
Efficacy and safety evaluation of complete intrafascial prostatectomy in suspected prostate cancer patients with dysuria: a retrospective cohort study
Highlight box
Key findings
• Complete intrafascial prostatectomy (CIP) may be a prudent option for suspected localized prostate cancer (PCa) patients with dysuria if such patients are unwilling to undergo a biopsy and have no desire to engage in sexual activity.
What is known and what is new?
• Precious few studies have explored the application of CIP in patients with suspected localized PCa who also presented with serious dysuria.
• In the absence of a needle biopsy, if selected with caution, CIP may be a safe option to treat underlying PCa and relieve the symptoms of dysuria in suspected localized PCa patients.
What is the implication, and what should change now?
• CIP represents an alternative treatment for specific suspected localized PCa patients. Prostate-specific membrane antigen positron emission tomography can be performed if necessary to reduce the positivity of surgical margin and unwarranted surgical trauma in benign patients.
Introduction
A prostate needle biopsy is the gold standard for the diagnosis of prostate cancer (PCa) (1). However, due to the non-specificity of prostate-specific antigen (PSA), the biopsy-positive rate of localized PCa is only 22.8–42.0% (2,3). With the development of magnetic resonance imaging–ultrasound fusion prostate biopsy, the accuracy of prostate biopsy has increased to approximately 60%, but missed diagnoses still occur (4,5). Radical prostatectomy (RP) is recommended as a curative method for localized PCa confirmed by needle biopsy. However, suspected PCa patients with dysuria caused by enlarged prostate gland are usually suggested to undergo transurethral resection of the prostate (TURP) when the negative results of needle biopsy come out. TURP does not provide the final accurate pathological diagnosis, which may lead to the delays in treatment due to missed PCa diagnoses. Meanwhile, tissue adhesion around the prostate after TURP increases the operative difficulty of subsequent RP and even the incidence of urinary incontinence following RP. Based on the above reasons, some, though not many, patients with localized suspected PCa refuse to undergo repeated prostate needle biopsies, and thus require en bloc resection of the prostate gland to identify and treat the underlying PCa and relieve the symptoms of dysuria (6).
The entire resection of the prostate gland is effectively equivalent to a RP, and the main postoperative complications are urinary incontinence and erectile dysfunction (7,8). Improvements in surgical techniques, such as the preservation of neurovascular bundles (NVBs), pelvic floor structures, bladder sphincter, and functional urethra, can reduce the complication rates of urinary incontinence and erectile dysfunction to a certain extent (8-10). Complete intrafascial prostatectomy (CIP) can maximize the preservation of adjacent normal tissues around the prostate, which is conducive to the returns of urinary continence and even erectile function. At present, CIP is often used as a radical surgical approach for localized PCa. However, precious few studies have explored the application of CIP in patients with suspected localized PCa who also presented with serious dysuria, and the cancer cure and complications of CIP are unclear (6). Thus, we performed a CIP in these specific patients to evaluate the efficacy and safety of this technique in this study. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-26/rc).
Methods
Clinical information
This study was a retrospective single-arm cohort study. The study was approved by the Hainan General Hospital Medical Ethics Committee (No. Med-Eth-Re[2022] 267), and conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all the individual participants in this study.
There were a total of 22 eligible PCa patients who had undergone CIP without needle biopsy at our department from September 2018 to January 2022, and the clinical data of all these patients were retrospectively collected in this study. The clinical data included age, PSA, free-serum PSA (fPSA), prostate volume, Eastern Cooperative Oncology Group (ECOG) performance status score, multiparametric magnetic resonance imaging (mpMRI), operation-related data, perioperative complications, and postoperative pathology. The perioperative complications were assessed using the Clavien-Dindo grading system (11).
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have presented with dysuria requiring surgical intervention; (II) have a PSA level ≤20 ng/mL; (III) have a prostate imaging reporting and data system (PI-PADS) score for mpMRI score ≥3, and tumor lesions that did not involve the prostate capsule; (IV) have no pathological diagnosis; (V) have a life expectancy of ≥5 years; (VI) have a ECOG performance status score of 0; and (VII) have refused to undergo a prostate biopsy.
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a clinical stage ≥ T3; (II) had lymph node, bone, or visceral metastasis; (III) had a desire to engage in sexual activity; (IV) had an American Society of Anesthesiologists score >2; and/or (V) had a history of TURP.
Surgical approach
All the operations were performed using the laparoscopic/robot extraperitoneal approach. The pelvic fascia was not incised, the puboprostatic ligament was not severed, and the deep dorsal vein complex (DVC) was not ligated. The junction between the bladder neck and prostate was separated using a combination of blunt and sharp methods, and the posterior urethra at the base of the prostate was exposed and then severed, leaving the bladder neck intact. After the bilateral vas deferens were severed, the space between the anterior layer of Denonvilliers’ fascia and the posterior capsule of the prostate was bluntly dissociated to the apex and lateral wall of the prostate. The prostatic lateral ligament was separated along the posterior capsule of the prostate, and the prostatic lateral capsule was then exposed. The lateral capsule was freed anteriorly to the apex of the prostate and up to the anterior fibromuscular stroma of the prostate. The DVC was divided, and the membranous urethra was exposed and then cut off close to the apex of the prostate (Figure 1). The specimen was removed, the urethra and bladder neck were anastomosed, and the puboprostatic ligament was sutured continuously to the anterior layer of the bladder detrusor.
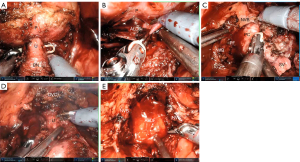
Perioperative management and follow-up
Postoperatively, a retropubic drainage tube was indwelled and removed when the drainage fluid volume was ≤10 mL. The urinary catheter was routinely indwelled for 2 weeks. The follow-up times were 2 weeks, 6 weeks, and then every 3 months for the 1st year after the operation at the outpatient department. Outpatient or telephone follow-ups were irregularly performed for 1 year after the operation according to each patient’s condition. The follow-up procedures included postoperative PSA, and the recoveries of urinary continence and erectile function. The undetectable PSA level (<0.1 ng/mL) at 6 weeks after CIP was defined as the main criterion to evaluate the efficacy of CIP. On the basis of the International Consultation on Incontinence Questionnaire Short Form, the loose criterion for urinary incontinence was defined as the use of diapers during daily activities, and the strict criterion was defined as urine leakage during coughing. Erectile function was assessed by the International Index of Erectile Function 5 (IIEF-5) (12). All the questionnaires were completed at the outpatient department to avoid response bias.
Statistical analysis
The statistical analysis of this study was performed using R4.1.0 software. The continuous variables are expressed as the mean ± standard deviation. According to Shapiro-Wilk test, all of the continuous variables were judged to be non-normally distributed continuous variables except the age. The t test was used to compare the differences between normally distributed continuous variables, the Wilcoxon rank sum test between non-normally distributed continuous variables, and the Fisher’s exact test between PI-PADS scores. A 2-tailed P value <0.05 was considered statistically significant.
Results
General clinical data
The patients had an average age of 71.91±8.29 years, an average preoperative PSA level of 10.75±4.25 ng/mL, an average fPSA of 2.08±1.59 ng/mL, and an average free-serum/total-serum PSA (f/tPSA) of 0.18±0.10. In relation to the PI-PADS scores for mpMRI, 22.73% (5/22) of the patients had a score of 3, 54.55% (12/22) had a score of 4, and 22.73% (5/22) had a score of 5.
All 22 patients completed the operation as expected without conversion to open surgery. The patients had an average operation time of 135.20±41.44 min (range, 40.0–215.0 min), an average hospital stay of 7.00±3.06 days (range, 4–19 days), and an average intraoperative blood loss volume of 128.64±145.09 mL. According to the Clavien-Dindo complication grading system, the perioperative complications of grade I were 45.5% (10/22), and grade II were 4.5% (1/22). The patient with grade II complication required an intraoperative blood transfusion. In total, 77.27% (17/22) of the patients had PCa confirmed by postoperative pathology, and 22.73% (5/22) had benign prostatic hyperplasia (BPH). There were statistically significant differences in the f/tPSA, prostate volume, and PSA density between the PCa group and the BPH group (Table 1). Among the 17 patients with PCa, the positivity of surgical margin was 11.76% (2/17). According to the International Society of Urological Pathology guidelines, 23.5% (4/17) of the patients had grade 1 PCa, 11.8% (2/17) had grade 2, 47.1% (8/17) had grade 3, and 17.6% (3/17) had grade 4.
Table 1
| Group | Age (year) | PSA (ng/mL) | f/tPSA | Prostate volume (mL) | PSA density (ng/mL2) | PI-PADS score | ||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | ||||||
| PCa (n=17) | 73.1±8.16 | 10.73±4.13 | 0.16±0.086 | 44.47±25.91 | 0.30±0.155 | 2 | 11 | 4 |
| BPH (n=5) | 67.8±8.23 | 10.82±5.15 | 0.27±0.108 | 86.42±54.94 | 0.15±0.070 | 3 | 1 | 1 |
| P value | 0.2467 | 1 | 0.039 | 0.039 | 0.048 | 0.060 | ||
| Statistics | t=−1.2727 | W=43 | W=69 | W=69 | W=17 | – | ||
Data are reported as mean value with standard deviation. PCa, prostate cancer; BPH, benign prostatic hyperplasia; PSA, prostate-specific antigen; f/tPSA, free-serum/total-serum prostate-specific antigen; PI-PADS, prostate imaging reporting and data system.
Follow-up data
The follow-up duration ranged from 10–50 months (median: 23 months), and no patient was lost to follow-up within 1 year of the operation. The postoperative PSA level decreased to an undetectable level below 0.1 ng/mL at 6 weeks (average: 0.010±0.004 ng/mL; range, 0.008–0.021 ng/mL). According to the loose criterion for assessing urinary incontinence, the patients achieved continence rates of 63.6% immediately after the operation, 95.5% at 1 month, and 100% at 3 months. According to the strict criterion for assessing urinary incontinence, the continence rates immediately after surgery, and at 1, 3, 6, and 9 months after surgery were 27.3%, 63.6%, 90.9%, 95.5%, and 100%, respectively (Table 2). None of the patients complained of urinary obstruction symptoms after surgery.
Table 2
| Group | Immediate | 1 month | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|---|---|
| Loose criteria (n) | 14 | 7 | 1 | 0 | 0 | 0 |
| Strict criteria (n) | 6 | 8 | 6 | 1 | 1 | 0 |
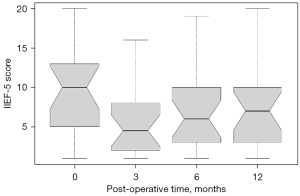
Before CIP, all the patients had erectile dysfunction and an average IIEF-5 score of 9.64±5.91. After surgery, the patients had IIEF-5 scores at 3, 6, and 12 months of 5.45±4.43, 6.95±5.30, and 7.57±5.69, respectively. Erectile function was significantly worse at 3 months after surgery (P=0.019) but then recovered at 6 and 12 months (P>0.05). However, there was a certain decrease in the IIEF-5 score, even at 12 months, compared to that before the operation (Figure 2).
Discussion
PSA is usually used for the primary screening of PCa, but a variety of factors, such as inflammation and ejaculation, can cause abnormal PSA results, which is why PSA has a high sensitivity for diagnosing PCa but a relatively low specificity (13). The results of this study showed that there was no significant difference in PSA between the early PCa and BPH patients; however, if considering prostate volume, the PSA density of the PCa patients was significantly lower than that of the BPH patients. Similar to other findings, we found that f/tPSA and PSA density could be used to improve the diagnostic specificity of identifying early PCa, but its accuracy remained unsatisfactory (14,15).
To improve the diagnostic accuracy of PCa, routine mpMRI is needed. According to a recent meta-analysis, the sensitivity, specificity, and accuracy of mpMRI for PCa were 0.84, 0.87, and 0.91, respectively (16). In this study, the specificity of mpMRI was 0.77, which was lower than the above-mentioned results; however, this might be related to the small number of patients included in this study and the inclusion of patients with early-stage tumors. Prostate-specific membrane antigen positron emission tomography has also begun to be used in clinical practice, but it is more suitable for the diagnosis of lymph nodes and distant metastases of PCa, and its diagnostic performance is not better than that of mpMRI for primary tumors (17).
RP is an important approach for curing localized PCa. However, unlike radical nephrectomy, which only requires imaging diagnosis, a Pca diagnosis needs to be supported by pathological evidence before RP is performed due to the postoperative complications that might affect the quality of life of the patients. For early PCa, there is still a certain missed diagnosis rate even with magnetic resonance imaging–ultrasound fusion prostate biopsy (4,5). Pathak et al. performed robotic total prostatectomy in patients with lower urinary tract symptoms who were still highly suspected to have PCa after the first negative biopsy, and the postoperative pathology diagnosed 90.91% (10/11) of the patients with PCa (6). In clinical practice, some patients will always be too concerned about tumor progression caused by missed diagnoses and the pain and complications caused by repeated needle biopsies to agree to undergo biopsies. If these patients have dysuria requiring surgical intervention, the performance of TURP after a missed diagnosis might seriously affect the efficacy of subsequent RP and increase the incidence of postoperative urinary incontinence. For these reasons, such patients require non-puncture prostatectomy to clarify the prostate pathology and relieve dysuria, which was also the original intention of this study.
The most common complications after RP were erectile dysfunction and urinary incontinence. Previous studies have confirmed that NVB preservation reduces the incidence of postoperative erectile dysfunction and urinary incontinence (9,18). However, even with preservation of the NVBs, accessory pudendal artery or other structures, and the postoperative use of drugs, such as statins, erectile dysfunction appeared to be uncontrollable (7,18,19). Thus, all the patients enrolled in this study had erectile dysfunction and a lack of desire to engage in sexual activity. In addition, all the patients had symptoms of dysuria. The mean age of the patients in this study was nearly 72 years old, which is older than that reported in other related studies (3,4,15). We found that the IIEF-5 scores were the worst at 3 months postoperatively, and while they recovered at 6 and 12 months postoperatively, the scores were still slightly worse than those before surgery. Seetharam et al. considered that normal preoperative sexual function, an age <55 years, and NVB retention were the most influential factors affecting potency recovery following RP (20). In this study, the NVBs were preserved; however, potency recovery after the operation was not ideal, which was a result of the patients being older and having a certain degree of erectile dysfunction before CIP.
Previous studies have shown that the complete preservation of the pelvic floor structure (including the intact Denonvilliers’ fascia), bladder sphincter, and functional urethra to preserve NVBs could further improve postoperative urinary continence (8-10). Retaining the Retzius space can significantly improve short-term urinary continence, but it is difficult to retain the Retzius space using conventional laparoscopy because the technical requirements are relatively high, and its application has been limited (21,22). In this study, CIP was performed using the conventional approach, with no incision of the pelvic fascia, no suture of DVC, the preservation of the Denonvilliers’ fascia, and the retention of the bladder neck, which facilitated postoperative urinary continence recovery. Regardless of whether the loose or strict criteria were applied, none of the patients experienced urinary incontinence at 9 months after CIP, and the urinary continence rate was no less than that achieved by patients undergoing Retzius space-preserving surgery (22).
In this study, all the patients underwent successful operations, and except in 1 patient who required an intraoperative blood transfusion, all the perioperative complications were Clavien-Dindo grade I. In total, 22.7% of the patients had BPH, which was related to the diagnostic accuracy of preoperative mpMRI. All the patients had undetectable PSA levels at 6 weeks postoperatively (PSA <0.1 ng/mL), but 11.7% (2/17) of the patients had positive surgical margins, which is similar to the results of another study on NVB preservation (23). A previous study showed that the en bloc resection of the prostate gland did not increase the positivity of surgical margin without preopereaive biopsy pathology (6).
This study had some limitations. This study was a retrospective single-arm cohort study, but the enrolled patients were strictly screened, which might have created a selectivity bias. Additionally, CIP requires a certain degree of surgical technical experience and cannot always be performed without issue. However, our study sought to use en bloc prostate gland resection to treat suspected PCa patients without preoperative pathology.
Conclusions
CIP may be a prudent option for patients with suspected localized PCa with dysuria who are unwilling to undergo a biopsy and have no desire to engage in sexual activity. This procedure has good safety and efficacy, but it is still insufficient at preserving potency.
Acknowledgments
Funding: This study was financially supported by the Hainan Province Science and Technology Special Fund (No. ZDYF2020114) and the Hainan Province Clinical Medical Center. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-26/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-26/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-26/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Hainan General Hospital Medical Ethics Committee (No. Med-Eth-Re[2022] 267), and conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all the individual participants in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Chun FK, Epstein JI, Ficarra V, et al. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol 2010;58:851-64. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Song G, Ruan M, Wang H, et al. How Many Targeted Biopsy Cores are Needed for Clinically Significant Prostate Cancer Detection during Transperineal Magnetic Resonance Imaging Ultrasound Fusion Biopsy? J Urol 2020;204:1202-8. [Crossref] [PubMed]
- Vėželis A, Platkevičius G, Kinčius M, et al. Systematic and MRI-Cognitive Targeted Transperineal Prostate Biopsy Accuracy in Detecting Clinically Significant Prostate Cancer after Previous Negative Biopsy and Persisting Suspicion of Malignancy. Medicina (Kaunas) 2021;57:57. [Crossref] [PubMed]
- Pathak RA, Hemal AK. Management of low-risk prostate cancer in patients with enlarged glands and lower urinary tract symptoms: robotic total prostatectomy, a novel technique. World J Urol 2020;38:829-36. [Crossref] [PubMed]
- Siltari A, Riikonen J, Fode M, et al. Effects of Preoperative Atorvastatin Treatment On Erectile Function After Radical Prostatectomy: Results From a Subgroup of ESTO1, a Randomized, Double-Blind, Placebo-Controlled Study. J Sex Med 2019;16:1597-605. [Crossref] [PubMed]
- Umari P, Eden C, Cahill D, et al. Retzius-Sparing versus Standard Robot-Assisted Radical Prostatectomy: A Comparative Prospective Study of Nearly 500 Patients. J Urol 2021;205:780-90. [Crossref] [PubMed]
- Budäus L, Isbarn H, Schlomm T, et al. Current technique of open intrafascial nerve-sparing retropubic prostatectomy. Eur Urol 2009;56:317-24. [Crossref] [PubMed]
- Lu X, He C, Zhang S, et al. Denonvilliers' fascia acts as the fulcrum and hammock for continence after radical prostatectomy. BMC Urol 2021;21:176. [Crossref] [PubMed]
- Mbaeri TU, Abiahu JA, Obiesie EA, et al. Assessment of Complications of Transurethral Resection of the Prostate Using Clavien-Dindo Classification in South Eastern Nigeria. Niger J Surg 2020;26:142-6. [PubMed]
- Schoentgen N, Marolleau J, Delage F, et al. Prospective four years of evaluation of erectile function after low-dose-rate prostate brachytherapy using baseline IIEF-5 >16. J Contemp Brachytherapy 2019;11:195-200. [Crossref] [PubMed]
- Merriel SWD, Pocock L, Gilbert E, et al. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med 2022;20:54. [Crossref] [PubMed]
- Nan LB, Yin XT, Gao JP. Significant Diagnostic Value of Free-Serum PSA (FPSA)/Prostate-Specific Antigen Density (PSAD) and (F/T)/PSAD for Prostate Cancer of the Chinese Population in a Single Institution. Med Sci Monit 2019;25:8345-51. [Crossref] [PubMed]
- Wu Q, Li F, Yin X, et al. Development and validation of a nomogram for predicting prostate cancer in patients with PSA ≤ 20 ng/mL at initial biopsy. Medicine (Baltimore) 2021;100:e28196. [Crossref] [PubMed]
- Li C, Li N, Li Z, et al. Diagnostic accuracy of high b-value diffusion weighted imaging for patients with prostate cancer: a diagnostic comprehensive analysis. Aging (Albany NY) 2021;13:16404-24. [Crossref] [PubMed]
- Szigeti F, Schweighofer-Zwink G, Meissnitzer M, et al. Incremental Impact of [68 Ga]Ga-PSMA-11 PET/CT in Primary N and M Staging of Prostate Cancer Prior to Curative-Intent Surgery: a Prospective Clinical Trial in Comparison with mpMRI. Mol Imaging Biol 2022;24:50-9. [Crossref] [PubMed]
- Kyriazis I, Spinos T, Tsaturyan A, et al. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers (Basel) 2022;14:1601. [Crossref] [PubMed]
- Williams SB, Morales BE, Huynh LM, et al. Analysis of Accessory Pudendal Artery Transection on Erections During Robot-Assisted Radical Prostatectomy. J Endourol 2017;31:1170-5. [Crossref] [PubMed]
- Seetharam Bhat KR, Moschovas MC, Sandri M, et al. Stratification of Potency Outcomes Following Robot-Assisted Laparoscopic Radical Prostatectomy Based on Age, Preoperative Potency, and Nerve Sparing. J Endourol 2021;35:1631-8. [Crossref] [PubMed]
- Madi R, Sayyid RK, Hiffa A, et al. Early Experience with Salvage Retzius-sparing Robotic-assisted Radical Prostatectomy: Oncologic and Functional Outcomes. Urology 2021;149:117-21. [Crossref] [PubMed]
- Rosenberg JE, Jung JH, Edgerton Z, et al. Retzius-sparing versus standard robot-assisted laparoscopic prostatectomy for the treatment of clinically localized prostate cancer. BJU Int 2021;128:12-20. [Crossref] [PubMed]
- Moris L, Gandaglia G, Vilaseca A, et al. Evaluation of Oncological Outcomes and Data Quality in Studies Assessing Nerve-sparing Versus Non-Nerve-sparing Radical Prostatectomy in Nonmetastatic Prostate Cancer: A Systematic Review. Eur Urol Focus 2022;8:690-700. [Crossref] [PubMed]


