS volume measurement over TRUS volume measurement was assumed, a non-inferiority analysis was performed to assess their equivalence within a limit that would unlikely affect biopsy decisions. The non-inferiority limit was set at ≤25% of the mean TRUS-measured prostate volume. Using the mean TRUS-measured prostate volume of 38.8 mL within the Rotterdam section of the ERSPC study (23), the power calculation (90% power, 5% one-sided significance level) showed a required sample size of 116 men. Equivalence was assessed using the paired t-test. Absolute prostate volume differences (|TAUS - TRUS|) categorised for TAUS quality were plotted per case against the experience of the general practitioners with TAUS (caseload). The correlation between prostate volume difference and TAUS experience and prostate volume was assessed as the Spearman’s rho correlation coefficient. The potential reduction of referrals to the urologist by risk stratification with TAUS and the RPCRC was assessed compared to standard clinical care (referral in case of PSA ≥3.0 and/or abnormal DRE). Statistical analyses were performed with SPSS for Windows (Version 21.0; Armonk, NY: IBM Corp., New York, USA).
Results
Patient characteristics
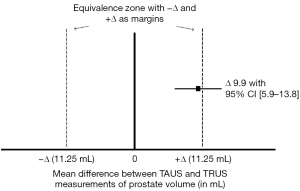
A total of 130 men were eligible for inclusion and provided written informed consent, see Figure 1. Twenty-five men were excluded from analyses either due to an inability to measure prostate volume with TAUS (large abdominal circumference) (n=1) or a no-show for their blood draw (n=5), TAUS consultation (n=5) or urologist consultation (n=14). The remaining 105 men were included in the analyses of the present study. These men had a mean age of 69 years and a median PSA of 1.9 ng/mL (Table 1). The mean TAUS-measured prostate volume measured by the general practitioner was 55 mL and the mean TRUS-measured prostate volume by the urologist was 45 mL. Therefore, the non-inferiority limit was 11.25 mL.
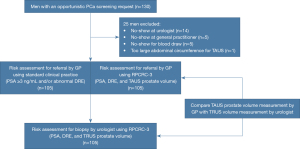
Table 1
| Parameter | Value |
|---|---|
| Age (years), mean ± SD | 69±9 |
| TAUS measured prostate volume (mL), mean ± SD | 55±25 |
| TRUS measured prostate volume (mL), mean ± SD | 45±22 |
| PSA (ng/mL), median [IQR] | 1.9 [0.8–4.2] |
| Suspicious DRE at primary care physician, n (%) | 5 [5] |
| Suspicious DRE at urologist, n (%) | 5 [5] |
| Total number of patients, n (%) | 105 [100] |
SD, standard deviation; IQR, interquartile range; DRE, digital rectal examination; TAUS, transabdominal ultrasound; TRUS, transrectal ultrasound.
Transabdominal versus transrectal ultrasound measured prostate volume
The mean difference between TAUS and TRUS prostate volume measurements was 9.9 mL (95% CI: 5.9–13.8), with a 95% confidence interval exceeding the predefined 11.25 mL positive equivalence margin (Figure 2). Thus, TAUS was inequivalent and more frequently overestimated than underestimated prostate volume compared with TRUS. Figure 3 shows the absolute prostate volume differences per case, plotted against the total number of TAUS performed by the general practitioners (caseload): the prostate volume differences decreased with increasing TAUS experience of the general practitioners (R2=0.19, P<0.05). Furthermore, the prostate volume differences between TAUS and TRUS increased with increasing prostate volume (R2=0.42, P<0.01).

Comparison of referral strategies by the general practitioner
According to standard clinical practice (PSA ≥3 ng/mL and/or abnormal DRE), 41 (39%; 95% CI: 30–49%) out of 105 men would have been referred by the primary care physician to the urologist for further risk assessment (Figure 4). In contrast, only 12 (11%; 95% CI: 6.7–19%) out of 105 men would have been referred after TAUS prostate volume measurement and RPCRC-based stratification. Thus, TAUS and RPCRC-based stratification avoided 29 (71%; 95% CI: 20–37%) out of 41 referrals. At the urology office, TRUS and RPCRC-based risk stratification yielded a biopsy indication in 11 [10%; 95% CI: 6.0–18%)] out of the total 105 men. Standard clinical practice (PSA ≥3 ng/mL and/or abnormal DRE) referred all 11 men who were found to have a biopsy indication. However, the TAUS and RPCRC-based referral strategy would not have referred 5 (45%; 95% CI: 21–72%) out of the 11 men who were found to have a biopsy indication. Two of these five “missed referrals” were biopsied in which no cancer was found. In addition, four out of these five men with the TAUS and RPCRC-based referral strategy occurred in the first half of the study period and were caused by an overestimation of the TAUS-measured prostate volume. Only three of the 11 men with a biopsy indication directly received a prostate biopsy of which one man was diagnosed with clinically insignificant PCa. Three men without a biopsy indication at first, received a prostate biopsy after follow-up. One of these men was diagnosed with clinically significant PCa, and one man was diagnosed with clinically insignificant PCa.

Discussion
To our knowledge, the present study is the first study to assess the feasibility of prostate cancer risk stratification using TAUS-measured prostate volume in the primary care setting. Our study showed that TAUS-prostate volume measurement with a handheld ultrasound device by the general practitioner is feasible. However, TAUS frequently overestimated the prostate volume (mean difference of 10 mL) compared to the TRUS by the urologist as the reference test. It was shown that the inaccuracy of the prostate volume by TAUS increased with increasing (TRUS-measured) prostate volume and decreased with operator experience. As expected, a learning curve phenomenon is associated with TAUS prostate volume measurement.
Due to increasing health care costs and workload in secondary care, governments stimulate diagnostics in the primary care setting to reduce unnecessary referrals to secondary care (13,14). According to the Dutch General Practitioners guideline, men should be referred to the urologist for risk-stratification with the RPCRC in case of a PSA ≥3 ng/mL and/or an abnormal DRE (24,25). It is known that risk stratification with the RPCRC in the secondary care setting prevents one-third of unnecessary TRUS-biopsies and pre-biopsy MRIs (5-12). Therefore, we hypothesized that risk-stratification with the RPCRC using TAUS in the primary care setting could cause a similar reduction of referrals to the urologist. Such a referral strategy would have caused a referral reduction of no less than 71% in the present study compared to Dutch clinical practice. However, this reduction of referrals to the urologist would be at the expense of non-referral of 45% of men with a biopsy indication according to a TRUS and RPCRC-based risk stratification. Although, no clinically significant prostate cancer was found in the “missed referrals” who were biopsied, the clinical consequences of TAUS prostate volume measurement with RPCRC-based risk stratification in such a low-risk primary care target population need to be studied further in a larger cohort with longer follow-up.
The accuracy of the risk assessment with TAUS in the present study might be improved by sufficient training and centralization to achieve a higher volume of opportunistic screening consultations in the concerning primary care facilities. The two general practitioners performing the TAUS in the present study only received two one-hour training sessions in TAUS prostate volume measurement before starting this study. More extensive training in TAUS prostate volume measurement might have shortened their learning curve, resulting in less non-referral of men with a biopsy indication with a risk-based referral strategy. Furthermore, the learning curve of the TAUS performers might have shortened with a higher caseload. Within our feasibility study, only 105 patients from two primary care centers were included for analysis and received an ultrasound from one of the two TAUS performers within a time-frame of four years. A higher caseload could be established by centralizing prostate cancer screening consultations from all regional general practitioners’ offices to a central primary care facility within a regional care pathway. Such a regional care pathway has been established in Rotterdam by the Urology Department of the Erasmus University Medical Center in collaboration with the primary care laboratory STAR-SHL (26). At the STAR-SHL primary care laboratory, prostate cancer screening consultations are performed by specially trained Erasmus MC personnel using TRUS and the RPCRC. A previous study by Osses et al. showed excellent results of these screening consultations, with a 46% reduction of referrals to secondary care and a high positive predictive value for prostate cancer (79%) in those referred after multivariable risk-stratification (26).
Our feasibility had some limitations. First, MRI prostate volume was unavailable for comparison as this study was conducted before the wide-spread adaption of prostate MRI. However, the clinical relevance between TRUS- and MRI prostate volume seems to be marginal (27). Secondly, the exact number of possible underdiagnosed prostate cancers is unknown as not all men were biopsied. Nonetheless, the RPCRC which was also applied in this study, has previously showed its ability in the primary care setting to reduce referrals and prostate biopsies while missing almost no significant prostate cancers after a median follow-up of 43 months (26,28). Lastly, as the urologist in our study only measured the prostate volume with TRUS (not TAUS), no comparison could be made between TAUS-measured prostate volume by the general practitioner and the urologist.
Conclusions
RPCRC-based risk stratification in primary care using TAUS prostate volume measurement is feasible and may prevent unnecessary referrals to the urologist and reduce costs. The accuracy of the prostate cancer risk assessment in primary care is likely to be improved by centralizing opportunistic screening consultations within a regional care pathway. In addition, TAUS prostate volume measurement with RPCRC-based risk stratification in the primary care population needs to be studied further in a larger cohort with longer follow-up.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-640/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-640/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-640/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-640/coif). MJR serves as an unpaid Associate Editor-in-Chief of Translational Andrology and Urology from January 2022 to December 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All men provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by both primary care centers and the Ethics Committee of the Erasmus University Medical Center, the Netherlands (MEC-2014-069).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Merriel SWD, Funston G, Hamilton W. Prostate Cancer in Primary Care. Adv Ther 2018;35:1285-94. [Crossref] [PubMed]
- Grossman DC, Curry SJ, Owens DK, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319:1901-13. [Crossref] [PubMed]
- Jefferies ER, Brewster SF. BAUS Section on Oncology. Urological recommendations from the National Institute for Health and Care Excellence (NICE) Guideline, June 2015: Suspected cancer: recognition and referral. BJU Int 2016;117:857-60. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Louie KS, Seigneurin A, Cathcart P, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol 2015;26:848-64. [Crossref] [PubMed]
- Roobol MJ, Steyerberg EW, Kranse R, et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol 2010;57:79-85. [Crossref] [PubMed]
- Roobol MJ, Schröder FH, Hugosson J, et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: results from the prostate biopsy collaborative group. World J Urol 2012;30:149-55. [Crossref] [PubMed]
- van Vugt HA, Roobol MJ, Kranse R, et al. Prediction of prostate cancer in unscreened men: external validation of a risk calculator. Eur J Cancer 2011;47:903-9. [Crossref] [PubMed]
- van Vugt HA, Kranse R, Steyerberg EW, et al. Prospective validation of a risk calculator which calculates the probability of a positive prostate biopsy in a contemporary clinical cohort. Eur J Cancer 2012;48:1809-15. [Crossref] [PubMed]
- Gayet M, Mannaerts CK, Nieboer D, et al. Prediction of Prostate Cancer: External Validation of the ERSPC Risk Calculator in a Contemporary Dutch Clinical Cohort. Eur Urol Focus 2018;4:228-34. [Crossref] [PubMed]
- Mannaerts CK, Gayet M, Verbeek JF, et al. Prostate Cancer Risk Assessment in Biopsy-naïve Patients: The Rotterdam Prostate Cancer Risk Calculator in Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound (TRUS) Fusion Biopsy and Systematic TRUS Biopsy. Eur Urol Oncol 2018;1:109-17. [Crossref] [PubMed]
- Alberts AR, Roobol MJ, Verbeek JFM, et al. Prediction of High-grade Prostate Cancer Following Multiparametric Magnetic Resonance Imaging: Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculators. Eur Urol 2019;75:310-8. [Crossref] [PubMed]
- Jones R, Newbold M, Reilly J, et al. The future of primary and secondary care. Br J Gen Pract 2013;63:379-82. [Crossref] [PubMed]
- Kringos DS, Boerma W, van der Zee J, et al. Europe's strong primary care systems are linked to better population health but also to higher health spending. Health Aff (Millwood) 2013;32:686-94. [Crossref] [PubMed]
- Van Poppel H, Hogenhout R, Albers P, et al. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur Urol Oncol 2021;4:731-9. [Crossref] [PubMed]
- Roobol MJ, van Vugt HA, Loeb S, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 2012;61:577-83. [Crossref] [PubMed]
- Huang Foen Chung JW, de Vries SH, Raaijmakers R, et al. Prostate volume ultrasonography: the influence of transabdominal versus transrectal approach, device type and operator. Eur Urol 2004;46:352-6. [Crossref] [PubMed]
- Feng KK, Chiang IN, Huang CY, et al. Analysis of transrectal and suprapubic ultrasonography for prostate size evaluation. Urological Science 2017;28:166-8. [Crossref]
- Babaei Jandaghi A, Shakiba M, Nasseh H, et al. Application of bland-altman method in comparing transrectal and transabdominal ultrasonography for estimating prostate volume. Iran J Med Sci 2015;40:34-9. [PubMed]
- Yuen JS, Ngiap JT, Cheng CW, et al. Effects of bladder volume on transabdominal ultrasound measurements of intravesical prostatic protrusion and volume. Int J Urol 2002;9:225-9. [Crossref] [PubMed]
- Pate WR, Garg N, Wang LB, et al. Comparison of Transabdominal and Transrectal Ultrasound for Sizing of the Prostate. Urology 2020;141:125-9. [Crossref] [PubMed]
- Pereira-Azevedo N, Braga I, Verbeek JF, et al. Prospective evaluation on the effect of interobserver variability of digital rectal examination on the performance of the Rotterdam Prostate Cancer Risk Calculator. Int J Urol 2017;24:826-32. [Crossref] [PubMed]
- Roobol MJ, Kranse R, Bangma CH, et al. Screening for prostate cancer: results of the Rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol 2013;64:530-9. [Crossref] [PubMed]
- Blanker MH, Klomp MA, van den Donk M, et al. Summary of the NHG practice guideline 'Lower urinary tract symptoms in men'. Ned Tijdschr Geneeskd 2013;157:A6178. [PubMed]
- Blanker MH, de Reijke TM, van Moorselaar RJ, et al. Changes to Dutch College of General Practitioners guideline 'Micturition problems in men'. Ned Tijdschr Geneeskd 2014;158:A8070. [PubMed]
- Osses DF, Alberts AR, Bausch GCF, et al. Multivariable risk-based patient selection for prostate biopsy in a primary health care setting: referral rate and biopsy results from a urology outpatient clinic. Transl Androl Urol 2018;7:27-33. [Crossref] [PubMed]
- Choe S, Patel HD, Lanzotti N, et al. MRI vs Transrectal Ultrasound to Estimate Prostate Volume and PSAD: Impact on Prostate Cancer Detection. Urology 2022; Epub ahead of print. [Crossref] [PubMed]
- Hogenhout R, Osses DF, Alberts AR, et al. Shifting risk-stratified early prostate cancer detection to a primary healthcare setting. BJU Int 2022; [Crossref] [PubMed]


