
Accuracy of actual stage prediction using Vesical Imaging Reporting and Data System (VI-RADS) before radical cystectomy for urothelial carcinoma in SUPER-UC-Cx
Highlight box
Key findings
• Validation of Vesical Imaging Reporting and Data System (VI-RADS) using Radical Cystectomy (RC) Specimens.
What is known and what is new?
• There is consensus on the efficacy and accuracy of VI-RADS with multiparametric magnetic resonance imaging (MRI) for bladder cancer.
• However, few studies have been validated with RC specimens. Accordingly, the authors confirmed the diagnostic accuracy of VI-RADS using RC specimens.
What is the implication, and what should change now?
• VI-RADS is a good predictor of muscle invasion as well as perivesical fat infiltration in bladder cancer.
Introduction
Bladder cancer is the fourth most common cancer among all cancers in the United States, and its incidence is steadily increasing. Especially in men, it accounts for approximately 4% of all cancer-related deaths (1). Regarding bladder cancer, the prognosis and treatment differ depending on whether the muscle is involved; therefore, it is important to evaluate whether the muscle is involved (2). The 5-year survival rate of patients with non-muscular invasive bladder cancer (NMIBC) is greater than 90%, whereas that of patients with muscle-invasive bladder cancer (MIBC) is less than 60% (3).
Most bladder cancers are histologically diagnosed through transurethral resection of bladder tumors (TUR-BT), and clinical staging is determined through imaging tests. If clinically T2 is suspected but pathologically lower than T1, TUR-BT is performed again for restaging. This is known as the second TUR-BT (4). Pathological T2 represents muscle involvement, and clinicians must decide whether to perform radical cystectomy (RC). Computed tomography (CT) is a widely used imaging technique. Although this test is useful in examining the entire abdomen to determine propagation to other organs or lymph nodes, it has limitations in predicting the depth of tumor involvement, that is, stage T (5).
Currently, the importance of magnetic resonance imaging (MRI) in bladder cancer is emerging, and the quantified reporting system is the Vesical Imaging Reporting and Data System (VI-RADS). Multiparametric MRI (mp-MRI) is a method of obtaining information about tumors by comparing T2-weighted images, diffusion-weighted images, apparent diffusion coefficients, and dynamic contrast-enhanced images (6). The VI-RADS identifies the mp-MRI and classifies tumors into five categories. On a scale of 1 to 5, a score closer to 5 indicates more severe tumor involvement. In particular, a score of 3 or higher may suggest muscle invasion, and a score of 4 or higher may strongly suggest muscle invasion (7).
Despite this reporting system, the rate of MRI scans in patients with bladder cancer remains low. This is because there were limitations in staging through TUR-BT in previous studies. Therefore, the researchers retrospectively evaluated the VI-RADS scores of patients who underwent RC and patients who underwent preoperative mp-MRI to confirm whether the VI-RADS score and actual stage matched using the Seoul National University Prospectively Enrolled Registry for Urothelial Cancer-Radical Cystectomy (SUPER-UC-Cx) (8). This is expected to provide a theoretical basis for mp-MRI imaging in patients with bladder cancer in the future. We present the following article in accordance with the STARD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-704/rc).
Methods
Ethics approval and informed consent
This study was approved by the Seoul National University Hospital (SNUH) Institutional Review Board (IRB) (approval number: 2207-194-1345). The prospectively collected cohort was approved by the SNUH IRB (approval number: 1506-122-682) for use with clinical data for scientific purposes. Informed consent was obtained from all participants.
Study design
This study used data from a prospective, multidisciplinary, and biobank-linked cohort, the SUPER-UC-Cx. Between March 2020 and March 2022, patients who underwent mp-MRI before RC were included in this study. Patients with non-urothelial carcinoma, insufficient bladder filling during MRI, and inadequate mp-MRI were excluded. Basic information and imaging information of patients included in the study were reviewed retrospectively.
VI-RADS scores were noted by two experienced radiologists. Patients’ basic characteristics and clinical information were blinded during the review. In cases of disagreement between the two readers, two urologists participated in the review and reached a consensus. The final pathological results were confirmed by an experienced pathologist.
MRI protocol
A 3-Tesla MRI scanner equipped with a multichannel phased-array coil was used in this study. Proper bladder distension is important to prevent false positives and negatives due to upstaging and downstaging. Patients were instructed to urinate 1–2 h before imaging and drink 500–1,000 mL of water 30 min before the test, depending on their tolerance, to reach the optimal bladder volume of approximately 300 mL. T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images were all included. This method is currently the most standardized method when using VI-RADS (6).
Patients’ clinical data and statistical analysis
The following clinical data were assessed: age, sex, body mass index, medical history, tumor location, size, number, clinical stage, previous urothelial cancer diagnosis, preoperative chemotherapy, follow-up period, perioperative outcomes, and MRI findings. The baseline characteristics were presented as descriptive statistics. The performance of qualitative and quantitative variables in predicting muscle layer invasion or perivesical fat infiltration was verified by receiver operating characteristic (ROC) curve analysis. This method calculates the positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity of the diagnostic test and investigate the discriminatory ability of this diagnostic test through area under the curve (AUC). All statistical analyses were performed using the R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 208 patients, 182 (87.5%) underwent mp-MRI before RC. Nine patients with non-urothelial carcinoma, six who underwent MRI that did not include all phases, and eight with insufficient bladder filling were excluded (Figure 1). The median interval between MRI and RC was 17 days [interquartile range (IQR) 11–24 days]. The patient was scheduled for RC due to T2 on TUR-BT or Bacillus Calmette-Guerin (BCG) unresponsiveness. The median age of the patients was 70 (IQR 63–76) years, and 132 patients (83%) were men. Forty-seven patients (29.6%) were non-smokers. Fifty-four patients (34%) received neoadjuvant chemotherapy and 33 patients (20.8%) had a prior history of BCG. A total of 99 patients (62.2%) underwent robotic surgery, and 134 patients (84.2%) underwent urinary diversion with an orthotopic neobladder. The distribution of VI-RADS scores on mp-MRI before surgery is shown in Table 1.
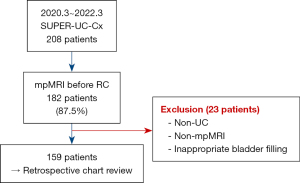
Table 1
| Characteristics | Total patients (N=159) |
|---|---|
| Age, median (IQR), years | 70.0 (63.0–76.0) |
| Gender, male (%) | 132 (83.0) |
| BMI (IQR), kg/m2 | 24.2 (22.4–26.0) |
| Non-smoker (%) | 47 (29.6) |
| ECOG PS 0 (%) | 156 (98.1) |
| Charlson Comorbidity Index (IQR) | 1 (0–2) |
| Neoadjuvant chemotherapy (%) | 54 (34.0) |
| Previous BCG (%) | 33 (20.8) |
| Type of surgery (%) | |
| Open | 60 (37.8) |
| Robot-assisted | 99 (62.2) |
| Type of diversion (%) | |
| Conduit | 25 (15.8) |
| Neobladder | 134 (84.2) |
| VI-RADS score (%) | |
| 0 | 65 (40.9) |
| 1 | 5 (3.1) |
| 2 | 8 (5.0) |
| 3 | 32 (20.1) |
| 4 | 23 (14.5) |
| 5 | 26 (16.4) |
IQR, interquartile range; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; BCG, Bacillus Calmette-Guerin; VI-RADS, Vesical Imaging-Reporting and Data System.
The median interval between TUR-BT and RC was 58 (IQR 43–136) days. Clinical tumor nodes metastases (TNM) stages of patients considering TUR-BT result and imaging before RC are shown in Table 2. The median operation time was 290 (IQR 225–335) min and the median estimated blood loss was 500 (IQR 357.5–730) mL. The median number of extracted lymph nodes was 11 (IQR 7–16). Table 3 shows the pathological T and N stages confirmed by an experienced pathologist.
Table 2
| Characteristics | Total patients (N=159) |
|---|---|
| From TUR-BT to RC time [IQR], days | 58 [43–136] |
| Clinical T stage (%) | |
| Tis | 3 (1.9) |
| Ta | 6 (3.8) |
| T1 | 43 (27.0) |
| T2 | 77 (48.4) |
| T3 | 23 (14.5) |
| T3 | 7 (4.4) |
| Clinical N stage (%) | |
| N0 | 135 (84.9) |
| N1 | 12 (7.6) |
| N2 | 12 (7.6) |
| Clinical M stage (%) | |
| M0 | 158 (99.4) |
| M1 | 1 (0.6) |
TUR-BT, transurethral resection of bladder tumors; RC, radical cystectomy; IQR, interquartile range; Tis, carcinoma in situ.
Table 3
| Outcomes | Total patients (N=159) |
|---|---|
| Operation time (IQR), min | 290.0 (225.0–335.0) |
| EBL (IQR), mL | 500.0 (357.5–730.0) |
| Extracted lymph node [IQR], N | 11 [7–16] |
| Pathologic T stage (%) | |
| T0 | 43 (27.0) |
| Tis | 25 (15.7) |
| Ta | 10 (6.3) |
| T1 | 33 (20.8) |
| T2 | 16 (10.1) |
| T3 | 24 (15.1) |
| T4 | 8 (5.0) |
| Pathologic N stage (%) | |
| N0 | 132 (83.0) |
| N1 | 8 (5.0) |
| N2 | 12 (7.6) |
| Nx | 7 (4.4) |
IQR, Interquartile range; EBL, estimated blood loss.
In the analysis indicating the diagnostic accuracy of muscle invasion, the cut-off for a VI-RADS score of 4 had the highest AUC and accuracy (sensitivity 0.84, specificity 0.93, accuracy 0.90, PPV 0.84, NPV 0.93, AUC 0.88) The cut-off for VI-RADS 3 was as follow: sensitivity 0.94, specificity 0.68, accuracy 0.76, PPV 0.57, NPV 0.96, AUC 0.81. Furthermore, the cut-off values for VI-RADS 5 were as follows: sensitivity, 0.51; specificity, 0.99; accuracy, 0.84; PPV, 0.96; NPV, 0.81; AUC, 0.75 (Table 4). In the analysis indicating the diagnostic accuracy of perivesical fat infiltration, the cut-off for a VI-RADS score of 5 had the highest AUC and accuracy (sensitivity 0.78, specificity 0.99, accuracy 0.95, PPV 0.96, NPV 0.95, AUC 0.89). The cut-off for VI-RADS 3 was as follows: sensitivity 0.96, specificity 0.60, accuracy 0.68, PPV 0.38, NPV 0.99, AUC 0.79. Furthermore, the cut-off values for VI-RADS 4 were as follows: sensitivity, 0.91; specificity, 0.84; accuracy, 0.86; PPV, 0.59; NPV, 0.97; AUC, 0.87 (Table 5).
Table 4
| Cut-off | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC |
|---|---|---|---|---|---|---|
| VI-RADS 3 | 0.94 | 0.68 | 0.76 | 0.57 | 0.96 | 0.81 |
| VI-RADS 4 | 0.84 | 0.93 | 0.90 | 0.84 | 0.93 | 0.88 |
| VI-RADS 5 | 0.51 | 0.99 | 0.84 | 0.96 | 0.81 | 0.75 |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; VI-RADS, Vesical Imaging-Reporting and Data System.
Table 5
| Cut-off | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC |
|---|---|---|---|---|---|---|
| VI-RADS 3 | 0.96 | 0.60 | 0.68 | 0.38 | 0.99 | 0.79 |
| VI-RADS 4 | 0.91 | 0.84 | 0.86 | 0.59 | 0.97 | 0.87 |
| VI-RADS 5 | 0.78 | 0.99 | 0.95 | 0.96 | 0.95 | 0.89 |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; VI-RADS, Vesical Imaging-Reporting and Data System.
Discussion
Since VI-RADS was first introduced in 2018, research on its effectiveness has been continuously conducted. These studies focused on muscle invasion. It is known to have excellent diagnostic performance in several studies (9-11). As muscle invasion in bladder cancer is a major indicator of treatment direction and patient prognosis, it is natural to focus on this. Furthermore, VI-RADS is now widely used worldwide because it is clinically familiar with the reporting systems used for other cancers, such as prostate and breast cancers (12). In this study, when the cutoff was set to VI-RADS 3 or 4, the diagnostic performance of VI-RADS for muscle invasion was similar to that in previous studies (13-16). Moreover, this study found diagnostic accuracy beyond the muscle layer, because it was detected in all RC specimens.
However, VI-RADS still has some weaknesses. One of the weaknesses is the role of VI-RADS score of 3 in predicting muscle involvement. In this study, the sensitivity of VI-RADS score of 3 was 0.94 and the specificity was 0.68. However, the sensitivity rapidly decreased and reached the optimal specificity in VI-RADS score of 4. Some researchers use tumor contact length to overcome this limitation of VI-RADS (17). Another problem is that the three variables (T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images) in MRI are sometimes inconsistent. In this case, a study has shown that using dynamic contrast-enhanced images is advantageous for predicting muscle invasion (10). These are ongoing issues and reasons to modify VI-RADS.
Much research has already been conducted on the inter-reader agreement of VI-RADS, and consensus has almost been reached (18-20). In these studies, the differences between the most experienced radiologists were negligible. However, the VI-RADS is an objective indicator of clinical use. The difference between the validators was not statistically significant (21). In addition, VI-RADS is not a major hurdle for experienced radiologists and produces similar results in most cases. This study did not specifically contribute to the study on inter-reader agreement.
In this study, VI-RADS was confirmed using mp-MRI immediately before RC. Therefore, patients with conditions after neoadjuvant chemotherapy, BCG, or TUR-BT were included. Assessing VI-RADS in these patients was not challenging. For example, a study of VI-RADS after chemotherapy has been introduced, and attempts are being made to evaluate its response (22). Moreover, since they were all RC specimens, the exact pathologic stage could be determined even if these patients were included. Moreover, it showed almost identical diagnostic performance in this study.
To the best of our knowledge, most of the literature has confirmed the performance of VI-RADS with TUR-BT specimens (14,20,23). This is important because the treatment direction varies according to muscle invasion in bladder cancer; however, it has limitations in predicting the overall pathological T stage. Therefore, we attempted to verify the effectiveness of VI-RADS in patients who underwent RC and found the effect of VI-RADS in not only muscle invasion but also a range beyond the muscle. This is a strength of the present study.
Recently, a new treatment algorithm using VI-RADS for bladder cancer has been proposed (24). If bladder cancer is diagnosed by cystoscopy or urine cytology, it is argued that MRI should be performed before TUR-BT and approached according to VI-RADS. In particular, VI-RADS 4 recommends performing neoadjuvant chemotherapy and RC after tissue sampling. This study has the potential to support this perspective. This might also help reduce the interval delay between the initial TUR-BT and RC. In some cases, RC was delayed due to the tight schedule and time required for pathological confirmation. Longer delays from bladder cancer diagnosis to RC can significantly worsen survival outcomes (25). However, this algorithm still requires further discussion.
This study had some limitations. First, it was a retrospective, single-center study and did not include many patients. Therefore, the results of our study must be confirmed and validated using a prospective, large-scale, multicenter study. However, the cohort used in this study was prospectively collected and judged to have less bias than the original retrospective study. Furthermore, in the study, whether VI-RADS scores depended on differences in experience has not been conducted. The two radiologists had similar experiences and, in most cases, agreed. Further research is needed on how much VI-RADS scoring can be performed for inexperienced radiologists or urologists. Finally, comparisons with conventional and widely used CT were not performed. Many factors must be considered in terms of cost, time, and space; however, the performances of the two modalities should be compared later.
Conclusions
Preoperative mp-MRI and VI-RADS scores are good predictors of bladder cancer staging before RC and are especially helpful in predicting muscle invasion and perivesical fat infiltration. However, further well-designed randomized, multicenter, and larger-scale studies are needed. We hope that our study supports the efficacy of mp-MRI and provides a basis for its use.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-704/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-704/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-704/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-704/coif). JHK serves as an unpaid editorial board member of Translational Andrology and Urology from January 2022 to December 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and the Ethical Guidelines for Clinical Studies. The study protocol was reviewed and approved by Seoul National University Hospital (SNUH) Institutional Review Board (IRB) (approval number: 2207-194-1345) and the prospectively collected cohort was approved by the SNUH IRB (approval number: 1506-122-682) for use with clinical data for scientific purposes. Informed consent for this study was obtained from each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Abern M, et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2.2022. J Natl Compr Canc Netw 2022;20:866-78. [Crossref] [PubMed]
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Blindheim AJ, Fosså SD, Babigumira R, et al. The use of reTURB in T1 bladder cancer: a Norwegian population-based study. Scand J Urol 2021;55:268-74. [Crossref] [PubMed]
- Vargas HA, Akin O, Schöder H, et al. Prospective evaluation of MRI, 11C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012;81:4131-7. [Crossref] [PubMed]
- Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol 2018;74:294-306. [Crossref] [PubMed]
- Woo S, Panebianco V, Narumi Y, et al. Diagnostic Performance of Vesical Imaging Reporting and Data System for the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Oncol 2020;3:306-15. [Crossref] [PubMed]
- Jeong CW, Suh J, Yuk HD, et al. Establishment of the Seoul National University Prospectively Enrolled Registry for Genitourinary Cancer (SUPER-GUC): A prospective, multidisciplinary, bio-bank linked cohort and research platform. Investig Clin Urol 2019;60:235-43. [Crossref] [PubMed]
- Del Giudice F, Flammia RS, Pecoraro M, et al. The accuracy of Vesical Imaging-Reporting and Data System (VI-RADS): an updated comprehensive multi-institutional, multi-readers systematic review and meta-analysis from diagnostic evidence into future clinical recommendations. World J Urol 2022;40:1617-28. [Crossref] [PubMed]
- Meng X, Hu H, Wang Y, et al. Accuracy and Challenges in the Vesical Imaging-Reporting and Data System for Staging Bladder Cancer. J Magn Reson Imaging 2022;56:391-8. [Crossref] [PubMed]
- Ghanshyam K, Nachiket V, Govind S, et al. Validation of Vesical Imaging Reporting and Data System score for the diagnosis of muscle-invasive bladder cancer: A prospective cross-sectional study. Asian J Urol 2022;9:467-72. [Crossref] [PubMed]
- Panebianco V, Pecoraro M, Del Giudice F, et al. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J Magn Reson Imaging 2022;55:23-36. [Crossref] [PubMed]
- Wang Z, Shang Y, Luan T, et al. Evaluation of the value of the VI-RADS scoring system in assessing muscle infiltration by bladder cancer. Cancer Imaging 2020;20:26. [Crossref] [PubMed]
- Ueno Y, Takeuchi M, Tamada T, et al. Diagnostic Accuracy and Interobserver Agreement for the Vesical Imaging-Reporting and Data System for Muscle-invasive Bladder Cancer: A Multireader Validation Study. Eur Urol 2019;76:54-6. [Crossref] [PubMed]
- Kim SH. Validation of vesical imaging reporting and data system for assessing muscle invasion in bladder tumor. Abdom Radiol (NY) 2020;45:491-8. [Crossref] [PubMed]
- Marchioni M, Primiceri G, Delli Pizzi A, et al. Could Bladder Multiparametric MRI Be Introduced in Routine Clinical Practice? Role of the New VI-RADS Score: Results From a Prospective Study. Clin Genitourin Cancer 2020;18:409-415.e1. [Crossref] [PubMed]
- Wang X, Tu N, Sun F, et al. Detecting Muscle Invasion of Bladder Cancer Using a Proposed Magnetic Resonance Imaging Strategy. J Magn Reson Imaging 2021;54:1212-21. [Crossref] [PubMed]
- Ahn H, Hwang SI, Lee HJ, et al. Quantitation of bladder cancer for the prediction of muscle layer invasion as a complement to the vesical imaging-reporting and data system. Eur Radiol 2021;31:1656-66. [Crossref] [PubMed]
- Barchetti G, Simone G, Ceravolo I, et al. Multiparametric MRI of the bladder: inter-observer agreement and accuracy with the Vesical Imaging-Reporting and Data System (VI-RADS) at a single reference center. Eur Radiol 2019;29:5498-506. [Crossref] [PubMed]
- Huang S, Bain J, Yiu TW, et al. Accuracy of the Vesical Imaging-Reporting and Data System (VIRADS) for pre-treatment staging of bladder cancer in an Australian cohort. J Med Imaging Radiat Oncol 2022;66:370-6. [Crossref] [PubMed]
- Delli Pizzi A, Mastrodicasa D, Marchioni M, et al. Bladder cancer: do we need contrast injection for MRI assessment of muscle invasion? A prospective multi-reader VI-RADS approach. Eur Radiol 2021;31:3874-83. [Crossref] [PubMed]
- Pecoraro M, Del Giudice F, Magliocca F, et al. Vesical Imaging-Reporting and Data System (VI-RADS) for assessment of response to systemic therapy for bladder cancer: preliminary report. Abdom Radiol (NY) 2022;47:763-70. [Crossref] [PubMed]
- Liu S, Xu F, Xu T, et al. Evaluation of Vesical Imaging-Reporting and Data System (VI-RADS) scoring system in predicting muscle invasion of bladder cancer. Transl Androl Urol 2020;9:445-51. [Crossref] [PubMed]
- Taguchi S, Watanabe M, Tambo M, et al. Proposal for a New Vesical Imaging-Reporting and Data System (VI-RADS)-Based Algorithm for the Management of Bladder Cancer: A Paradigm Shift From the Current Transurethral Resection of Bladder Tumor (TURBT)-Dependent Practice. Clin Genitourin Cancer 2022;20:e291-5. [Crossref] [PubMed]
- Russell B, Liedberg F, Khan MS, et al. A Systematic Review and Meta-analysis of Delay in Radical Cystectomy and the Effect on Survival in Bladder Cancer Patients. Eur Urol Oncol 2020;3:239-49. [Crossref] [PubMed]


