
Identification and validation of immunohistochemical marker panels to predict the prognosis of muscle invasive bladder cancer
Highlight box
Key findings
• A prognostic model based on immunohistochemistry (IHC) markers to stratify muscle invasive bladder cancer (MIBC) prognosis was established.
What is known and what is new?
• The luminal/basal molecular subtype of bladder cancer has been reported to be predictors of prognosis, but the high-cost of sequencing limits its clinical application.
• In this retrospective study, we established a clinically-practical risk stratification model based on cost-efficient immunohistochemistry markers. MIBC were classified into luminal/basal groups by 4 IHC markers (CK5/6, CK14, CK20 and GATA3) with significantly distinct prognosis. Furthermore, luminal MIBC were classified into different risk groups by a 2-marker classifier (YAP1 and CCNB1).
What is the implication, and what should change now?
• This work established an economical, clinically-practical way to stratify the prognosis of bladder cancer patients, providing guidance to treatment and follow-up regimen.
Introduction
Bladder cancer is the 7th most commonly diagnosed cancer in males; 25% of patients have muscle invasive bladder cancer (MIBC) at the time of initial diagnosis (1). Radical cystectomy (RC) is the standard treatment for MIBC, neoadjuvant chemotherapy and adjuvant immunotherapy have been demonstrated to improve the survival of MIBC patients (2,3). However, despite the development of treatment strategy, the 5-year overall survival (OS) rate for MIBC is approximately 50% (1). Clinicopathological parameters may be insufficient to identify patients at high risk of progression, and patients with similar histopathological characteristics may belong to distinct molecular subgroups with different prognosis (4,5). Therefore, clinical-practicable molecular classification may help to stratify MIBC patients into different risk groups for personalized treatment and follow-up strategy (5).
Several studies classified MIBC into various distinct molecular subtypes using next generation sequencing (6-9). There is a general consensus that basal and luminal subtypes of MIBC is at the top-level separation (10). The basal-subtype MIBC was more aggressive with shorter survival compared with the luminal-subtype. On the other hand, basal-subtype was more sensitive to cisplatin-based chemotherapy and appeared to gain more benefits from immunotherapy compared with luminal-subtype (8,10,11). A set of immunohistochemical (IHC) markers have been developed to classify MIBC into luminal- and basal-subtypes. The common IHC markers include GATA3 and CK20 for luminal-subtype, CK5/6 and CK14 for basal-subtype (10,12-14). Nevertheless, few studies have evaluated the role of these IHC markers in clinical practice.
In the present study, we aimed to assess the applicability of basal/luminal molecular classification using IHC markers (GATA3, CK20, CK5/6 and CK14) in an independent retrospective cohort of bladder cancer patients. Meanwhile, a set of IHC markers that have been demonstrated to have prognostic value for MIBC in our previous study were explored as additional molecular classifier. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-538/rc).
Methods
Patients
In this study, patients who underwent RC were retrospectively identified from Changhai Hospital between 2006 and 2016. Patients were divided into training set and validation set. Specifically, training and validation sets were selected between 2006–2014 and 2011–2016 (patients assigned to training cohort were not included), respectively. Patients who received neoadjuvant chemotherapy, died within 30 days of surgery, had other tumor history, had no follow-up information or informed consent were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Committee on Ethics of Medicine, Second Military Medical University (No. CHEC2019-134). Individual consent for this retrospective analysis was waived.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) blocks of all patients were obtained from Department of Pathology, Changhai Hospital. For each FFPE block, 1 pathologist-defined tumor core was taken, and tissue microarrays (TMAs) of training set and validation set were constructed with these cores. Immunohistochemistry (IHC) were performed using antibodies for CK5/6 (MXB Biotechnologies, Fuzhou, China, MAB-0744), CK14 (MXB Biotechnologies, MAB-0832), CK20 (MXB Biotechnologies, MAB-0834), GATA3 (MXB Biotechnologies, MAB-0695), YAP1 (Abcam, Cambridge, UK, ab52771), CCNB1 (Abcam, ab72), NEK2 (Absin, Shanghai, China, abs133048), p53 (MXB Biotechnologies, MAB-0674), ANLN (Abcam, ab211872), CDC20 (Bio-immune, Shanghai, China, BM15656), PD-L1 (Cell Signaling, Massachusetts, USA, 13684), ARID1A (Santa Cruz, Texas, USA, sc-32761), IPO11 (Abcam, ab221615), and KLF15 (Abcam, ab22851). Dewaxation and rehydration were performed before heat-induced epitope retrieval in ethylenediaminetetraacetic acid or citric buffer. Slides were incubated with antibodies overnight at 4 ℃ then referred to secondary antibody incubation for 30 min at room temperature, followed by incubating in DAB solution (MXB Biotechnologies) and counterstaining with haematoxylin. Detailed procedures were described previously (15). After dehydration and mounting, the slides were scanned by Hamamatsu scanner and viewed with NDP.view software (Ver 2.9.22, Hamamatsu, Shizuoka, Japan).
Slides were stained and viewed by pathologist who was blinded to the patients’ information and prognosis. Staining scores were evaluated by both intensity (0–3, 0 for no staining, 1 for low staining, 2 for medium staining and 3 for strong staining) and percentage of positive cells (0–100%). The intensity score was multiplied by percentage score and recorded as final IHC score. Nuclear and cytoplasmic tumor specific IHC scores were evaluated separately for YAP1, CCNB1, NEK2, ANLN, CDC20, and IPO11, while others were evaluated only for nuclear, cytoplasmic or membrane based on previous reports (16-20).
Molecular subgrouping based on IHC score
Tumors positive for GATA3 and/or CK20 but negative for CK5/6 and CK14 were regarded as luminal, while those positive for CK5/6 and/or CK14 but negative for GATA3 and CK20 were regarded as basal. Tumors positive for both luminal markers (GATA3, CK20) and basal markers (CK5/6, CK14) were classified based on the higher combined IHC scores of the two markers in each subtype (e.g., patient with sum scores of GATA3 and CK20 > CK5/6 and CK14 was categorized as luminal). For other markers, the best cutoff to distinguish patients’ prognosis was determined by X-tile software (Ver 3.6.1, Rimm Lab, Yale School of Medicine, Connecticut, USA), and the tumors were classified into high- and low-expression groups according to cutoff values. Significant markers were applied to validation group for further verification.
Bioinformatic analysis
The bladder cancer samples in The Cancer Genome Atlas (TCGA) project (n=433) were selected as the external validation cohort. Patients who had follow-up time less than 30 days, non-muscle invasive bladder cancer (NMIBC), non-transitional cell carcinoma, non-primary tumor or received neoadjuvant chemotherapy were excluded. After scaling the transcripts per million expression levels of KRT5, KRT14, GATA3, and KRT20 across tumor samples, luminal score (GATA3 + KRT20) and basal score (KRT5 + KRT14) were calculated, respectively. Tumors with higher luminal score were identified as luminal subtype. To validate the combined prognostic value of YAP1 and CCNB1 on luminal MIBC, the reverse phase protein array (RPPA) data which quantify protein expression were downloaded from the TCGA-BLCA project. Patients with valid RPPA data were classified into different risk groups and survival analysis was performed where the OS was defined as end point due to the lack of cancer-specific survival (CSS) data.
Statistical analysis
Statistical analysis was performed with R software (Ver 4.1.1). Missing data were listwise deleted. Categorical data were compared using Chi-square test. Numerical data were compared using Student t-test. Time-to-event data were analyzed by Kaplan-Meier survival curve and log-rank test, and the end point was defined as CSS. Pearson’s χ2 test assessed associations between markers. Univariable and multivariable cox proportional hazards analysis was conducted with a backward step-down Wald selection method. P value <0.05 with 2 sides was considered statistically significant.
Results
Patient characteristics
A total of 236 patients who underwent RC had valid TMA spots after quality assessment and complete clinicopathological information was recorded for analysis. Clinical characteristics of the patients were shown in Table 1. There were 163 and 73 patients included as training and validation cohorts, respectively. The median follow-up time is 70.57 [interquartile range (IQR): 66.90–75.83] and 62.36 (IQR 56.40–65.77) months for training and validation cohorts, respectively.
Table 1
| Risk factors | Training cohort (n=163) | Validation cohort (n=73) | P value |
|---|---|---|---|
| Age (years, mean ± SD) | 66.31±10.06 | 66.05±10.19 | 0.86 |
| Gender | |||
| Male | 147 | 66 | 1 |
| Female | 16 | 7 | |
| Tumor size group | |||
| ≤3 cm | 87 | 42 | 0.65 |
| >3 cm | 76 | 31 | |
| Tumor grade | |||
| Low | 36 | 12 | 0.41 |
| High | 127 | 61 | |
| Tumor number | |||
| Single | 40 | 27 | 0.07 |
| Multiple | 123 | 46 | |
| T stage | |||
| Ta & T1 | 64 | 21 | 0.42 |
| T2 | 42 | 24 | |
| T3 | 39 | 21 | |
| T4 | 18 | 7 | |
| N stage | |||
| Negative | 139 | 57 | 0.24 |
| Positive | 24 | 16 | |
| Recurrent tumor | |||
| Primary | 109 | 54 | 0.35 |
| Recurrent | 54 | 19 | |
| Subtype | |||
| Luminal | 104 | 44 | 0.84 |
| Basal | 57 | 28 | |
| Double negative | 2 | 1 |
SD, standard deviation.
Basal and luminal classification
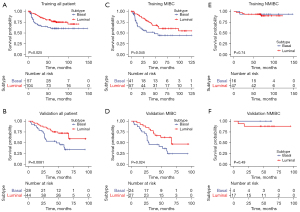
The IHC scores of TMA cores were analyzed using heatmap, as shown in Figure 1A,1B. It became evident that tumors clustered into two categories, corresponding to luminal and basal subtypes. The representative expression patterns of CK5/6, CK14, CK20 and GATA3 were illustrated in Figure 1C and Figure S1. The baseline patients’ characteristics of the 2 categories were shown in Table S1. As shown in Figure 2A,2B, the basal tumors were associated with a significantly shorter survival as compared to luminal tumors (P=0.025 and 0.0081 in training and validation cohorts, respectively). In line with previous studies, we identified a small group of tumors (n=3, 1.3%) with poor prognosis (median CSS, 6.6 months) expressed neither luminal nor basal markers, but were not included in further subgroup analysis due to small sample size. Subgroup analysis was further performed in MIBC subgroup (Figure 2C,2D), patients with basal tumor had significantly reduced CSS compared to luminal tumor in both training (median survival 47.9 months vs. not reached, P=0.045) and validation cohorts (median survival 28.9 vs. 67.2 months, P=0.024). Regarding NMIBC cohort (Figure 2E,2F), basal and luminal subtypes showed no correlation with prognosis in both training and validation sets (P=0.74 and 0.49). In multivariable analysis, age over 65 (HR =1.64, 95% CI: 1.02–2.63, P=0.04), MIBC stage (HR =5.28, 95% CI: 2.32–12.02, P<0.01) and basal subtype (HR =1.91, 95% CI: 1.19–3.05, P<0.01) were independent risk factors of CSS (Table S2).
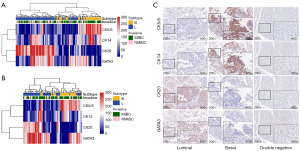
Molecular subtypes of luminal and basal tumors
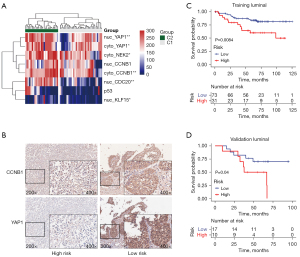
Correlation between selected IHC markers and CSS of luminal or basal MIBC patients were assessed in the training cohort. A few markers showed prognostic significance for basal tumors based on the IHC marker panel we developed (Figure S2). However, we found luminal tumors could be further classified into 2 distinct groups when clustered according to the IHC scores of these markers (Figure 3A). Specifically, lower expression of CCNB1 (cytoplasmic), NEK2 (cytoplasmic), YAP1 (cytoplasmic), CDC20 (nuclear), KLF15 (nuclear), and p53 (nuclear) was significantly correlated with decreased survival (Figure S3 and Table S3) in luminal subgroup. Next, these six markers were analyzed for correlations to assess if they interact with each other, and we revealed the expression of these markers were relatively independent (Figure S4). We further performed IHC analysis of these six markers on the validation cohort, and found patients with low expression of cytoplasmic CCNB1 and YAP1 had significantly shorter survival in line with the training cohort (Figure S5). Moreover, CCNB1 and YAP1 were combined to stratify luminal MIBC tumors. Patients with high expression of both CCNB1 and YAP1 were defined as low-risk luminal tumors, and those with low expression of either CCNB1 or YAP1 were defined as high-risk luminal tumors. Representative IHC images were shown in Figure 3B and examples of different staining intensity were shown in Figure S6. Compared to low-risk luminal type, the high-risk type was significantly associated with decreased CSS both in training (median survival 43.6 months vs. not reached, P=0.0084, Figure 3C) and validation cohorts (median survival 52.2 months vs. not reached, P=0.04, Figure 3D). Multivariable analysis revealed that advanced stage, metastasis and molecular subtypes were independent risk factors for prognosis of luminal MIBC patients (Table S4).
External validation of molecular subtypes using TCGA data
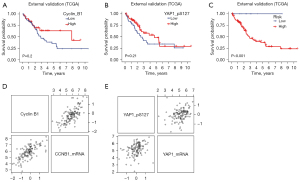
A total of 265 cases met the inclusion criteria and were finally included for validation. 156 were identified as luminal tumor due to the higher luminal score (Figure S7A,S7B). Among them, RRPA data were available for 125 patients in luminal group. The protein expression of YAP1_pS127 rather than YAP1 was selected because phosphorylated YAP1 tends to localize in the cytoplasm. Survival curve of YAP1_pS127 and Cyclin B1 of these patients showed that higher levels of either YAP1_pS127 or Cyclin B1 indicate tendency of better prognosis, although not statistically significant (Figure 4A,4B). While the combination of Cyclin B1 and YAP1_pS127 defined a group of patients with significantly good survival (Figure 4C), which is consistent with IHC result. Although the mRNA and protein expression level correlated well (Figure 4D,4E), we did not find the same trend at mRNA level (Figure S7C,S7D).
Discussion
RC is currently the standard treatment for MIBC, but it is associated with high postoperative complications, impaired quality of life, and the 5-year survival of MIBC patients after RC is only about 50% (21,22). Conventional pathological parameters such as tumor stage and grade have limited ability to predict the heterogenous behaviors of bladder cancer, especially for tumors with similar stage and grade (4). The present study showed that a four-marker IHC panel, including GATA3, CK20 as luminal markers, and CK5/6, CK14 as basal markers, is valuable to stratify MIBC patients into different molecular subgroups. Patients with basal-type tumor had worse prognosis compared with luminal-type tumor. Furthermore, we developed and validated a novel two-marker IHC panel (YAP1, CCNB1) to delineate luminal type as high-risk and low-risk subgroups.
The molecular classification of urothelial bladder cancer has made great progress in the last decade. Gaining insight into the biology of bladder cancer with the development of next-generation sequencing and bioinformatics analysis, distinct molecular subtypes of bladder cancer have been revealed. Sjödahl et al. (6) firstly reported five major subtypes: urobasal A, urobasal B, genomically unstable, squamous cell carcinoma like, and infiltrated. The TCGA identified four distinct molecular subtypes of bladder cancer (7). Choi et al. (8) classified bladder cancer into three categories: basal, luminal and p53-like. Damrauer et al. (9) proposed two molecular subsets of high-grade bladder cancer, termed luminal and basal-like subtypes. From these studies, it is obvious that bladder cancer is a heterogenous disease not only by clinicopathological characteristics, but also molecular alterations. These molecular subtypes revealed different carcinogenesis of bladder cancer and could be used to predict the prognosis. Currently, it is widely accepted that the top-level classification of bladder cancer is basal and luminal subtypes (9). This two-category classification resembles that originally identified in breast cancer (23). Basal-subgroup MIBC was associated with poorer overall- and progression-free survival in comparison to luminal subgroup, and basal tumors were found to be more sensitive to neoadjuvant cisplatin-based chemotherapy and immunotherapy (10,11,24,25). Although identifying molecular subtypes of bladder cancer with gene expression analysis by sequencing is ideal, it is not economically and technically feasible for routine clinical diagnostics. Studies have tried to develop a reliable IHC panel for classifying molecular subtypes of bladder cancer and predicting patient prognosis, because IHC markers would permit cost-effective and simple classification (10,12,14,26).
It has been suggested luminal tumors were positive for GATA3 and CK20, and basal tumors were positive for CK5/6 and CK14 (10,12). The expression of two markers, GATA3 and CK5/6, were sufficient to classify bladder cancer into basal and luminal subtypes with over 90% accuracy (10). In the present study, we used GATA3, CK20, CK5/6, and CK14 as surrogate for luminal and basal classification. There was significant overlap expression of these markers in tumors, with 86.02% expressing both luminal and basal markers. We further categorized them based on the higher sum of IHC scores of the two sets of markers as previously reported (26). In agreement with the previous studies (10,11,24,25), basal MIBC was correlated with decreased survival compared with luminal MIBC both in training and validation cohorts. Multivariable analysis revealed that basal/luminal molecular subtypes based on IHC classification was an independent risk factor. Although several studies revealed basal/luminal subtypes were also correlated with the prognosis of NMIBC (27,28), no association between subtypes and prognosis of NMIBC were found in the present study, and this might be due to small sample size and good prognosis of NMIBC patients, thus only a few patients reached end point.
The previous study reported that p53-like tumors was a subgroup of both luminal and basal tumors (10). However, Dadhania et al. suggested that the so-called p53 phenotype may result from the contamination of stromal cells in the tumor tissue, and they found no significant difference in clinical behavior of tumor with p53-like signature. In our previous work, we have discovered and validated a panel of IHC markers (TOP2A, ANLN, GTSE1, YAP1, CCNB1, etc.) through RNA-sequencing that could potentially stratify patients who underwent RC with different prognosis. Among these markers, we revealed that both YAP1 and CCNB1 could help to further categorize luminal MIBC tumor into subtypes with different prognosis. The biological function of biomarkers included in the signature has been previously reported. Cyclin B1 is a protein related with cell cycle, which localizes entirely in the cytoplasm during interphase and translocate into nuclear during mitosis. High expression of cyclin B1 in cytoplasm may suggest lower mitosis rate of tumor cells thus leading to a better prognosis (29). YAP1 plays a central role in the Hippo pathway, nuclear translocation of YAP1 functions as co-activator to multiple transcription factor that regulates multiple cell functions such as growth and stemness. While cytoplasmic retention of YAP1 results in proteasomal degradation, which may explain that high cytoplasmic YAP1 suggests better prognosis (30). The combined IHC classifier with the two markers was independent of TNM-stage, and could stratify luminal MIBC into low- and high-risk groups with distinctly different prognosis both in training and validation cohorts. The classifier was further validated in external cohort generated from TCGA database and showed similar results. Our findings provide a novel approach to further stratify luminal tumor into molecular subtypes using two IHC markers classifier. This is the first study to demonstrate that molecular subtypes of luminal MIBC assessed by IHC markers could be beneficial for risk stratification. Previously, Robertson et al. (11) clustered luminal MIBC into luminal-papillary (35%), luminal-infiltrated (19%) and luminal (6%) subtypes based on mRNA expression. Luminal-papillary subtype was characterized by FGFR3 mutation and low risk for progression, low likelihood of neoadjuvant chemotherapy responsiveness; while luminal-infiltrated subtype was more likely to respond to immune checkpoint therapy but resistant to cisplatin-based chemotherapy.
This study had several limitations. First, this is a retrospective study with modest sample size. Second, although neoadjuvant chemotherapy helps to prolong survival for some MIBC patients, it was not routinely performed for MIBC patients in our center. Few data regarding neoadjuvant chemotherapy limited analysis of relationship between molecular subtypes and response to chemotherapy. Third, this study was only validated in an individual center and public database, external validation from multiple centers and across different populations is warranted (4).
Conclusions
This study confirmed basal and luminal molecular subtypes of MIBC could be assessed using two sets of IHC markers (GATA3, CK20, CK5/6, CK14), and the basal type MIBC had worse survival compared with luminal type. We developed and validated a two-marker IHC classifier (YAP1 and CCNB1) allowing selection of patients with poor prognosis within luminal MIBC cohort. Molecular subtypes of MIBC based on IHC classification is readily available and could help to develop treatment strategy and follow-up schedule in clinical practice.
Acknowledgments
We thank Department of Pathology, Changhai Hospital, for providing the FFPE blocks.
Funding: This research was financed by grants from Qihang program of Second Military Medical University (2021), National Natural Science Foundation of China (Nos. 81802515, 81801854, 82172871, 81972391, 82272950), Discipline Development Plan of Changhai Hospital (No. 2019YXK041), Science and Technology Commission of Shanghai Municipality (Nos. 20Y11904800, 22140903700), Shanghai Municipal Health Commission (No. 2022YQ010).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-538/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-538/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-538/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-538/coif). CX serves as an unpaid editorial board member of Translational Andrology and Urology from March 2021 to February 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Committee on Ethics of Medicine, Second Military Medical University (No. CHEC2019-134). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- Khalife N, Chahine C, Kordahi M, et al. Urothelial carcinoma in the era of immune checkpoint inhibitors. Immunotherapy 2021;13:953-64. [Crossref] [PubMed]
- Wu J, Wen JM, Wang YC, et al. Prognostic Value of an Immunohistochemical Signature in Patients With Bladder Cancer Undergoing Radical Cystectomy. Front Oncol 2021;11:641385. [Crossref] [PubMed]
- Dyrskjøt L, Reinert T, Algaba F, et al. Prognostic Impact of a 12-gene Progression Score in Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Validation Study. Eur Urol 2017;72:461-9. [Crossref] [PubMed]
- Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377-86. [Crossref] [PubMed]
- Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [Crossref] [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [Crossref] [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [Crossref] [PubMed]
- Dadhania V, Zhang M, Zhang L, et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016;12:105-17. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-556.e25. [PubMed]
- Jangir H, Nambirajan A, Seth A, et al. Prognostic stratification of muscle invasive urothelial carcinomas using limited immunohistochemical panel of Gata3 and cytokeratins 5/6, 14 and 20. Ann Diagn Pathol 2019;43:151397. [Crossref] [PubMed]
- Volkmer JP, Sahoo D, Chin RK, et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A 2012;109:2078-83. [Crossref] [PubMed]
- Rebola J, Aguiar P, Blanca A, et al. Predicting outcomes in non-muscle invasive (Ta/T1) bladder cancer: the role of molecular grade based on luminal/basal phenotype. Virchows Arch 2019;475:445-55. [Crossref] [PubMed]
- Liu A, Zeng S, Lu X, et al. Overexpression of G2 and S phase-expressed-1 contributes to cell proliferation, migration, and invasion via regulating p53/FoxM1/CCNB1 pathway and predicts poor prognosis in bladder cancer. Int J Biol Macromol 2019;123:322-34. [Crossref] [PubMed]
- Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015;26:812-7. [Crossref] [PubMed]
- Bejrananda T, Kanjanapradit K, Saetang J, et al. Impact of immunohistochemistry-based subtyping of GATA3, CK20, CK5/6, and CK14 expression on survival after radical cystectomy for muscle-invasive bladder cancer. Sci Rep 2021;11:21186. [Crossref] [PubMed]
- Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 2005;6:678-86. [Crossref] [PubMed]
- Cao Q, Wang C, Ding Y, et al. ARID1A upregulation predicts better survival in patients with urothelial bladder carcinoma. J Int Med Res 2020;48:300060519895687. [Crossref] [PubMed]
- Yoda T, McNamara KM, Miki Y, et al. KLF15 in breast cancer: a novel tumor suppressor? Cell Oncol (Dordr) 2015;38:227-35. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [Crossref] [PubMed]
- Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol 2006;24:296-304. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Hodgson A, Liu SK, Vesprini D, et al. Basal-subtype bladder tumours show a 'hot' immunophenotype. Histopathology 2018;73:748-57. [Crossref] [PubMed]
- Seiler R, Ashab HAD, Erho N, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol 2017;72:544-54. [Crossref] [PubMed]
- Rodriguez Pena MDC, Chaux A, Eich ML, et al. Immunohistochemical assessment of basal and luminal markers in non-muscle invasive urothelial carcinoma of bladder. Virchows Arch 2019;475:349-56. [Crossref] [PubMed]
- Breyer J, Wirtz RM, Otto W, et al. In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch 2017;470:267-74. [Crossref] [PubMed]
- Sikic D, Taubert H, Wirtz RM, et al. High Androgen Receptor mRNA Expression Is Associated with Improved Outcome in Patients with High-Risk Non-Muscle-Invasive Bladder Cancer. Life (Basel) 2021;11:642. [Crossref] [PubMed]
- Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol 2000;12:658-65. [Crossref] [PubMed]
- Nguyen CDK, Yi C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019;5:283-96. [Crossref] [PubMed]


