
Study on the mechanism of aging-related erectile dysfunction based on bioinformatics and experimental verification
Highlight box
Key findings
• The phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (AKT) pathway, endothelial dysfunction, and fibrosis are involved in the pathogenesis of aging-related erectile dysfunction (ED).
What is known and what is new?
• Erectile function is impaired with aging. Aging-related ED is widely considered to be the result of a combination of factors.
• Biglycan may be the key gene regulating pathological changes in the penis.
What is the implication, and what should change now?
• Our study provides a basis to further explore the mechanism of aging-related ED, and also proposes new potential treatment ideas. Due to the relatively limited understandings of aging-related ED, more in-depth and detailed research is needed.
Introduction
Erectile dysfunction (ED) refers to the inability of the penis to achieve or maintain enough hardness for satisfactory sex. As a common affliction in men, ED directly affects the quality of life of both spouses, including those in the elderly population (1,2). With improvements in people’s living standards and the aging population, an increasing number of patients are troubled by ED. As one of the causative factors of ED, aging has gradually been attracting attention. Multiple studies have shown that the incidence of ED increases with age and is close to 100% in men aged >70 years (3,4). Moreover, ED appears to be a risk marker for other underlying metabolic or cardiovascular diseases in the body (5). Aging-related ED should be considered one of the most important health problems in the elderly population.
To date, relatively few studies have been conducted on aging-related ED, and its mechanisms have not yet been fully elucidated. Aging-related ED is widely thought to be the result of a combination of factors. Oxidative stress, increased vasoconstriction, and venous occlusive dysfunction appear to be involved in the development of aging-related ED (6). In addition, due to their older age, patients with aging-related ED often have other comorbidities, such as hypertension, diabetes, and Alzheimer’s disease. These diseases themselves could lead to the occurrence of ED. Consequently, patients with these comorbidities are often prescribed therapeutic drugs, such as hypotensors and diuretics. These drugs are also associated with ED (1).
As a first-line treatment for ED, the overall effective rate of the phosphodiesterase type 5 inhibitor (PDE5i) is about 87% (7). However, PDE5i is only effective in roughly 65.7% of patients with aging-related ED (8). The side effects of PDE5i, such as headache, rhinitis and indigestion (9), tend to be more pronounced in the elderly population. Notably, the use of PDE5i is also restricted to varying degrees, as elderly patients tend to take other drugs. Thus, understandings of the pathological mechanisms of aging-related ED need to be urgently extended to achieve better therapeutic effects and improve the quality of life of the elderly.
In this study, we conducted a bioinformatics analysis of data from the Gene Expression Omnibus (GEO) database to examine the molecular and pathway changes associated with age-related ED. We then validated our findings in aged rats. Our results suggest that the phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (AKT) pathway, endothelial dysfunction, and fibrosis are involved in the pathogenesis of aging-related ED. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-511/rc).
Methods
Data sets
A microarray data set [GSE10804 (10)] was downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo) to obtain candidate genes related to ED. It contained data on endothelial cells from both ED and non-ED. Other microarray data sets [GSE13712 (11), GSE37091 (12) and GSE77239 (13)] were also downloaded from the GEO database to obtain candidate genes related to aging. These data sets contained data on both young and senescent endothelial cells in vitro.
Identification of differentially expressed genes (DEGs)
The DEGs were identified using “limma” package in R project (version 4.1.0; http://www.r-project.org/). For the GSE10804 data set, genes with P values <0.05 and |log fold changes (FCs)| ≥1 were considered DEGs. For the GSE13712 data set, genes with P values <0.05 and |log FCs| ≥2 were considered DEGs. For the other 2 data sets (GSE37091 and GSE77239), the screening criteria were set as adjusted P values <0.05 and |log FCs| ≥2. A Venn diagram was drawn to identify the intersection of the DEGs among GSE13712 and the 3 other data sets. The shared genes were used for the subsequent analysis.
Construction of protein-protein interaction (PPI) network and identification of hub genes
The PPI network of the DEGs was created in the STRING database (https://cn.string-db.org/) using a minimum interaction score of 0.40. Next, Cytoscape software (version 3.8.2; https://cytoscape.org/) was used to analyze and better visualize the PPI network.
CytoHubba, a plugin of Cytoscape, was used to identify the hub genes. Four algorithms [i.e., degree, maximal clique centrality (MCC), maximum neighborhood component (MNC), and density of maximum neighborhood component (DMNC)] were applied to calculate the top 10 hub genes, respectively. The intersection of all the results was the final hub genes (14-16).
Functional enrichment analysis
To clarify the biological function of DEGs, a functional enrichment analysis was performed using Sangerbox (http://vip.sangerbox.com/). Next, the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of DEGs were conducted, and a P value <0.05 was considered significant.
Animals
A total of 16 male Sprague-Dawley rats (aged 8-week-old from the Laboratory Animal Center of Tongji Medical College, Wuhan, China) were included in our study. The rats were kept in a specific pathogen-free environment with free access to food and water. The erectile function of all the animals was confirmed by mating experiments. All the rats were randomly divided into two groups (n=8 per group) and bred under the above conditions to the congruent age. This study included the following two groups: young rats as the control group (aged 5-months-old) and aged rats (aged 24-month-old) (17).
The experiments were performed under a project license (No. TJH-201907004) granted by the Committee on Animal Experiments of Tongji Hospital and conducted in accordance with the Guidelines for the Ethical Review of Laboratory Animal Welfare, People’s Republic of China National Standard GB/T 35892-2018.
Erection evaluation in vivo
After the rats reached the predetermined age, erectile function was evaluated under electrical stimulation. First, the carotid artery and cavernous nerve were exposed after the rats had been anesthetized. Subsequently, the intracavernous pressure (ICP) and arterial pressure were recorded (BL-420F, Taimeng, China) under the electrical stimulation of the cavernous nerve (15 Hz; 5.0 V; 1 min). Finally, the corpus cavernosum was harvested, embedded in paraffin, and frozen at –80 ℃.
Western blot (WB) analysis
The expression levels of the related proteins in the frozen corpus cavernosum were detected by WB analysis. The following primary antibodies were used: PI3K, regulatory subunit 3 (gamma) (PI3K p55; 1:500; 55036-1-AP; Proteintech, Wuhan, China), AKT (1:500; 60203-2-Ig; Proteintech), phospho-AKT (p-AKT; 1:500; BM4838; Boster, Wuhan, China), endothelial nitric oxide synthase (eNOS; 1:1,000; ab5589; Abcam, USA), phospho-eNOS (p-eNOS; 1:500; 9571; Cell Signaling Technology, USA), transforming growth factor β1 (TGFβ1; 1:1,000; 21898-1-AP; Proteintech), α-smooth muscle actin (α-SMA; 1:1,000; GB111364; Servicebio, Wuhan, China), and β-actin (1:1,000; GB11001; Servicebio). The membranes were then incubated with the secondary antibody (anti-rabbit: 1:5,000; GB23303, Servicebio; anti-mouse: 1:5,000; ab6789, Abcam). The immunoblots were detected using the ChemiDocTM MP Image System (Bio-Rad Laboratories, CA, USA).
Quantitative real-time-polymerase chain reaction (qRT-PCR)
The total ribonucleic acid (RNA) of the frozen corpus cavernosum was isolated with RNA extraction reagent (G3013; Servicebio). Reverse transcription and qRT-PCR (Yeasen) were carried out to determine the messenger RNA (mRNA) levels of the relevant genes. The 2-ΔΔCT method was used to analyze the data. The primer sequences used are listed in Table 1.
Table 1
| Genes | Primer sequences (5'→3') |
|---|---|
| COL1A2 | F TGTCGATGGCTGCTCCAAAA |
| R CCGATGTCCAGAGGTGCAAT | |
| COL3A1 | F GCCTACATGGATCAGGCCAA |
| R CACCAGTGTGTTTAGTGCAGC | |
| COL6A2 | F TGGAAGACGTCCTTTGTCCG |
| R GTGACACCTCCTCCACGAAG | |
| BGN | F TCTCAAACTCCTCCAGGTTGTC |
| R AGTAGGGCACAGGGTTGTTG | |
| β-actin | F GGAGATTACTGCCCTGGCTCCTA |
| R GACTCATCGTACTCCTGCTTGCTG |
qRT-PCR, quantitative real-time-polymerase chain reaction; COL1A2, collagen type I alpha 2 chain; COL3A1, collagen type III alpha 1 chain; COL6A2, collagen type VI alpha 2 chain; BGN, biglycan.
Detection of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP)
NO and cGMP of the corpus cavernosum were detected following the respective protocols. A total NO assay kit (Beyotime Biotechnology, Shanghai, China) and an enzyme-linked immunosorbent assay (ELISA) kit of cGMP (Mbbiology, Jiangsu, China) were used to assess the level of these molecules.
Histologic examination
Immunofluorescence (IF) was carried out to determine the level of the corresponding molecules. The primary antibodies against cluster of differentiation 31 (CD31; 1:100; GB113151, Servicebio) and α-SMA (1:100; GB111364, Servicebio) were used in this study.
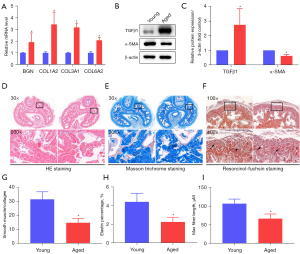
Hematoxylin and eosin (H&E) staining was employed to examine the gross changes in histomorphology; the chromatin in the nucleus and nucleic acids in the cytoplasm were colored violet-blue, and the cytoplasm and extracellular matrix (ECM) were colored red.
Masson trichrome staining was performed to evaluate the ratio of the corpus cavernosum to collagen in the penis. The red part represented the smooth muscle, while the blue part represented collagen. The above results indicated the degree of fibrosis in the penile tissue.
Resorcinol-fuchsin staining was used to show the level of elastin in the penile tissue. The elastic fibers were purple-black, the collagen fibers were red, and other components in the background were yellow.
Statistical analysis
All the in vitro experiments were conducted at least 3 times. The results are expressed as the mean ± standard deviation, and the data were analyzed using GraphPad Prism (version 8.0). An unpaired t-test was used to test the data, and a P value <0.05 was considered statistically significant.
Results
Identification of the DEGs
The details of the 4 data sets used in this study are listed in Table 2. After a normalized analysis of each data set, the DEGs of each data set were obtained using R project. Next, a Venn diagram was drawn to identify the intersection of the DEGs among the GSE13712 and the other 3 data sets. A total of 100 genes were identified as the final DEGs (Figure 1A).
Table 2
| Data set | Type of samples | Platform | Case samples | Control samples | Gene numbers |
|---|---|---|---|---|---|
| GSE10804 | HCCECs, HCAECs, and HUVECs from ED and non-ED population | GPL571 | 4 | 8 | 12,640 |
| GSE13712 | Senescent HUVECs were prepared by continuous subculture in vitro | GPL570 | 6 | 6 | 20,864 |
| GSE37091 | Senescent HUVECs were prepared by continuous subculture in vitro | GPL6480 | 3 | 2 | 19,595 |
| GSE77239 | Senescent HCAECs were prepared by continuous subculture in vitro | GPL570 | 3 | 3 | 20,864 |
GEO, Gene Expression Omnibus; HCCECs, human corpus cavernosum endothelial cells; HCAECs, human coronary artery endothelial cells; HUVECs, human umbilical vein endothelial cells; ED, erectile dysfunction.
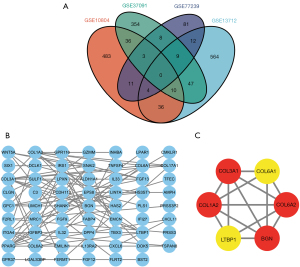
Construction of PPI network and identification of the hub genes
The 100 DEGs were analyzed via the STRING database, and the PPI network was constructed. Cytoscape was used to analyze and present the results (Figure 1B). The highest scoring PPI network was also generated with the CytoHubba plugin (Figure 1C). We then used 4 algorithms, including degree (Table S1), MCC (Table S2), MNC (Table S3), and DMNC (Table S4), to retrieve the top 10 hub genes, respectively. A Venn diagram was drawn to identify the intersection of the above hub genes under different algorithms (Figure S1), including collagen type I alpha 2 chain (COL1A2), collagen type III alpha 1 chain (COL3A1), collagen type VI alpha 2 chain (COL6A2), and biglycan (BGN).
Functional enrichment analysis
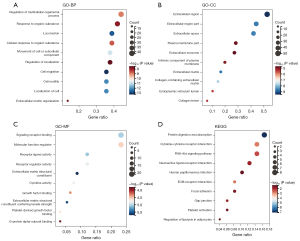
GO and KEGG enrichment analyses of the 100 DEGs were performed to identify their biological functions (Figure 2). The GO profiles revealed that these DEGs were associated with the ECM, especially collagen-containing ECM. The KEGG profiles revealed that these DEGs were associated with the ECM-receptor interaction and PI3K/AKT pathway.
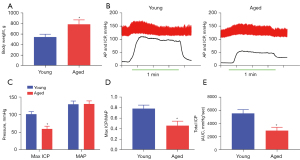
Physiological parameters for the rats
To validate the bioinformatics results, we constructed a model of aged rats. The body weight of the aged rats was significantly higher than that of the young rats (P<0.05; Figure 3A). The mean arterial pressure (MAP) did not differ between the two groups (P>0.05; Figure 3B,3C), but the aged rats had a lower maximum ICP/MAP and total ICP than the younger rats (all P<0.05; Figure 3B-3E). The above results indicated that the erectile function of the aged rats was impaired compared to that of the young rats.
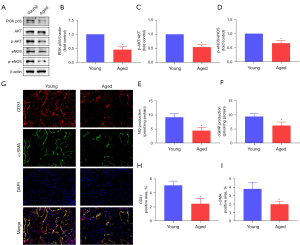
The PI3K/AKT pathway and endothelial function in vivo
Given that the PI3K/AKT pathway was included in the KEGG analysis, we validated it in the rat penis. The eNOS/NO/cGMP pathway is crucial for penile erection and is downstream of the PI3K/AKT signaling pathway. As a result, we also verified the expression of the eNOS/NO/cGMP pathway in the rat penis. The expression of PI3K p55, the ratio of p-AKT to AKT, and the ratio of p-eNOS to eNOS in the aged rats were lower than those in the young rats (all P<0.05; Figure 4A-4D). The levels of NO and cGMP in the two groups were also in line with the above trends (both P<0.05; Figure 4E,4F). In addition, IF revealed that the content of the endothelial and smooth muscle cells was also decreased in the aged rats (all P<0.05; Figure 4G-4I).
The level of the hub genes and fibrosis in penis
The mRNA levels of the hub genes in the penile tissue were detected by qPCR. The results showed that the levels of COL1A2, COL3A1, COL6A2, and BGN of the aged rats were upregulated compared to those of the young rats (all P<0.05; Figure 5A). Since these hub genes were all associated with fibrosis and the results of the functional analysis were related to the formation of the ECM, we examined the level of fibrosis in penile tissue. The expression of TGFβ1 was increased in the penis of the aged rats, while the expression of α-SMA was decreased (both P<0.05; Figure 5B,5C). The H&E staining results indicated that blood sinuses were disordered in the aged rats, and the thickness of the smooth muscle layer was thin and discontinuous (Figure 5D). In addition, the ratio of smooth muscle to collagen, the content of elastin, and the length of the largest fibers also showed decreasing trends in the aged rats (all P<0.05; Figure 5E-5I).
Discussion
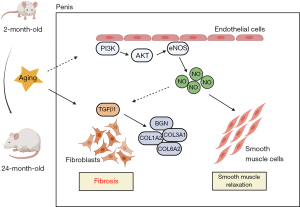
Aging-related ED, which is an important type of ED, has a high incidence in the elderly population. Given that the pathogenesis of aging-related ED is complex and not yet fully understood, as well as the medication limitations for the elderly, the current conventional treatments for ED are not very effective for this disease (18). Thus, this experiment sought to explore the potential key genes and pathways involved in the pathophysiology of aging-related ED. We conducted a bioinformatics analysis of the data sets related to ED and aging and found that 4 hub genes (COL1A2, COL3A1, COL6A2, and BGN) and related pathways were associated with the generation of the ECM and PI3K/AKT pathway. Subsequently, we validated the above findings in aged rats, and found that the expression of the PI3K/AKT pathway was indeed decreased and was accompanied by endothelial dysfunction and fibrosis in the penises of the aged rats. We believed that these results may inform future research on aging-related ED.
The PI3K/AKT pathway has become a major focus of attention in the medical community because of its important role in regulating various cellular functions, including metabolism, growth, proliferation, survival, transcription, and protein synthesis (19). The dysregulation of this pathway has been observed in a variety of human diseases, including geriatric diseases (20,21). The PI3K/AKT pathway is also important for erectile function (22). As one of the downstream pathways of the PI3K/AKT pathway, the activation of the eNOS/NO/cGMP pathway could directly promote the relaxation of vascular smooth muscles, which results in normal penile erection. The above process depends on the normal function of the endothelial cells and smooth muscle cells (3). Several studies have reported that the phosphorylation of AKT and eNOS in the penis of aged rats is decreased (23,24). In the present study, our results indicated that PI3K/AKT and eNOS/NO/cGMP pathways were downregulated in the aged rats, and this downregulation was accompanied by endothelial dysfunction.
Fibrosis, resulting from diabetes and nerve damage, is also involved in the pathophysiological process of ED (7,25). The process of fibrosis has also been reported to be involved in aging-related ED (18,23). Fibrosis in penile tissue often involves a decrease in the content of cells, such as smooth muscle cells, an increase in the content of collagen, and the fragment and decrease of elastic fibers (26). The hub genes in this study happened to contain collagen (COL1A2, COL3A1, and COL6A2). COL1A2 and COL3A1 have been shown to be involved in the development of fibrosis in ED (27). COL6A2 has also been reported to be involved in the development of fibrosis in the liver (28).
BGN is also upregulated during fibrosis in the kidney and heart (29,30). In our study, we found the aged rats had elevated expressions of all 4 hub genes and increased levels of fibrosis in the penis. Notably, NO produced by endothelial cells has also been shown to have anti-fibrotic effects (31). Further, the single-cell RNA sequencing of the human corpus cavernosum revealed a unique subtype of endothelial cells with the hallmarks of fibroblasts (32). Combined with the epithelial to mesenchymal transition in the kidney and heart (33,34), we hypothesize that endothelial cells may transform into fibroblasts and exacerbate the development of fibrosis. We just observed both impaired endothelial dysfunction and the presence of fibrosis in aged rats.
This study summarized and elucidated the partial pathogenesis of aging-related ED (Figure 6). As age increases, the level of fibrosis in the penis increases. As one of the hub genes, BGN may play an important role in this process. In addition, the PI3K/AKT pathway is inhibited in aged rats, and one of its downstream pathways, the eNOS/NO/cGMP pathway, is also downregulated. These changes in turn exacerbate the process of fibrosis and ultimately lead to aging-related ED.
We validated the bioinformatics results in vitro; however, this study still had several limitations. First, we only confirmed the expression levels of the hub genes, especially BGN, and did not explore their subsequent pathways. Second, no gene or pathway was modulated in the animal or cellular experiments to observe the subsequent effects. Finally, factors such as metabolic syndrome or low levels of testosterone, which were important for ED, were not examined in this study. We intend to address these issues in our future research.
Conclusions
Erectile function was confirmed to decreased in aged rats, which were also accompanied by an increased level of fibrosis. The upregulation of BGN may play an important role in this process. In addition, the downregulation of the PI3K/AKT pathway and endothelial dysfunction were found to be involved in the mechanism of aging-related ED. Our study provides a basis for the further exploration of the mechanism of aging-related ED, and also proposes new potential treatment ideas.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Nos. 81873831 and 82001536).
Footnote
Reporting Checklist: The authors have completed the ARRIVE checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-511/rc).
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-511/dss
Conflicts of Interest: All the authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-511/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work appropriately investigated and resolved. The experiments were performed under a project license (No. TJH-201907004) granted by the Committee on Animal Experiments of Tongji Hospital and conducted in compliance with the Guidelines for the Ethical Review of Laboratory Animal Welfare, People’s Republic of China National Standard GB/T 35892-2018.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muneer A, Kalsi J, Nazareth I, et al. Erectile dysfunction. BMJ 2014;348:g129. [Crossref] [PubMed]
- Porst H, Burnett A, Brock G, et al. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med 2013;10:130-71. [Crossref] [PubMed]
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381:153-65. [Crossref] [PubMed]
- Wang W, Fan J, Huang G, et al. Meta-Analysis of Prevalence of Erectile Dysfunction in Mainland China: Evidence Based on Epidemiological Surveys. Sex Med 2017;5:e19-30. [Crossref] [PubMed]
- Saigal CS, Wessells H, Pace J, et al. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207-12. [Crossref] [PubMed]
- Ferrini MG, Gonzalez-Cadavid NF, Rajfer J. Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl Androl Urol 2017;6:20-7. [Crossref] [PubMed]
- Liu K, Sun T, Luan Y, et al. Berberine ameliorates erectile dysfunction in rats with streptozotocin-induced diabetes mellitus through the attenuation of apoptosis by inhibiting the SPHK1/S1P/S1PR2 and MAPK pathways. Andrology 2022;10:404-18. [Crossref] [PubMed]
- Chen L, Staubli SE, Schneider MP, et al. Phosphodiesterase 5 inhibitors for the treatment of erectile dysfunction: a trade-off network meta-analysis. Eur Urol 2015;68:674-80. [Crossref] [PubMed]
- Lin G, Hayashi N, Carrion R, et al. Improving erectile function by silencing phosphodiesterase-5. J Urol 2005;174:1142-8. [Crossref] [PubMed]
- Wessells H, Sullivan CJ, Tsubota Y, et al. Transcriptional profiling of human cavernosal endothelial cells reveals distinctive cell adhesion phenotype and role for claudin 11 in vascular barrier function. Physiol Genomics 2009;39:100-8. [Crossref] [PubMed]
- Mun GI, Lee SJ, An SM, et al. Differential gene expression in young and senescent endothelial cells under static and laminar shear stress conditions. Free Radic Biol Med 2009;47:291-9. [Crossref] [PubMed]
- Jong HL, Mustafa MR, Vanhoutte PM, et al. MicroRNA 299-3p modulates replicative senescence in endothelial cells. Physiol Genomics 2013;45:256-67. [Crossref] [PubMed]
- Costarelli L, Giacconi R, Malavolta M, et al. Different transcriptional profiling between senescent and non-senescent human coronary artery endothelial cells (HCAECs) by Omeprazole and Lansoprazole treatment. Biogerontology 2017;18:217-36. [Crossref] [PubMed]
- Cheng Q, Chen X, Wu H, et al. Three hematologic/immune system-specific expressed genes are considered as the potential biomarkers for the diagnosis of early rheumatoid arthritis through bioinformatics analysis. J Transl Med 2021;19:18. [Crossref] [PubMed]
- Luan H, Zhang C, Zhang T, et al. Identification of Key Prognostic Biomarker and Its Correlation with Immune Infiltrates in Pancreatic Ductal Adenocarcinoma. Dis Markers 2020;2020:8825997. [Crossref] [PubMed]
- Yang X, Li Y, Lv R, et al. Study on the Multitarget Mechanism and Key Active Ingredients of Herba Siegesbeckiae and Volatile Oil against Rheumatoid Arthritis Based on Network Pharmacology. Evid Based Complement Alternat Med 2019;2019:8957245. [Crossref] [PubMed]
- Yang J, Zhang Y, Zang G, et al. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology 2018;6:498-509. [Crossref] [PubMed]
- Hu D, Ge Y, Cui Y, et al. Upregulated IGFBP3 with Aging Is Involved in Modulating Apoptosis, Oxidative Stress, and Fibrosis: A Target of Age-Related Erectile Dysfunction. Oxid Med Cell Longev 2022;2022:6831779. [Crossref] [PubMed]
- Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal 2011;23:1515-27. [Crossref] [PubMed]
- Wang D, Wang E, Li Y, et al. Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway. Molecules 2022;27:4502. [Crossref] [PubMed]
- Sun J, Zhao H, Shen C, et al. Tideglusib promotes wound healing in aged skin by activating PI3K/Akt pathway. Stem Cell Res Ther 2022;13:269. [Crossref] [PubMed]
- Luan Y, Cui K, Tang Z, et al. Human Tissue Kallikrein 1 Improves Erectile Dysfunction of Streptozotocin-Induced Diabetic Rats by Inhibition of Excessive Oxidative Stress and Activation of the PI3K/AKT/eNOS Pathway. Oxid Med Cell Longev 2020;2020:6834236. [Crossref] [PubMed]
- Wang Y, Wang Y, Cong R, et al. Restoration of erectile function by suppression of corporal apoptosis and oxidative stress with losartan in aged rats with erectile dysfunction. Andrology 2020;8:769-79. [Crossref] [PubMed]
- Musicki B, Kramer MF, Becker RE, et al. Age-related changes in phosphorylation of endothelial nitric oxide synthase in the rat penis. J Sex Med 2005;2:347-55; discussion 355-7. [Crossref] [PubMed]
- Wang J, Song J, Song G, et al. Acetyl-L-carnitine improves erectile function in bilateral cavernous nerve injury rats via promoting cavernous nerve regeneration. Andrology 2022;10:984-96. [Crossref] [PubMed]
- Lei H, Xin H, Guan R, et al. Low-intensity Pulsed Ultrasound Improves Erectile Function in Streptozotocin-induced Type I Diabetic Rats. Urology 2015;86:1241.e11-8. [Crossref] [PubMed]
- Chen ZB, Li G, Lin H, et al. Low androgen status inhibits erectile function by increasing pyroptosis in rat corpus cavernosum. Andrology 2021;9:1264-74. [Crossref] [PubMed]
- Chen W, Zhao W, Yang A, et al. Integrated analysis of microRNA and gene expression profiles reveals a functional regulatory module associated with liver fibrosis. Gene 2017;636:87-95. [Crossref] [PubMed]
- Nakamura T, Bonnard B, Palacios-Ramirez R, et al. Biglycan Is a Novel Mineralocorticoid Receptor Target Involved in Aldosterone/Salt-Induced Glomerular Injury. Int J Mol Sci 2022;23:6680. [Crossref] [PubMed]
- Beetz N, Rommel C, Schnick T, et al. Ablation of biglycan attenuates cardiac hypertrophy and fibrosis after left ventricular pressure overload. J Mol Cell Cardiol 2016;101:145-55. [Crossref] [PubMed]
- Wang C, Kemp-Harper BK, Kocan M, et al. The Anti-fibrotic Actions of Relaxin Are Mediated Through a NO-sGC-cGMP-Dependent Pathway in Renal Myofibroblasts In Vitro and Enhanced by the NO Donor, Diethylamine NONOate. Front Pharmacol 2016;7:91. [Crossref] [PubMed]
- Fang D, Tan XH, Song WP, et al. Single-Cell RNA Sequencing of Human Corpus Cavernosum Reveals Cellular Heterogeneity Landscapes in Erectile Dysfunction. Front Endocrinol (Lausanne) 2022;13:874915. [Crossref] [PubMed]
- Gwon MG, An HJ, Kim JY, et al. Anti-fibrotic effects of synthetic TGF-β1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal transitions. FASEB J 2020;34:333-49. [Crossref] [PubMed]
- Peng Q, Shan D, Cui K, et al. The Role of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Cells 2022;11:1834. [Crossref] [PubMed]


