
Asymptomatic Rosai-Dorfman-Destombes disease presenting as isolated bilateral perinephric infiltration: a case report and review of the literature
Highlight box
Key findings
• We reported a rare case of asymptomatic renal RDD.
What is known and what is new?
• It’s diagnosis and treatment are very challenging.
• We summarized the imaging features of renal RDD.
What is the implication, and what should change now?
• It’s necessary to distinguish RDD from other renal tumors to avoid unnecessary misdiagnosis and nephrectomy.
Introduction
Rosai-Dorfman-Destombes disease (RDD) is a rare histiocytosis disease with unknown etiology. It commonly occurs in young people, and the typical manifestation is painless cervical lymph node enlargement. According to early reports, extra-nodal involvement has been observed in 43% of RDD cases, while kidney involvement has only been reported in 4% of RDD cases, but the involvement of this vital organ often leads to a poor prognosis (1,2).
It is difficult to make a clear diagnosis in some extra-nodal RDD cases without lymph node enlargement. Pathological diagnosis is the main method of diagnosis, which largely depends on the emperipolesis of characteristic histocytes and S100 protein positive immunohistochemistry results. However, some cases of extra-nodal variation in RDD lack the characteristic of histiocytes, which increases the difficulty of pathological diagnosis (3).
In this article, we report a rare case of extra-nodal RDD involving both kidneys. Perinephric lesions were found in an asymptomatic elderly male patient during a medical examination. After a tortuous diagnosis, the patient was finally diagnosed with RDD. To date, only a few cases of renal RDD have been reported (4-7). To improve understanding of the disease and reduce misdiagnosis, we conducted a comprehensive literature review to summarize its imaging features. We present the following article in accordance with the CARE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-742/rc).
Case presentation
The patient was a 67-year-old Chinese male with a history of kidney calculi, he denied smoking and family history, and no symptoms of urinary system diseases, such as lumbago, backache and hematuria, before he came to the hospital. He underwent regular medical examinations on the advice of doctors due to abnormalities in both kidneys. He came to us for further examination on March 29th, 2022.
A color doppler ultrasound showed that there were kidney calculi and hydronephrosis in the left kidney, and both kidneys were wrapped by hypoechoic soft tissue lesions with well-defined boundaries. A hypoechoic mass (5.4 cm × 4.9 cm in size) with a clear boundary was also observed outside the upper pole of the left kidney. Further, the contrast-enhanced ultrasound (CEUS) results indicated the same contrast-enhanced pattern of left renal mass and bilateral perirenal lesions (Figure 1), while the computed tomography urography (CTU) showed no enhancement in the bilateral perirenal lesions (Figure 2).
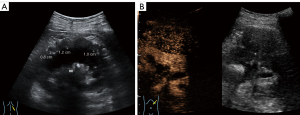
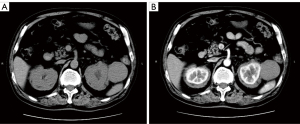
The patient underwent additional comprehensive examinations, and no abnormalities were found in the routine blood, routine urine, tumor marker, and renal function index results. His serum creatinine of 75 µmol/L, uric acid of 306.6 µmol/L, and with the negative results in test of inspecting urine albumen, urine occult blood and white blood cell. The patient’s immunoglobulin A, immunoglobulin G (IgG) and immunoglobulin M levels were in the normal range. His erythrocyte sedimentation rate was 23.0 mm/h. His immunoglobulin G4 (IgG4) was 2.15 g/L at the first test, but decreased to 1.82 g/L 5 days later. Due to the uncertainty of the imaging and laboratory examination results, a percutaneous biopsy under ultrasound-guided was performed on the left perirenal mass. However, the pathological result only indicated that the tissue contained fibrous adipose tissue and a few lymphocytes. The immunohistochemistry results were as follows: IgG4(–) and IgG(–). To obtain more tissue, the urologists removed the left perirenal mass and part of the perirenal tissues. The pathologists found that the microscopic appearance of the perirenal mass and perirenal tissues are the same, but a diagnosis remained difficult (Figure 3). Combined with immunohistochemical results of S100(+) and CD68(+), a final diagnosis of extra-nodal RDD was made in consultation with pathologists from a superior hospital. A V600E mutation in the V-raf murine sarcoma viral oncogene homolog B1 (BRAF) gene in the tumor was also detected.
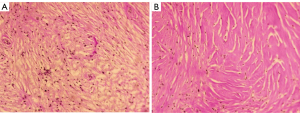
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
RDD is also known as sinus histiocytosis with massive lymphadenopathy, which is a rare type of non-Langerhans cell histiocytosis. It was first described by Destombes in 1965 and later by Rosai and Dorfman in 1969, at which time the disease was also named (8). RDD is a rare disease with unknown etiology. Based on the clinical manifestations, there are 3 types of RDD: that is, the lymph node type, extra-nodal type, and mixed type. The disease occurs mainly in children and young people. Its typical clinical manifestations include painless bilateral neck lymph node enlargement with associated fevers, night sweats, weight loss, malaise, leukocytosis and increased erythrocyte sedimentation rate (9). Extra-nodal involvement has been reported in 43% of RDD cases, and almost all organs are affected (2). It often occurs in the skin, bones, head and neck, central nervous system; however, kidney involvement accounts for about only 4% of extra-nodal cases (2). A diagnosis of RDD mainly depends on histopathology and immunohistochemistry results, and the pathological features of the disease vary (e.g., some extra-nodal lesions contain few RDD tissue cells and are covered by fibrotic or inflammatory cells), which increases the difficulty of diagnosis (3). Because of this, the diagnosis of our case was tortuous. The significance of the BRAF gene mutation is still unclear. Previous studies have found that some RDD cases are clonal and involve multiple gene mutations; however, the BRAF gene mutation is very rare (9,10).
RDD is considered to be a self-limiting disease, and has no standard treatment plan at present. It has been noted that the symptoms of 20–50% of RDD patients with skin lesions or lymph nodes will be relieved during the observation period without treatment. The main purpose of surgery is to take a biopsy or treat a single mass. Steroids, chemotherapy, and immunomodulatory therapy have been used to treat refractory or recurrent cases, but their efficacy requires further study (2). It appears that patients with kidney involvement often have a poor prognosis, and a mortality rate of about 40% (2). The patient in the present case has now been diagnosed for about 5 months, continues to be asymptomatic, and is not receiving any treatment. A follow-up ultrasound on September 27th, 2022 showed no change in the appearance of the kidneys. We will continue to follow up with the patient.
Very few cases of extra-nodal RDD with kidney involvement have been reported (Table 1). By analyzing the 15 cases, we found that only 1 patient had no clinical symptoms, and cases with kidney involvement tended to occur in the elderly. The imaging findings showed bilateral renal masses in 7 cases, bilateral perirenal masses in 4 cases (1 of which was accompanied by a huge mass in the left kidney), solitary renal masses in 3 cases, and soft tissue surrounding the right kidney and an isolated mass in the left kidney in 1 case. Brown et al. (13) in 2001 analyzed the CT findings of 3 cases, found that the imaging manifestations of renal RDD included perirenal soft tissue infiltration or a mass at the renal hilum, and noted that this unique pattern of perirenal soft tissue infiltration may be related to the lymphatic drainage of the kidney from the renal envelope to the hilum. Two of the 15 cases did not mention the treatment and prognosis. Only 1 case was reported of a patient who died of uremia several years later. Three patients underwent surgical resection, but the prognosis was not mentioned. Eleven patients were treated with surgery or steroids or chemotherapy, and even combination therapy, but most of the results are unsatisfactory. They were followed up for only 3 to 18 months, maybe the treatment and prognosis of these patients need more time to verify. Based on the literature review, our case had no clinical symptoms, but had typical imaging findings, perhaps due to the early stage of the RDD. Future follow-up should focus on changes in renal function and perirenal lesions.
Table 1
| Reference | Age, years | Gender | Size (cm) | Symptom | Image findings |
|---|---|---|---|---|---|
| Bain et al. (11) | 75 | F | 13 | Yes | Bilateral renal masses |
| Lai et al. (12) | 57 | F | N/A | Yes | Bilateral renal masses |
| Brown et al. (13) | 24 | F | N/A | Yes | Bilateral renal masses |
| Brown et al. (13) | 61 | M | N/A | Yes | Bilateral renal masses |
| Brown et al. (13) | 24 | M | 3 | Yes | Bilateral renal masses |
| Izadyar et al. (14) | 28 | F | N/A | Yes | Bilateral renal masses |
| Krishnan et al. (4) | 76 | M | 9 | Yes | Right perinephric masses and left renal mass |
| El Majdoub et al. (15) | 68 | F | 8 | Yes | Right renal mass |
| Kmetz et al. (16) | 60 | M | 22 | Yes | Bilateral perinephric masses |
| Bassa et al. (17) | 64 | F | 5 | Yes | Bilateral perinephric masses and left renal mass |
| Danisious et al. (18) | 12 | F | N/A | Yes | Left renal mass |
| Wang et al. (19) | 77 | M | 3.5 | Yes | Left renal mass |
| Abrishami et al. (6) | 67 | F | 12.6 | No | Bilateral renal masses |
| Synghal et al. (7) | 81 | M | N/A | Yes | Bilateral perinephric masses |
| Razanamahery et al. (5) | 67 | M | N/A | Yes | Bilateral perinephric masses |
RDD, Rosai-Dorfman-Destombes disease; F, female; M, male.
We conclude that renal RDD usually occurs in both kidneys, mainly presents as diffuse soft tissue masses around the kidney, and/or may be accompanied by a renal mass, which can cause hydronephrosis in severe cases. Generally, the renal mass is huge, but its malignant features are not significant, which can help distinguish it from a renal tumor. However, El Majdoub et al. (15) reported a case in which a patient presented with a right renal mass with invasion of the renal vein and several small lymph nodes in the hilar area, which was easily misdiagnosed as renal carcinoma. This disease can generally be differentiated from renal carcinoma, lymphoma, Erdheim-Chester disease, and IgG4-related diseases (2,20).
When we first encountered the present case, we were very confused. We initially suspected that the soft tissues around both kidneys and the left perinephric mass were caused by 2 diseases. Thus, we performed CEUS, and the same pattern of enhancement made us confirm that the different ultrasound findings were caused by the same disease. Given that the patient had a slightly increased IgG4 index, we initially suspected an IgG4-related disease, but the laboratory examinations and pathological diagnosis did not support such a diagnosis. Interestingly, no significant enhancement was observed on CTU, while the CEUS showed hypo-enhancement. This may be because CEUS is more sensitive than CTU. Additionally, the perirenal lesions had more fibrotic cells than lymph node type, which affects the effect of CT enhancement.
Conclusions
This case appears to be the first reported case of renal RDD in which CEUS was performed and may be the second report of an asymptomatic case. Its diagnosis and treatment are very challenging. however, we found that some cases have certain specificity in imaging. If the bilateral kidneys are involved and have a “hairy kidney” appearance, the possibility of renal RDD should be considered after other known diseases are excluded. Additionally, a percutaneous biopsy is recommended to avoid surgical resection. If a solitary mass is observed, CEUS or/and CTU may be helpful to differentiate the mass from a common tumor.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-742/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-742/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-742/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 1969;87:63-70. [PubMed]
- Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 2018;131:2877-90. [Crossref] [PubMed]
- Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica 2020;105:348-57. [Crossref] [PubMed]
- Krishnan A, Nassar A, Nieh PT. Rosai-Dorfman disease presenting as extranodal renal mass. Urology 2005;66:1319. [Crossref] [PubMed]
- Razanamahery J, Humbert S, Emile JF, et al. Immune Thrombocytopenia Revealing Enriched IgG-4 Peri-Renal Rosai-Dorfman Disease Successfully Treated with Rituximab: A Case Report and Literature Review. Front Med (Lausanne) 2021;8:678456. [Crossref] [PubMed]
- Abrishami A, Ziaeefar P, Ebrahimi S, et al. Rosai-Dorfman disease: A case report of asymptomatic isolated renal involvement. Clin Case Rep 2021;9:e04132. [Crossref] [PubMed]
- Synghal G, Bhavan R, Jain SK, et al. Rosai-Dorfman disease in a symptomatic elderly man. Proc (Bayl Univ Med Cent) 2021;35:78-9. [Crossref] [PubMed]
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer 1972;30:1174-88. [Crossref] [PubMed]
- Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol 2020;73:697-705. [Crossref] [PubMed]
- Richardson TE, Wachsmann M, Oliver D, et al. BRAF mutation leading to central nervous system rosai-dorfman disease. Ann Neurol 2018;84:147-52. [Crossref] [PubMed]
- Bain ES, Kinney TB, Gooding JM, et al. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman Disease): a rare cause of bilateral renal masses. AJR Am J Roentgenol 1999;172:995-6. [Crossref] [PubMed]
- Lai FM, To KF, Szeto CC, et al. Acute renal failure in a patient with Rosai-Dorfman disease. Am J Kidney Dis 1999;34:e12. [Crossref] [PubMed]
- Brown WE, Coakley FV, Heaney M. Renal involvement by Rosai-Dorfman disease: CT findings. Abdom Imaging 2002;27:214-6. [Crossref] [PubMed]
- Izadyar S, Samiei F, Gholamrezanezhad A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease)--imaging manifestations of renal involvement. Nucl Med Rev Cent East Eur 2014;17:44-6. [Crossref] [PubMed]
- El Majdoub A, El Houari A, Chbani L, et al. Isolated localization of Rosai Dorfman disease as renal mass: a case report and review of literature. Pan Afr Med J 2016;24:64. [Crossref] [PubMed]
- Kmetz DJ, Van Leeuwen B, Bentley D. Symptomatic Rosai-Dorfman Disease Presenting as Isolated Bilateral Perinephric Infiltration. Rev Urol 2019;21:41-4. [PubMed]
- Bassa C, Tagle R, Claro E, et al. Rosai-Dorfman-Destombes disease with renal involvement and secondary glomerulopathy: Report of an exceptional case. Urol Case Rep 2018;22:17-8. [Crossref] [PubMed]
- Danisious T, Hettiarachchi M, Dharmadasa C, et al. Rosai-Dorfman disease with renal involvement and associated autoimmune haemolytic anaemia in a 12-year-old girl: A case report. BMC Pediatr 2020;20:470. [Crossref] [PubMed]
- Wang F, Xu J, Chen J, et al. A case report of Rosai-Dorfman disease with kidney involvement. J Xray Sci Technol 2018;26:141-6. [Crossref] [PubMed]
- Purysko AS, Westphalen AC, Remer EM, et al. Imaging Manifestations of Hematologic Diseases with Renal and Perinephric Involvement. Radiographics 2016;36:1038-54. [Crossref] [PubMed]


