
Effects of the body mass index of males on hormone levels, sperm and embryo parameters, and clinical outcomes in non-obstructive azoospermia: a systematic review and meta-analysis
Highlight box
Key findings
• The body mass index (BMI) of males affected reproductive hormones, sperm parameters, and clinical outcomes in non-obstructive azoospermia (NOA).
What is known and what is new?
• Serum testosterone and gonadotropins, rather than sperm retrieval outcomes, are related to the BMI in NOA. No comprehensive assessment had been conducted on the effect of the BMI of males in NOA.
• The BMI ≥25 kg/m2 group had a lower follicle-stimulating hormone (FSH) level, total testosterone (TT) level, and live-birth rate than the BMI <25 kg/m2 group; the testicular volume of the BMI ≥25 kg/m2 group was larger than that of the BMI <25 kg/m2 group, and the average BMI of patients with successful sperm extraction was lower than that of those with failed sperm extraction.
What is the implication, and what should change now?
• Clinicians may advise NOA patients with a BMI ≥25 kg/m2 to lose weight.
Introduction
Non-obstructive azoospermia (NOA) is a serious and common cause of male infertility in which no sperm is found in the ejaculate due to spermatogenesis failure (1,2). This disease affects around 1% of men in the general population, and is characterized by clinical heterogeneity, which suggests that different acquired and genetic factors are involved (3). For most male patients with NOA, the only available treatment option for having biological children is assisted reproductive technology (ART) (4). Intracytoplasmic sperm injection (ICSI) combined with surgical sperm extraction is the only method by which NOA patients can achieve fertility (5).
Some factors affect fertility in men with NOA. Testicular histology has been reported to be related to sperm retrieval and fertilization rates but not pregnancy and live-birth rates in NOA (6). Zhang et al. (7) reported that high smoking and alcohol consumption rates are contributing factors affecting the infertility of patients with NOA and severe oligozoospermia. Recently, increasing research has been conducted on the effect of obesity on male infertility. Obese men with a body mass index (BMI) ≥35 kg/m2 have been reported to have decreased fertility compared to those with a normal BMI (<25 kg/m2) (8). Anifandis et al. (9) showed that the BMI of males affected the quality of embryos and pregnancy rates after in-vitro fertilization (IVF). However, some studies have reported that the BMI of men is not associated with the fertilization rate, early embryo quality, and clinical pregnancy rate after ART (10,11). A high BMI is associated with an elevated risk of azoospermia (12,13). Pavan-Jukic et al. (14) and Iwatsuki et al. (15) found no correlation between BMI and sperm retrieval outcomes for patients with NOA, but found that serum testosterone and gonadotropins were correlated to the BMI, which could affect male fertility. The results of the published studies were inconsistent, and the sample size in each study was relatively small, based on which a systematic review and meta-analysis should be done.
This study sought to comprehensively evaluate the effects of the BMI of males on hormone levels, sperm parameters, embryo parameters, and clinical outcomes in NOA patients via a meta-analysis and systematic review. We present the following article in accordance with the MOOSE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-125/rc).
Methods
Search strategy
The PubMed, Embase, Cochrane Library, and Web of Science databases were comprehensively searched by 2 independent authors (XC Mai, and XY Xu) to retrieve relevant articles published up to May 27, 2022. Any disagreements were resolved through discussion with another author (YH Dong). The English search terms were as follows: “body mass index” or “index, body mass” or “Quetelet index” or “index, Quetelet” or “Quetelet’s index” or “Quetelets index” or “BMI” or “overweight” or “obesity” or “obese” and “non-obstructive azoospermia” or “nonobstructive azoospermia” or “azoospermia” or “azoospermic” or “aspermia” or “testicular failure.” The retrieved studies were imported into Endnote X9 (Clarivate, Philadelphia, PA, USA), and preliminarily screened by reading the titles and abstracts. The ineligible studies were then excluded after reading the full texts, and the remaining studies were ultimately included in the meta-analysis.
Eligibility criteria
To be eligible for inclusion in this meta-analysis, the articles had to meet the following inclusion criteria based on the population, intervention, comparator, outcome and study design (PICOS) framework: (I) examine patients with NOA (P); (II) examine the BMI of patients, and include a control group of patients with a BMI <25 kg/m2 (the normal weight group) (C), and an observation group of patients with a BMI ≥25 kg/m2 (I); (III) examine at least 1 of the following outcomes: reproductive hormones [luteinizing hormone (LH, mIU/mL), follicle-stimulating hormone (FSH, mIU/mL), total testosterone (TT, ng/mL), free testosterone (FT, pg/mL), calculated bioavailable testosterone (cBAT, ng/mL), sex hormone-binding globulin (SHBG, nmol/mL), and prolactin (PRL, ng/mL)], sperm parameters [testicular volume, ejaculate volume, sperm motility degree (A + B) (16), the sperm retrieval rate, and sperm retrieval success], embryo parameters (embryo transfer, the embryo implantation rate, the fertilization rate, and the good-quality embryo rate), and clinical outcomes (the clinical pregnancy rate, pregnancy, livebirth rate, and abortion rate) (O); and (IV) be a cohort study published in the English language (S). Reproductive hormones were baseline indicators; sperm parameters, embryo parameters, and clinical outcomes were follow-up outcome indicators.
Articles were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) examined male patients with infertility of unspecified causes; (II) examined the influence of genetic factors on the following outcomes: chromosome deletion and abnormality; (III) examined the following female factors affecting egg quality: advanced age, premature ovarian failure, recurrent abortion, and luteinized unruptured follicle syndrome; (IV) relate to animal experiments; (V) had incomplete or non-extractable data; and/or (VI) comprised a case report, abstract, conference summary, letter, review, or meta-analysis.
Data extraction and quality assessment
Data on the first author, year of publication, country, study design, patient, BMI (kg/m2) group, sample size, age (years), testicular volume (mL), ejaculation volume (mL), infertility duration (months), orchiopexy, varicocele, smoking, follow-up time and outcome of NOA patients were obtained from the included articles. The modified Newcastle-Ottawa Scale (NOS) was used to assess the quality of the observational studies. The NOS has a total possible score of 9, with a score of 0–3 indicating poor quality, 4–6 indicating fair quality, and 7–9 indicating good quality (17).
Statistical analysis
The statistical analysis was conducted via Stata 15.1 (Stata Corporation, College Station, TX, USA). Weighted mean differences (WMDs) were used as the effect size for the measurement data, and relative risks (RRs) were used as the effect size for the enumeration data. The effect size was expressed with the 95% confidence interval (CI). The effect size of each outcome was tested for heterogeneity. If the heterogeneity statistic of I2 was ≥50%, then the random-effects model was used for the analysis; otherwise, the fixed-effects model was used for the analysis. The normal weight group (BMI <25 kg/m2) was used as the control group, and the BMI ≥25 kg/m2 group was used as the observation group for comparisons between the 2 groups. We also compared the overweight group (BMI =25–30 kg/m2) with the normal weight group (BMI <25 kg/m2), the obese group (BMI >30 kg/m2) with the normal weight group (BMI <25 kg/m2), and the overweight group (BMI = 25–30 kg/m2) with the obese group (BMI >30 kg/m2). A sensitivity analysis was carried out for all the outcomes. As for publication bias, meta-analyses should include at least 10 studies for each outcome (18,19). This meta-analysis did not meet the above requirements for publication bias assessment, and thus publication bias was not assessed in this study. A P value <0.05 indicated a statistically significant difference.
Results
Characteristics of the included studies
A total of 893 studies were identified from the 4 databases. After removing duplicate articles and screening the articles according to the eligibility criteria, 12 studies (14-16,20-28) comprising 2,994 NOA patients were included in the analysis. A flowchart of the study selection process is illustrated in Figure 1. All the included studies were cohort studies, covering the period of 2013 to 2022. The follow-up time of each cohort study was not available. All the patients underwent testicular sperm extraction (TESE). In the study of Li et al. (16), clinical pregnancy was determined by gestational sacs under B-ultrasonography or specific clinical signs of pregnancy 35 days after embryo transplantation. In the study of Shrem et al. (27), pregnancy was confirmed by positive beta Human Chorionic Gonadotropin (bHCG) test 14 days after embryo transfer. The results of the literature quality assessment showed that 9 of the studies were of fair quality and 3 were of high quality. Table 1 sets out the characteristics of the included studies.
Table 1
| Author | Year | Country | Study design | Patient | Group of BMI (kg/m2) | Sample size | Age (years) | Testicular volume (mL) | Method for estimating testicular volume | Ejaculation volume (mL) | Infertility duration (months) | Orchiopexy | Varicocele | Smoking | Follow-up time | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ramasamy (25) | 2013 | USA | Cohort study | Patients with NOA undergoing TESE | <25 | 298 | 34.1±2.0 | 8.9±1.4 | Orchidometer | NA | NA | NA | 66 | 35 | NA | Testicular volume, FSH, sperm retrieval rate, pregnancy rate, live birth rate | 6 |
| 25–30 | 400 | 36.6±1.4 | 9.2±1.1 | NA | NA | NA | 80 | 55 | |||||||||
| >30 | 272 | 34.9±1.6 | 9.2±1.6 | NA | NA | NA | 53 | 14 | |||||||||
| Xu (28) | 2016 | China | Cohort study | Patients with NOA who underwent micro-TESE | – | 52 | 33.3±5.8 | 7.8±2.5 | Ultrasound | NA | NA | NA | 24 | NA | NA | Success of sperm retrieval | 5 |
| Iwatsuki (15) | 2017 | Japan | Cohort study | Patients with NOA who underwent micro-TESE | <25 | 151 | 35.4±5.3 | 9.0±5.2 | NA | NA | NA | NA | NA | NA | NA | Sperm retrieval rate, testicular volume, LH, FSH, TT, FT, cBAT, SHBG | 7 |
| ≥25 | 66 | 35.4±5.3 | 8.2±4.9 | NA | NA | NA | NA | NA | |||||||||
| Karamazak (21) | 2018 | Turkey | Cohort study | Patients who underwent TESE with the diagnosis of azoospermia | <25 | 101 | 32.9±6.3 | 13.3±5.4 | Orchidometer | 2.7±1.3 | 16.23 | 3 | 7 | 50 | NA | Testicular volume, ejaculate volume, FSH, LH, TT | 6 |
| 25–29.9 | 141 | 33.3±5.5 | 13.3±4.9 | 2.8±1.5 | 14.39 | 13 | 6 | 69 | |||||||||
| ≥30 | 40 | 35.7±6.6 | 13.6±5.4 | 2.9±2.1 | 15.44 | 2 | 1 | 26 | |||||||||
| Li (16) | 2019 | China | Cohort study | Patients with NOA undergoing TESE | <25 | 355 | 30.94±5.808 | 7.14±4.10 | NA | NA | NA | NA | 0 | 0 | a* | Sperm retrieval rate, testicular volume, sperm motility degree, FSH, LH, TT, PRL, fertilisation rate, good quality embryo rate, embryo transfer, embryo implantation rate, clinical pregnancy rate, live birth rate, abortion rate | 8 |
| 25–29.9 | 206 | 32.27±6.560 | 7.13±4.91 | NA | NA | NA | 0 | 0 | |||||||||
| ≥30 | 144 | 31.84±6.630 | 7.38±4.16 | NA | NA | NA | 0 | 0 | |||||||||
| Shrem (27) | 2019 | Israel | Cohort study | Patients with NOA undergoing TESE | – | 52 | 31 [29–36] | NA | NA | NA | NA | NA | NA | 34 | b* | Pregnancy | 5 |
| Liu (22) | 2020 | China | Cohort study | Patients with NOA had undergone TESE or micro-TESE | – | TESE: 155 | NA | 8.75±3.86 | Ultrasound | NA | NA | NA | NA | NA | NA | Success of sperm retrieval | 6 |
| – | micro-TESE: 139 | NA | 6.17±3.60 | NA | NA | NA | NA | NA | |||||||||
| Osaka (23) | 2020 | Japan | Cohort study | Patients with NOA who underwent micro-TESE | – | 36 | 38.63 (24.5–53) | 9.65 (5–16) | Orchidometer | NA | 30.84 (6–120) | 36 | 0 | NA | NA | Success of sperm retrieval | 5 |
| Pavan-Jukic_1 (14) | 2020 | Croatia | Cohort study | Patients with NOA undergoing TESE | <25 | 18 | 35.7±4.0 | right: 11.6±9.3; left: 9.4±7.7 | NA | NA | NA | NA | NA | NA | NA | Sperm retrieval rate | 7 |
| 25–30 | 25 | ||||||||||||||||
| 30–35 | 11 | ||||||||||||||||
| 35–40 | 10 | ||||||||||||||||
| >40 | 11 | ||||||||||||||||
| Pavan-Jukic_2 (24) | 2020 | Croatia | Cohort study | Patients with NOA undergoing TESE | – | 62 | 36.8±4.9 | right: 11.9±9.8; left: 8.7±7.1 | NA | NA | NA | NA | 18 | 15 | NA | Success of sperm retrieval | 5 |
| Rehnitz (26) | 2020 | Germany | Cohort study | Patients with NOA undergoing TESE | – | 90 | (28–73) | NA | NA | NA | NA | NA | NA | NA | NA | Pregnancy | 6 |
| Bastug (20) | 2022 | Turkey | Cohort study | Patients with NOA who underwent micro-TESE | – | 159 | 34 [30–38] | NA | NA | NA | NA | NA | 0 | 81 | NA | Success of sperm retrieval | 6 |
For variables age, testicular volume, ejaculation volume, and infertility duration, data were presented as median [interquartile range] or mean (extremum range), and other data were presented as mean ± standard deviation. a*, clinical pregnancy was established by gestational sacs under B-ultrasonography or specific clinical signs of pregnancy 35 days after embryo transplantation; b*, pregnancy was determined by positive beta Human Chorionic Gonadotropin (bHCG) test 14 days after embryo transfer. NOA, non-obstructive azoospermia; TESE, testicular sperm extraction; BMI, body mass index; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TT, total testosterone; FT, free testosterone; cBAT, calculated bioavailable testosterone; SHBG, sex hormone-binding globulin; PRL, prolactin; NOS, Newcastle-Ottawa scale; NA, not available.
Association between the BMI of males and hormone levels
LH
Among the studies, 3 provided data on the association between the BMI and LH. Overall the analysis showed that patients with a BMI ≥25 kg/m2 had a similar LH level to those with a BMI <25 kg/m2 (pooled WMD: 0.29, 95% CI: –0.30 to 0.89, I2=0.0%, P=0.335) (Figure 2A). According to the subgroup analysis, no significant differences were found in the LH levels between the overweight group and the normal weight group (WMD: 0.31, 95% CI: –0.34 to 0.95, P=0.354), between the obese group and the normal weight group (WMD: 0.10, 95% CI: –1.72 to 1.52, P=0.904), and between the overweight group and the obese group (WMD: 0.57, 95% CI: –1.41 to 2.55, P=0.573) (Table 2, Table S1).
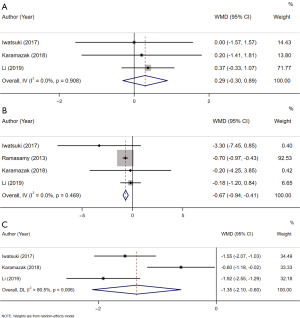
Table 2
| Outcome | Outcome indicator | WMD/RR (95% CI) | P | I2 (%) |
|---|---|---|---|---|
| Hormone levels | LH (mIU/mL) | |||
| Overall | 0.29 (−0.30, 0.89) | 0.335 | 0.0 | |
| Comparison | ||||
| 25–30 vs. <25 | 0.31 (−0.34, 0.95) | 0.354 | 0.0 | |
| >30 vs. <25 | 0.10 (−1.72, 1.52) | 0.904 | 44.2 | |
| 25–30 vs. >30 | 0.57 (−1.41, 2.55) | 0.573 | 58.1 | |
| FSH (mIU/mL) | ||||
| Overall | −0.67 (−0.94, −0.41) | <0.001 | 0.0 | |
| Comparison | ||||
| 25–30 vs. <25 | −0.83 (−1.84, 0.19) | 0.110 | 0.0 | |
| >30 vs. <25 | 0.16 (−2.51, 2.82) | 0.909 | 30.2 | |
| 25–30 vs. >30 | −0.09 (−4.53, 4.36) | 0.970 | 64.3 | |
| TT (ng/mL) | ||||
| Overall | −1.35 (−2.10, −0.60) | <0.001 | 80.5 | |
| Comparison | ||||
| 25–30 vs. <25 | −0.96 (−2.07, 0.15) | 0.088 | 82.0 | |
| >30 vs. <25 | −1.84 (−3.10, −0.59) | 0.004 | 87.5 | |
| 25–30 vs. >30 | 0.92 (0.57, 1.26) | <0.001 | 0.0 | |
| Sperm parameters | Testicular volume (mL) | |||
| Overall | 0.26 (0.09, 0.44) | 0.003 | 0.0 | |
| Comparison | ||||
| 25–30 vs. <25 | 0.28 (0.09, 0.46) | 0.003 | 0.0 | |
| >30 vs. <25 | 0.30 (0.06, 0.53) | 0.014 | 0.0 | |
| 25–30 vs. >30 | −0.02 (−0.23, 0.20) | 0.881 | 0.0 | |
| Sperm retrieval rate | ||||
| Overall | 1.02 (0.93, 1.11) | 0.698 | 39.9 | |
| Comparison | ||||
| 25–30 vs. <25 | 1.02 (0.92, 1.13) | 0.715 | 49.9 | |
| >30 vs. <25 | 1.02 (0.91, 1.14) | 0.729 | 45.6 | |
| 25–30 vs. >30 | 1.00 (0.89, 1.11) | 0.944 | 34.5 | |
| Success of sperm retrieval | ||||
| Overall | −0.97 (−1.89, −0.04) | 0.041 | 8.3 | |
| Clinical outcomes | Clinical pregnancy rate | |||
| Overall | 0.94 (0.85, 1.04) | 0.236 | 0.0 | |
| Comparison | ||||
| 25–30 vs. <25 | 0.97 (0.87, 1.09) | 0.596 | 0.0 | |
| >30 vs. <25 | 0.89 (0.78, 1.02) | 0.084 | 0.0 | |
| 25–30 vs. >30 | 1.09 (0.96, 1.25) | 0.196 | 0.0 | |
| Pregnancy | ||||
| Overall | −1.33 (−4.79, 2.13) | 0.451 | 83.1 | |
| Live birth rate | ||||
| Overall | 0.88 (0.78, 0.99) | 0.031 | 14.9 | |
| Comparison | ||||
| 25–30 vs. <25 | 0.90 (0.79, 1.03) | 0.134 | 0.0 | |
| >30 vs. <25 | 0.83 (0.71, 0.97) | 0.020 | 16.3 | |
| 25–30 vs. >30 | 1.09 (0.93, 1.29) | 0.292 | 0.0 | |
BMI, body mass index; WMD, weighted mean difference; RR, risk ratio; CI, confidence interval; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TT, total testosterone.
FSH
Based on 4 studies, patients with a BMI ≥25 kg/m2 had a lower FSH level than those with a BMI <25 kg/m2 (pooled WMD: –0.67, 95% CI: –0.94 to –0.41, I2=0.0%, P<0.001) (Figure 2B). Comparable FSH levels were observed in the overweight group and the normal weight group (WMD: –0.83, 95% CI: –1.84 to 0.19, P=0.110), in the obese group and the normal weight group (WMD: 0.16, 95% CI: –2.51 to 2.82, P=0.909), and in the overweight group and the obese group (WMD: –0.09, 95% CI: –4.53 to 4.36, P=0.970) (Table 2, Table S1).
TT
The TT level was evaluated by 3 studies. The combined analysis demonstrated that the TT level was reduced in the BMI ≥25 kg/m2 group compared to the normal weight group (pooled WMD: –1.35, 95% CI: –2.10 to –0.60, I2=80.5%, P<0.001) (Figure 2C). The overweight group was found to have an equivalent TT level to the normal weight group (WMD: –0.96, 95% CI: –2.07 to 0.15, P=0.088). The patients in the obese group had a lower TT level than those in the normal weight group (WMD: –1.84, 95% CI: –3.10 to –0.59, P=0.004). The patients in the overweight group had a higher TT level than those in the obese group (WMD: 0.92, 95% CI: 0.57 to 1.26, P<0.001) (Table 2, Table S1).
FT, cBAT, and SHBG
Iwatsuki et al. (15) assessed the differences among FT, cBAT, and SHBG between a BMI ≥25 kg/m2 group and a normal weight group with a BMI <25 kg/m2. The results showed that there was no significant difference between the 2 groups in the levels of the above 3 hormones (all P>0.05) (Table S1).
PRL
The PRL levels of the BMI ≥25 kg/m2 group and the normal weight group were assessed by Li et al. (16), and no significant difference was found (P=0.847). The overweight group and the normal weight group, the obese group and the normal weight group, and the overweight group and the obese group had similar PRL levels (all P>0.05) (Table S1).
Association between the BMI of males and sperm parameters
Testicular volume
Among the studies, 4 examined testicular volume. According to the pooled analysis, the testicular volume of the BMI ≥25 kg/m2 group was larger than that of the normal weight group (pooled WMD: 0.26, 95% CI: 0.09 to 0.44, I2=0.0%, P=0.003) (Figure 3A). The subgroup analysis showed that the overweight group (WMD: 0.28, 95% CI: 0.09 to 0.46, P=0.003) and the obese group (WMD: 0.30, 95% CI: 0.06 to 0.53, P=0.014) had a greater testicular volume than the normal weight group. The overweight group had a comparable testicular volume to the obese group (WMD: –0.02, 95% CI: –0.23 to 0.20, P=0.881) (Table 2).

Ejaculate volume
Karamazak et al. (21) found no significant difference in the ejaculate volume between the BMI ≥25 kg/m2 group and the normal weight group (P=0.569). Further, similar ejaculate volumes were identified in the overweight group and the normal weight group, in the obese group and the normal weight group, and in the overweight group and the obese group (all P>0.05).
Sperm motility degree (A + B)
In relation to sperm motility degree (A + B), Li et al. (16) showed that patients in the BMI ≥25 kg/m2 group and the normal weight group had an equivalent sperm motility degree (A + B) (P=0.171). The subgroup analysis showed no significant difference in the sperm motility degree (A + B) between the overweight group and the normal weight group, between the obese group and the normal weight group, and between the overweight group and the obese group (all P>0.05).
Sperm retrieval rate
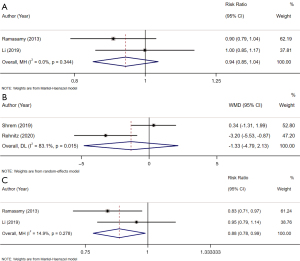
The sperm retrieval rate was evaluated in 4 studies. There were no significant differences in the sperm retrieval rates between the BMI ≥25 kg/m2 group and the normal weight group (pooled RR: 1.02, 95% CI: 0.93 to 1.11, I2=39.9%, P=0.698) according to the overall analysis (Figure 3B). No significant differences were observed in the sperm retrieval rate between the overweight group and the normal weight group (RR: 1.02, 95% CI: 0.92 to 1.13, P=0.715), between the obese group and the normal weight group (RR: 1.02, 95% CI: 0.91 to 1.14, P=0.729), and between the overweight group and the obese group (RR: 1.00, 95% CI: 0.89 to 1.11, P=0.944) (Table 2).
Sperm retrieval success
Among the studies, 3 examined sperm retrieval success and reported that the average BMI of the group with successful sperm extraction was lower than that of the group with failed sperm extraction (pooled WMD: –0.97, 95% CI: –1.89 to –0.04, I2=8.3%, P=0.041) (Figure 3C, Table 2).
Association between the BMI of males and embryo parameters
Embryo transfer, the embryo implantation rate, the fertilization rate, and the good-quality embryo rate
Based on the study of Li et al. (16), embryo transfer, the embryo implantation rate, the fertilization rate, and the good-quality embryo rate of the BMI ≥25 kg/m2 group were equivalent to those of the normal weight group (all P>0.05). No significant differences were found in embryo transfer, the embryo implantation rate, the fertilization rate, and the good-quality embryo rate between the overweight group and the normal weight group, and between the obese group and the normal weight group (all P>0.05). The overweight group had greater embryo transfer than the obese group (P=0.015), and the overweight group had a similar embryo implantation rate, fertilization rate, and good-quality embryo rate to the obese group (all P>0.05).
Association between the BMI of males and clinical outcomes
Clinical pregnancy rate
A combined analysis of 2 studies showed that the clinical pregnancy rate of the BMI ≥25 kg/m2 group was comparable to that of the normal weight group (pooled RR: 0.94, 95% CI: 0.85 to 1.04, I2=0.0%, P=0.236) (Figure 4A). Equivalent clinical pregnancy rates were observed in the overweight group and the normal weight group (RR: 0.97, 95% CI: 0.87 to 1.09, P=0.596), in the obese group and the normal weight group (RR: 0.89, 95% CI: 0.78 to 1.02, P=0.084), and in the overweight group and the obese group (RR: 1.09, 95% CI: 0.96 to 1.25, P=0.196) (Table 2).
Pregnancy
Based on the 2 qualified studies, no difference existed in the BMIs between the successful pregnancy group and the failed pregnancy group (pooled WMD: –1.33, 95% CI: –4.79 to 2.13, I2=83.1%, P=0.451) (Figure 4B, Table 2).
Live-birth rate
The live-birth rate was investigated in 2 studies. The overall analysis showed that the live-birth rate of the group with a BMI ≥25 kg/m2 was lower than that of the group with a normal weight (pooled RR: 0.88, 95% CI: 0.78 to 0.99, I2=14.9%, P=0.031) (Figure 4C). The subgroup analysis did not reveal any significant differences in the live-birth rates between the overweight group and the normal weight group (RR: 0.90, 95% CI: 0.79 to 1.03, P=0.134), and between the overweight group and the obese group (RR: 1.09, 95% CI: 0.93 to 1.29, P=0.292). The obese group had a decreased live-birth rate compared to the normal weight group (RR: 0.83, 95% CI: 0.71 to 0.97, P=0.020) (Table 2).
Abortion rate
Li et al. (16) showed that the BMI ≥25 kg/m2 group had a similar abortion rate to the normal weight group (P=0.286). No significant differences in the abortion rates were found between the overweight group and the normal weight group, between the obese group and the normal weight group, and between the overweight group and the obese group (all P>0.05).
Sensitivity analysis
A sensitivity analysis was performed by deleting 1 study at a time and comprehensively analyzing the remaining studies. The results showed that the 1-study removal did not significantly influence the combined results, indicating that the findings of this meta-analysis were stable and robust.
Discussion
This study first comprehensively assessed the effects of the BMI of males on NOA in terms of hormone levels, sperm parameters, embryo parameters, and clinical outcomes, and demonstrated that male patients with a BMI ≥25 kg/m2 had lower FSH and TT levels than those with a BMI <25 kg/m2, the testicular volume of the BMI ≥25 kg/m2 group was larger than that of the BMI <25 kg/m2 group, the average BMI of patients with successful sperm extraction was lower than that of those with failed sperm extraction, and the live-birth rate of the BMI ≥25 kg/m2 group was lower than that of the group with the BMI <25 kg/m2 group.
In relation to the hormone levels, Stárka et al. (29) divided 224 participants into 3 groups with BMIs of 18–25, 25–29, and 30–39 kg/m2, and found that the levels of the gonadotropins LH and FSH were not correlated with BMI. In a study of 98 samples with an overall prevalence of azoospermia of 30.61%, BMI was reported to have no effect on LH, FSH, testosterone, or PRL (30). In the current study, we initially compared patients with a BMI <25 kg/m2 to those with a BMI ≥25 kg/m2, and found that the patients with a BMI ≥25 kg/m2 had a similar LH to those with a BMI <25 kg/m2, and had reduced FSH and TT levels compared to those with a BMI <25 kg/m2. The differences in the findings of the above-mentioned studies may be related to differences in the study populations, sample sizes, and groupings.
Evidence has shown that hormone levels are affected by obesity in men of childbearing age (31,32). An increased BMI is directly associated with a reduction in both TT and FT levels (33,34). This is most likely due to function changes in the hypothalamic-pituitary-gonadal (HPG) axis and crosstalk between the hypothalamic-pituitary-adrenal (HPA) and the HPG axes. Obesity is reported to affect the activities of the HPA and HPG axes (35). Low testosterone levels are due to overactivity of the aromatase cytochrome P450 enzyme, and this enzyme is highly expressed in white adipose tissue and plays an essential role in estrogen synthesis (36,37). In consideration of the established estrogen receptor in the male hypothalamus, estrogen has negative feedback at the hypothalamic level, leading to a decrease in testosterone and FSH levels (38). We further found that NOA patients with a BMI >30 kg/m2 had a lower TT level than those with a BMI <25 kg/m2 and a BMI of 25–30 kg/m2. Obese patients may need to manage their weight to keep their TT at a suitable level.
In relation to the relationship between the BMI of males and sperm parameters, this study suggested that the testicular volume of patients with a BMI ≥25 kg/m2 was greater than that of those with a BMI <25 kg/m2, and patients with successful sperm extraction had lower average BMI than those with failed sperm extraction. A possible explanation for the greater testicular volume in the obese versus normal weight group is that obese patients have lower testosterone levels, and the body may attempt to compensate for lower testosterone levels by secreting more pituitary hormones, which may relate to an increase in testicular volume; individual differences also exist in testicular volume. Ehala-Aleksejev et al. (39) found an association between testicular volume and BMI in men. A previous study on obese men with a BMI ≥30 kg/m2 found that BMI was a significant predictor of testicular volume (40). More studies need to be conducted to investigate the relationship between the BMI of males and testicular volume in NOA. Zeadna et al. (41) examined the role of the BMI of males in sperm extraction and included the BMI in their prediction model for sperm extraction in NOA patients.
In relation to the embryo parameters, only 1 included study provided data on embryo transfer, the embryo implantation rate, the fertilization rate, and the good-quality embryo rate, which limited the comprehensive analysis of these outcomes. Further studies need to be conducted to evaluate these factors. In relation to the clinical outcomes, patients with a BMI ≥25 kg/m2 had a lower live-birth rate than those with a BMI <25 kg/m2 in this study. A previous meta-analysis (42) reported that an increased BMI in males was related to a significant decline in the live-birth rate per IVF-ICSI cycle (autologous spermatozoa); however, some studies have reported that the BMI of males was not correlated with the live-birth rate of infertile couples undergoing IVF/ICSI cycles (autologous spermatozoa) (43,44). In a real-world setting, embryo parameters and clinical outcomes can also be affected by female factors, such as female age and BMI (45,46). Thus, future studies should pay more attention to the control of important clinical variables influencing embryo parameters and clinical outcomes.
Based on our finding that the BMI of males affected the FSH and TT levels, testicular volume, sperm retrieval success, and live-birth rate in NOA. Thus, clinicians may advise NOA patients with a BMI ≥25 kg/m2 to lose weight (e.g., by doing more exercise, controlling their diet, having healthy eating behaviors, keeping balanced and adequate nutrition). Lifestyle modification and weight management could exhibit a positive impact on reproductive outcomes for these obese patients. Patients with a BMI ≥25 kg/m2 who wish to have children should fully understand these risks and be motivated to adopt a healthy lifestyle. Targeting these patients who consult fertility clinics and stimulating them to make lifestyle changes prior to pregnancy could assist in lowering the cost of fertility treatment and failure of treatment. Healthcare providers and national healthcare systems should pay attention to obesity (e.g., via BMI monitoring) and help to mitigate obesity-associated infertility in NOA patients. Through developed intervention strategies and programs to realize normal weight, male patients could improve their overall health, especially reproductive health.
The limitations of this study should be noted in interpreting the results. First, few studies were conducted on each outcome, and some outcomes could only be described qualitatively, which may have affected the stability of the results. Second, factors, such as different sperm extraction technologies, and the living habits of patients, such as smoking/drinking, may have affected the sperm extraction and pregnancy results. However, the information provided by the included studies was insufficient to support further analysis. Besides, high heterogeneity may exist for some indicators. Age, lifestyle and previous medical history may be the source of heterogeneity, but due to the limitations of the study design, our data did not support the exploration of the source of heterogeneity. As for methods for estimating testicular volume, three included studies applied an orchidometer, two used ultrasound, and seven did not report specific methods, which may have affected the reliability of the results. Missing data about cryptorchidism and orchiopexy in some of the included studies may also have affected the reliability of the results. However, we could not perform subgroup analysis based on methods for estimating testicular volume, cryptorchidism and orchiopexy due to limited data, which requires future studies. Finally, this study only included English-language studies, which may have introduced a language bias.
Conclusions
The NOA patients with a BMI ≥25 kg/m2 had lower FSH and TT levels than those with a BMI <25 kg/m2, the testicular volume of the BMI ≥25 kg/m2 group was larger than that of the BMI <25 kg/m2 group, the average BMI of the patients with successful sperm extraction was lower than that of those with failed sperm extraction, and the live-birth rate of the BMI ≥25 kg/m2 group was lower than that of the BMI <25 kg/m2 group. More studies need to be conducted to confirm these findings.
Acknowledgments
Funding: This study was funded by the Open Project of Yunnan Provincial Reproductive and Obstetrics and Gynecology Clinical Medicine Center (No. 2020LCZXKF-SZ12).
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-125/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-125/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-125/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peña VN, Kohn TP, Herati AS. Genetic mutations contributing to non-obstructive azoospermia. Best Pract Res Clin Endocrinol Metab 2020;34:101479. [Crossref] [PubMed]
- Kherraf ZE, Cazin C, Bouker A, et al. Whole-exome sequencing improves the diagnosis and care of men with non-obstructive azoospermia. Am J Hum Genet 2022;109:508-17. [Crossref] [PubMed]
- Krausz C, Cioppi F. Genetic Factors of Non-Obstructive Azoospermia: Consequences on Patients' and Offspring Health. J Clin Med 2021;10:4009. [Crossref] [PubMed]
- Vij SC, Sabanegh E Jr, Agarwal A. Biological therapy for non-obstructive azoospermia. Expert Opin Biol Ther 2018;18:19-23. [Crossref] [PubMed]
- Arshad MA, Majzoub A, Esteves SC. Predictors of surgical sperm retrieval in non-obstructive azoospermia: summary of current literature. Int Urol Nephrol 2020;52:2015-38. [Crossref] [PubMed]
- Guler I, Erdem M, Erdem A, et al. Impact of testicular histopathology as a predictor of sperm retrieval and pregnancy outcome in patients with nonobstructive azoospermia: correlation with clinical and hormonal factors. Andrologia 2016;48:765-73. [Crossref] [PubMed]
- Zhang ZB, Jiang YT, Yun X, et al. Male infertility in Northeast China: a cytogenetic study of 135 patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet 2012;29:83-7. [Crossref] [PubMed]
- Sundaram R, Mumford SL, Buck Louis GM. Couples' body composition and time-to-pregnancy. Hum Reprod 2017;32:662-8. [Crossref] [PubMed]
- Anifandis G, Dafopoulos K, Messini CI, et al. The BMI of men and not sperm parameters impact on embryo quality and the IVF outcome. Andrology 2013;1:85-9. [Crossref] [PubMed]
- Colaci DS, Afeiche M, Gaskins AJ, et al. Men's body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril 2012;98:1193-9.e1. [Crossref] [PubMed]
- Thomsen L, Humaidan P, Bungum L, et al. The impact of male overweight on semen quality and outcome of assisted reproduction. Asian J Androl 2014;16:749-54. [Crossref] [PubMed]
- Belloc S, Cohen-Bacrie M, Amar E, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril 2014;102:1268-73. [Crossref] [PubMed]
- Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221-31. [Crossref] [PubMed]
- Pavan-Jukic D, Starc A, Stubljar D, et al. Obesity with High Body Mass Index Does Not Influence Sperm Retrieval in Males with Azoospermia. Med Sci Monit 2020;26:e923060. [Crossref] [PubMed]
- Iwatsuki S, Sasaki S, Taguchi K, et al. Effect of obesity on sperm retrieval outcome and reproductive hormone levels in Japanese azoospermic men with and without Klinefelter syndrome. Andrology 2017;5:82-6. [Crossref] [PubMed]
- Li F, Yang Q, Shi H, et al. Effects of obesity on sperm retrieval, early embryo quality and clinical outcomes in men with nonobstructive azoospermia undergoing testicular sperm aspiration-intracytoplasmic sperm injection cycles. Andrologia 2019;51:e13265. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000.
- Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat Med 2015;34:2781-93. [Crossref] [PubMed]
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [Crossref] [PubMed]
- Bastug Y, Tokuc E, Bastug N, et al. Systemic immune-inflammation index, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are predictors of sperm presence in microdissection testicular sperm extraction. Andrologia 2022;54:e14419. [Crossref] [PubMed]
- Karamazak S, Kızılay F, Bahçeci T, et al. Do body mass index, hormone profile and testicular volume effect sperm retrieval rates of microsurgical sperm extraction in the patients with nonobstructive azoospermia? Turk J Urol 2018;44:202-7. [Crossref] [PubMed]
- Liu YP, Qi L, Zhang NN, et al. Follicle-stimulating hormone may predict sperm retrieval rate and guide surgical approach in patients with non-obstructive azoospermia. Reprod Biol 2020;20:573-9. [Crossref] [PubMed]
- Osaka A, Iwahata T, Kobori Y, et al. Testicular volume in non-obstructive azoospermia with a history of bilateral cryptorchidism may predict successful sperm retrieval by testicular sperm extraction. Reprod Med Biol 2020;19:372-7. [Crossref] [PubMed]
- Pavan-Jukic D, Stubljar D, Jukic T, et al. Predictive factors for sperm retrieval from males with azoospermia who are eligible for testicular sperm extraction (TESE). Syst Biol Reprod Med 2020;66:70-5. [Crossref] [PubMed]
- Ramasamy R, Bryson C, Reifsnyder JE, et al. Overweight men with nonobstructive azoospermia have worse pregnancy outcomes after microdissection testicular sperm extraction. Fertil Steril 2013;99:372-6. [Crossref] [PubMed]
- Rehnitz J, Rösner S, Harsch J, et al. Factors Influencing Success Rate of Intracytoplasmic Sperm Injection with Azoospermic Male Patients. Geburtshilfe Frauenheilkd 2020;80:713-22. [Crossref] [PubMed]
- Shrem G, Brudner Y, Atzmon Y, et al. The influence of obesity, smoking, and serum follicular stimulating hormone in azoospermic patients on testicular sperm extraction-intra cytoplasmic sperm injection outcomes: A retrospective cohort study. Medicine (Baltimore) 2019;98:e14048. [Crossref] [PubMed]
- Xu T, Peng L, Lin X, et al. Predictors for successful sperm retrieval of salvage microdissection testicular sperm extraction (TESE) following failed TESE in nonobstructive azoospermia patients. Andrologia 2017; [Crossref] [PubMed]
- Stárka L, Hill M, Pospíšilová H, et al. Estradiol, obesity and hypogonadism. Physiol Res 2020;69:S273-8. [Crossref] [PubMed]
- Hadjkacem Loukil L, Hadjkacem H, Bahloul A, et al. Relation between male obesity and male infertility in a Tunisian population. Andrologia 2015;47:282-5. [Crossref] [PubMed]
- Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia 2011;43:121-8. [Crossref] [PubMed]
- MacDonald AA, Herbison GP, Showell M, et al. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update 2010;16:293-311. [Crossref] [PubMed]
- Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care 2004;27:861-8. [Crossref] [PubMed]
- Aggerholm AS, Thulstrup AM, Toft G, et al. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril 2008;90:619-26. [Crossref] [PubMed]
- Khodamoradi K, Parmar M, Khosravizadeh Z, et al. The role of leptin and obesity on male infertility. Curr Opin Urol 2020;30:334-9. [Crossref] [PubMed]
- Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010;7:153-61. [Crossref] [PubMed]
- Fejes I, Koloszár S, Závaczki Z, et al. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch Androl 2006;52:97-102. [Crossref] [PubMed]
- Hammoud AO, Gibson M, Peterson CM, et al. Obesity and male reproductive potential. J Androl 2006;27:619-26. [Crossref] [PubMed]
- Ehala-Aleksejev K, Punab M. Relationships between total testicular volume, reproductive parameters and surrogate measures of adiposity in men presenting for couple's infertility. Andrologia 2017; Epub ahead of print. [Crossref] [PubMed]
- Tang Fui MN, Hoermann R, Wittert G, et al. Testicular volume and clinical correlates of hypothalamic-pituitary-testicular function: A cross-sectional study in obese men. Asian J Androl 2020;22:354-9. [Crossref] [PubMed]
- Zeadna A, Khateeb N, Rokach L, et al. Prediction of sperm extraction in non-obstructive azoospermia patients: a machine-learning perspective. Hum Reprod 2020;35:1505-14. [Crossref] [PubMed]
- Mushtaq R, Pundir J, Achilli C, et al. Effect of male body mass index on assisted reproduction treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 2018;36:459-71. [Crossref] [PubMed]
- Le W, Su SH, Shi LH, et al. Effect of male body mass index on clinical outcomes following assisted reproductive technology: a meta-analysis. Andrologia 2016;48:406-24. [Crossref] [PubMed]
- Wang X, Hao J, Zhang F, et al. Effects of female and male body mass indices on the treatment outcomes and neonatal birth weights associated with in vitro fertilization/intracytoplasmic sperm injection treatment in China. Fertil Steril 2016;106:460-6. [Crossref] [PubMed]
- Sermondade N, Huberlant S, Bourhis-Lefebvre V, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update 2019;25:439-51. [Crossref] [PubMed]
- Kato K, Ueno S, Berntsen J, et al. Comparing prediction of ongoing pregnancy and live birth outcomes in patients with advanced and younger maternal age patients using KIDScore™ day 5: a large-cohort retrospective study with single vitrified-warmed blastocyst transfer. Reprod Biol Endocrinol 2021;19:98. [Crossref] [PubMed]



