Pharmacology of testosterone replacement therapy preparations
Introduction
Testosterone replacement is approved by the United States Food and Drug Administration (USA FDA) for use in men who lack or have low testosterone levels in conjunction with an associated medical condition (1,2). Medical conditions which necessitate testosterone replacement therapy (TRT) include primary hypogonadism, in which the condition originates in the testes, and secondary/hypogonadotropic hypogonadism, a disease of the hypothalamus or pituitary gland. The goals of TRT are to restore serum testosterone levels to within the mid-normal physiological range associated with the patient’s age group, generally considered to be between 400 and 700 ng/dL, and to improve symptoms in hypogonadal men (2,3). While there is no consensus as to what serum testosterone level to initiate TRT (<200 versus <300 ng/dL, for example), it is agreed that low levels be verified with repeat testing with early morning samples (3). In practice, initiation of TRT is usually driven by patient symptoms in addition to low serum level.
Approximately 2.4 million USA males aged 40–69 years old suffer from hypogonadism, and 2.3% of men in their 40s and 3.8% of men in their 60s were taking some form of TRT in 2011 (4,5). Positive effects of TRT show considerable variation in terms of length of time to detection. Most begin to occur between 3 to 6 weeks after initiation, but some can take up to 1 year (6). Time-course differences are likely attributed to varying testosterone preparation pharmacokinetics (PKs). Common adverse effects associated with TRT are generally preparation-specific as well. More serious complications associated with use include polycythemia and increased risk of cardiac or venous thromboembolism events; long-term risks of TRT remains undetermined (7).
There are several testosterone preparations currently USA FDA approved. These can be generally classified via route of delivery and include buccal, nasal, subdermal, transdermal, and intramuscular (IM). Oral formulations of testosterone are not approved in the USA, due to historically being linked with liver toxicity and fluctuations in testosterone levels (8,9). This article will review the different preparations of testosterone currently approved by the USA FDA and discuss their pharmacology/PKs, dosing, and preparation specific adverse effects. Available products are compared in Table 1.
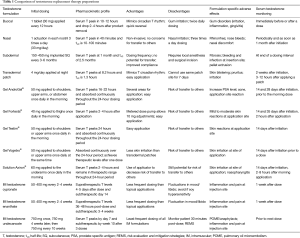
Full table
Oral administration
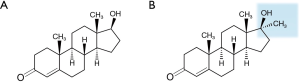
Historically, oral administration of physiological testosterone (Figure 1A) has been proven unsuccessful due to extensive first pass metabolism through the liver despite having good gastrointestinal absorption. Ingestion of supra-physiological doses was required to overcome this and allow for measurable amounts in the serum (10). Alkylation of testosterone at carbon 17α resulted in 17α-methyltestosterone (Figure 1B) with the ability to bypass first pass metabolism. However, this modification caused significant liver toxicity and lowering of HDL cholesterol (11). Esterification at carbon 17β yielded testosterone undecanoate (TU) (Figure 2A) which is absorbed via the lymphatic system and also bypasses liver degradation. This formulation required a high capsule burden due to low bioavailability along with gastrointestinal and liver adverse effects (12,13). Currently, oral testosterone preparations are not options for TRT in the USA due to these adverse effects.
Buccal administration
The novel route of buccal administration for TRT was initially approved by the USA FDA in 2003 (Striant®, Actient Pharmaceuticals LLC, Chesterbrook, PA, USA). Mucoadhesive tablets are applied to the gums of the mouth and provide controlled and sustained release of testosterone as the buccal system hydrates. This system delivers testosterone directly into the systemic circulation and bypasses the liver, avoiding first pass metabolism and increasing bioavailability. Each tablet contains 30 mg of testosterone and one tablet is applied every 12 hours, alternating sides of the mouth above the incisor teeth (14).
Levels of circulating testosterone peak within 10 to 12 hours of initial administration, and reach steady state within 24 hours (14). When the product is discontinued, testosterone levels drop below normal within two to 4 hours, allowing for quick reversal if necessary. The preparation mimics physiological circadian testosterone rhythm, with serum levels quickly increasing after insertion and peak levels obtained by the second dose with no accumulation over time (15). It is recommended to check a testosterone level between 4 to 12 weeks after therapy initiation prior to the morning dose (14). In a phase III trial assessing the PK profile and efficacy of buccal testosterone in 82 men over a 12-week treatment period, mean serum testosterone increased from a baseline of 149±88 ng/dL and was sustained between 580–700 ng/dL throughout the treatment period (16). About 80% of subjects maintained a Cavg above the lower normal limit at week 12 the majority (80%) of a 24-hour period (16).
Buccal testosterone has been found to be well tolerated in clinical trials lasting up to 12 months, with the most common adverse effect being gum-related (16,17). Approximately 18% of subjects reported irritation, inflammation, or gingivitis. After two years of usage in an open label phase III study, there was no additional increase of gum-related issues and only a 4.3% discontinuation rate (15). Advantages of buccal testosterone include non-invasive administration with minimal risk for secondary transfer to women or children.
Nasal administration
The most recently added product for TRT is a nasal gel formulation (Natesto™, Endo Pharmaceuticals, Malvern, PA, USA), granted USA FDA approval in May 2014. This product utilizes a metered dose pump applicator, allowing patients to self-administer into the nostrils. Each pump delivers 5.5 mg of testosterone, with the recommended dose of two pumps (one actuation per nostril) three times a day, for a total daily dose of 33 mg. Doses should be separated by 6 to 8 hours (18). Absorption occurs through the nasal mucosa, avoiding first pass metabolism, and the Cmax is reached within 40 minutes of administration (19).
In an open-label phase III trial assessing nasal testosterone usage in 306 hypogonadal men (testosterone <300 ng/dL) for 90 days, 90% achieved serum testosterone concentrations within the normal physiological range with a Cavg of 421 ng/dL, while 10% remained subtherapeutic (18). Treatment was well tolerated with a low discontinuation rate of 3.7%. Adverse effects occurring in more than 3% of subjects included increased prostate specific antigen (PSA), headache, rhinorrhea, nosebleed, nasal discomfort, upper respiratory tract infection, sinusitis, bronchitis, and nasal scab (18). Testosterone nasal gel is another non-invasive alternative with simple administration, low total daily dose, and no concern for secondary transfer.
Subdermal administration
Subdermal testosterone pellets were the first effective formulation for androgen replacement therapy, developed in the 1940s (20). Testosterone pellets consist of crystalline testosterone and are created through high-temperature molding and designed for consistent and prolonged release (21). Absorption occurs through uniform erosion of the pellet’s surface in correspondence to the solubility of testosterone in extracellular fluid. Dosing varies on patient age and diagnosis, and is adjusted to the patient’s response and manifestation of adverse reactions. General dosing recommendations are 150 to 450 mg implanted subdermally in the hip area or another fatty area at 3 to 6 month intervals (22). Testosterone pellets are available generically in 12.5, 25, 37.5, and 50 mg pellets. A 75 mg pellet is also available as Testopel® (Auxilium Pharmaceuticals Inc., Malvern, PA, USA).
In a randomized, cross-over clinical study, 43 men with primary or secondary hypogonadism received each of the following regimens: 100 mg ×6 pellets, 200 mg ×3 pellets, or 200 mg ×6 pellets (23). Each regimen was separated by at least 6 months, and the next one was not initiated until testosterone decreased to hypogonadal levels. Serum levels peaked at approximately one month and were sustained in the normal range for four to five months with either 600 mg dose and for 6 months with the 1,200 mg dose. The estimated half-life was 2.5 months.
The most common adverse event is pellet extrusion, with an incidence of 10% (21). Other adverse events include site infections, bleeding, and fibrosis at the insertion site (12). Potential advantages of pellet usage include the infrequency of dosing, guaranteed compliance, and lack of transference. However, administration is invasive requiring skin incision and local anesthesia. There are also concerns regarding pellet removal for patients experiencing androgen related side effects.
Transdermal administration
Transdermal patch
The first transdermal patches found to be efficacious were scrotal patches in the 1980s (21). This application allowed for maximal absorption through a thin-skinned area. Disadvantages included smaller skin surface area and application challenges (hair clipping). As such, scrotal testosterone patches fell out of favor for other transdermal options and are no longer available in the USA.
Non-scrotal transdermal patches, Androderm®, (Actavis, Parsippany, NJ, USA) achieved USA FDA approval in 1995. The patches are available in either 2 or 4 mg/day formulations (24). The recommended starting dose is one 4 mg/day patch (not 2×2 mg/day patches) every 24 hours applied nightly. The patch is to be applied to the back, abdomen, upper arms, or thighs. Sites should be rotated and not re-used within 7 days. Two weeks after initiation of therapy, a serum testosterone level should be measured (early morning after patch application the night prior) and patch dosing adjusted as necessary. Levels <400 ng/dL require a dose escalation to 6 mg/day (1×4 mg/day patch plus 1×2 mg/day patch), while levels >930 ng/dL should be reduced to 2 mg/day.
A multi-center, prospective randomized trial compared the PKs, efficacy, and safety of the transdermal system to IM testosterone enanthate (TE) injections (25). Treatment was for 24 weeks and 33 subjects were enrolled per group. For the transdermal system group, the starting dose was 5 mg/day (2×2.5 mg/day patches, no longer commercially available). Dose adjustments were allowed for adverse effects or serum testosterone levels not within physiologic range. PK analysis occurred at week 16. Twenty-seven transdermal subjects were eligible for PK analysis. Baseline serum testosterone was 55.4 ng/mL and increased to an average of 517 ng/dL. The Cmax was 765 ng/dL within 8.2 hours of application. Transdermal administration allows testosterone to be continuously absorbed for 24 hours with no dose accumulations, mimicking normal circadian pattern when applied nightly. The approximate half-life is 1.3 hours and hypogonadal concentrations are achieved within 24 hours of patch removal (26).
The efficacy of the transdermal patch is often limited by lack of adherence or discontinuation due to skin blistering, pruritus, or irritation. This is due to permeation enhancers included in the transdermal system which are necessary to increase absorption. Combined clinical trials data report the overall incidence of any application site reactions as 48% with pruritus being most common (24). Topical corticosteroid application to the affected area is recommended for alleviation of symptoms (24). Advantages to transdermal patch use include non-invasive, easy application, quick reversal after removal, and normal circadian pattern of testosterone. Risk of transference to others is not concerning as there is an occlusive backing film on the patch system preventing others from contact with the active ingredient.
Transdermal gel/liquid solution
Since 2002, several testosterone gels and liquids have been developed for transdermal TRT. Due to the concern for testosterone gel or liquid being transferred to females and children who come into contact with a patient’s skin after use, these formulations have received a USA Boxed Warning. Patients should be reminded to wash their hands after application and to avoid skin contact with others. Recommended sites of application for these agents are areas that will be covered by clothing to minimize transfer. The potential advantages of testosterone transdermal gels and liquids include ease of application, less skin irritation than patches, and more consistent serum testosterone levels than other formulations, such as IM testosterone (27). The four branded, USA FDA approved testosterone hydroalcoholic gels available include AndroGel® (AbbVie Inc., North Chicago, IL, USA), Fortesta® (Endo Pharmaceuticals, Malvern, PA, USA), Testim® (Auxilium Pharmaceuticals Inc., Malvern, PA, USA), and Vogelxo® (Upsher-Smith Laboratories Inc., Maple Grove, MN, USA). There are generic formulations available for some gel products. Additionally, there is one testosterone transdermal solution, Axiron® (Eli Lilly, Indianapolis, IN, USA).
AndroGel®
Androgel® is available in 1% and 1.62% concentrations. Dosage forms for the 1% concentration include a metered-dose pump, which delivers 12.5 mg of testosterone per actuation (generic only), and unit-dose packets that contain either 25 mg/2.5 g or 50 mg/5 g of testosterone (brand or generic) (28). The recommended starting dose is 50 mg applied topically once daily in the morning. Areas for application include the shoulders, upper arms, or abdomen. Based on serum testosterone levels, the dose can be increased in 25 mg increments up to 100 mg of testosterone daily. The 1.62% concentration is also available in a metered-dose pump and unit-dose packets (brand only). The metered-dose pump provides 20.25 mg of testosterone per actuation, while the unit-dose packets contain either 20.25 mg/1.25 g or 40.5 mg/2.5 g of testosterone (29). The recommended starting dose of AndroGel® 1.62% is 40.5 mg applied topically once daily in the morning. Serum testosterone levels should be measured 14 and 28 days after initiation prior to the morning dose. It should be noted that application sites for 1.62% gel only include the shoulders and upper arms, and not the abdomen. Dose adjustments between 20.25–81 mg increments are recommended for levels outside the range of 350 to 750 ng/dL.
The PK effects of AndroGel® 1% on serum testosterone levels was evaluated in a randomized, parallel study that compared gel 50 mg/day to gel 100 mg/day and patches 5 mg/day for three months in 227 hypogonadal men (30). After three months, the patients could receive either testosterone gel 50, 75, 100 mg/day or patch 5 mg/day. On day one, serum testosterone was within physiological range in both gel groups, reaching a Cmax of 560±31 ng/dL in the 50 mg/day group after 22 hours of application and 745±40 ng/dL in the 100 mg/day group after 16 hours. By day 30, the Cmax in the 50 mg/day gel group was 875±57 ng/dL with a Cmin of 360±39 ng/dL; for the 100 mg/day gel group, the serum testosterone Cmax was 1,198±56 ng/dL with a Cmin of 504±27 ng/dL. The 1% transdermal testosterone gel was able to increase serum testosterone to the upper range of normal after a few days of use and maintain levels with repeated daily use. The range of levels between the two doses illustrates the flexibility available with transdermal testosterone gel (30).
The most common adverse effect associated with AndroGel® 1% included acne (1% to 8% incidence) and application site reaction (3% to 5% incidence) (28). The most common adverse effect of AndroGel® 1.62% was increased PSA level (29). The incidence was 11.1% in a multi-phase, 364 day study of 234 hypogonadal men. Increased PSA was defined as an adverse event if there was a level >4 ng/mL or an increase >0.75 ng/mL from baseline on two separate occasions. All other reported adverse effects were <3% incidence.
Fortesta®
Fortesta® 2% testosterone gel is available (both brand and generic) as a metered-dose pump delivering 10 mg of testosterone per actuation. The recommended starting dose is 40 mg (four actuations) applied once daily in the morning to the thighs. The dose can be adjusted in increments of 10 mg, based on serum levels measured two hours after morning application 14 and 35 days after initiation or adjustments. Fortesta® dosing range is 10 to 70 mg/day (31).
The effects of Fortesta® 2% gel on serum testosterone levels was evaluated in a multicenter, open-label study of 129 men with hypogonadism (32). The subjects applied Fortesta® 2% gel initially at 40 mg/day to their front and inner thighs for 90 days. Dose adjustments between 10 to 70 mg/day were allowed at clinic visits on days 14, 35, and 60. Serum levels were measured on days 14, 35, 60, and 90. On day 90, the Cavg was 438±162 ng/dL with a mean Cmax of 827±356 ng/dL. Normal physiologic range of testosterone was defined as 300 to 1,140 ng/dL, and 77.5% of patients had Cavg within this range at 90 days. Additionally, the 24-hour PK profile showed testosterone peaked 2–4 hours after application. Fortesta® 2% gel at doses between 10 to 70 mg/day achieved physiologic serum testosterone levels (32).
Adverse effects of Fortesta® 2% gel were reported in a controlled multi-center, open 90-day study of 149 hypogonadal patients (31). Adverse effects occurred in 22.8% (34/149) of patients. Most common was skin reactions at site of application (16.1%); 79% were mild and the remainder were moderate. Only two patients discontinued therapy for skin related issues.
Testim®
Testim® 1% gel is supplied in unit-dose tubes containing 50 mg of testosterone/5 g (33). The recommended starting dose is 50 mg applied once daily in the morning to the shoulders or upper arms. Monitoring of serum testosterone should be performed 14 days after starting Testim®. The dose can be increased up to 100 mg of testosterone daily if indicated.
The PKs of Testim® gel was compared to AndroGel® in a randomized, open-label, two-way complete cross-over study that included 29 hypogonadal male subjects (34). Several blood samples were collected over a 48-hour period to evaluate serum testosterone levels. The dose of testosterone for both formulations was 50 mg. For Testim® gel, the mean Cmax was 480 ng/dL compared to 368 ng/dL for AndroGel®. The mean AUC0-24 was also higher with Testim® gel, measuring 5,864 ng·h/dL, compared to 4,499 ng·h/dL for AndroGel®. Since the ratios of Testim® gel to AndroGel® for Cmax and AUC0-24 were both 1.30, with 90% confidence intervals outside the bioequivalence limits of 0.80 to 1.25, it was concluded that Testim® gel is not equivalent to AndroGel® (34).
Adverse effects were reported from a controlled clinical study where 304 patients were treated with Testim® 50 mg, 100 mg or placebo up to 90 days (33). The only reported adverse effect with greater than 3% incidence was application site reactions at 4% in the 100 mg group.
Vogelxo®
Vogelxo® 1% gel is available in a multi-dose metered pump, unit-dose tubes and packets. All formulations are also available as generic. Each unit-dose tube or packet contains 50 mg of testosterone/5 g. The multi-dose metered pump provides 12.5 mg of testosterone per actuation. It is recommended to start Vogelxo® at a dose of 50 mg applied once daily to the shoulders or upper arms. Dose adjustments are based on serum testosterone levels measured 14 days after starting therapy. The dose of Vogelxo® can be increased to 100 mg/day to achieve therapeutic serum testosterone levels (35).
In a randomized, parallel treatment group study of 406 subjects with low serum testosterone, two doses of Vogelxo® were compared to a testosterone patch and placebo gel (36). Study subjects received either 50 or 100 mg of Vogelxo® daily, while the patch delivered 5 mg of testosterone daily. The study was conducted over 90 days, with measuring of serum testosterone to assess 24-hour PK profiles on days 30 and 90. At day 30, the mean Cavg for the 50 mg gel group increased 50% from baseline compared to a 173% increase in the 100 mg gel group. The patch group had an increase in Cavg similar to the 50 mg gel group. Normal adult range for testosterone was defined as 300–1,000 ng/dL, and 55% of subjects in the 50 mg gel group achieved a Cavg above 300 ng/dL compared to 95% in the 100 mg gel group, 68% in the patch group, and 8% of placebo. While 30 patients in the 100 mg gel group had a Cmax above 1,000 ng/dL, the Cavg remained within normal range for 87% of them. Both gel groups had significantly less fluctuation in testosterone levels over 24 hours compared to the patch group. On day 60, patients subtherapeutic in the 50 mg gel group were increased to 100 mg/day, while supratherapeutic subjects in the 100 mg group were decreased to 50 mg/day. On day 90, Cavg above 300 ng/dL was achieved in 75% of the 50 mg gel group and 80% of the 100 mg group compared to 57% in the patch group.
Overall, there was a significantly lower number of skin irritation events in the gel groups compared to the patch group. Only six of 205 subjects in the gel groups discontinued therapy; two were related to drug specific adverse effects (hypertension and mood swings). The study concluded that Vogelxo® gel is better tolerated than transdermal testosterone patches, and can better normalize serum testosterone levels with titration (36).
Axiron®
Axiron® is supplied as a solution in a metered-dose pump that provides 30 mg of testosterone per actuation. The solution must be applied to the underarms using the provided applicator and is not recommended for application on other body parts. The suggested starting dose of Axiron® is 60 mg applied once daily in the morning. Dose adjustments should be made based on serum testosterone levels measured 14 days after initiation, and drawn two to eight hours after dose application. The dose can be adjusted in 30 mg increments up to a maximum of 120 mg or a minimum of 30 mg/day (37).
A multicenter, open label study in men with documented androgen deficiency was conducted to evaluate the effects of Axiron® at a dose of 60 mg/day (38). Normal physiological range of serum testosterone was defined as 300–1,050 ng/dL, and doses were adjusted on days 45 and 90. On day 15, all subjects (n=135) were receiving 60 mg/day and the mean Cavg was 456 ng/dL with a Cmin of 257 ng/dL and a Cmax of 743 ng/dL. By day 120, 84.1% of patients had a Cavg within normal range. At this time, 10 patients were receiving 120 mg, 25 were titrated to 90 mg, 97 were still receiving 60 mg, and 3 patients had decreased to 30 mg/day. Mean Cavg and Cmax for all dosing regimens were within normal range on day 120. Adverse events occurring in greater than 3% of subjects who received at least one dose included application site irritation (7%), application site erythema (5%), headache (5%), increased hematocrit (4%), and nasopharyngitis (4%). The authors concluded Axiron® was well tolerated and achieved therapeutic testosterone levels with appropriate dose adjustments (38). Axiron® is novel in its use of an applicator which prevents users from touching the solution, potentially decreasing transmission risk to others unlike testosterone gels.
IM administration
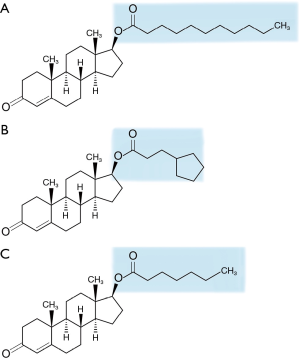
Preparations of IM testosterone have been used since the 1950s. Unmodified testosterone has an approximate half-life of 10 minutes when injected, which would necessitate unrealistic multi-dosing regimens to achieve and maintain therapeutic levels (21). Current formulations have a prolonged duration of action as they are synthesized through esterification of the 17β carbon of natural testosterone. Esterification increases the solubility of testosterone in oil, which allows for slower release once injected into the muscle. Testosterone esters are not biologically active until the ester group is cleaved off. The three IM preparations that are USA FDA approved are testosterone cypionate (TC), TE, and TU. These products differ based on the carbon side chain esterified to the 17β position of testosterone (Figure 2). TU has the longest carbon side chain, consisting of 11 carbon atoms compared to seven and eight for TC and TE respectively, which accounts for its longer duration of action (39,40). One disadvantage of these formulations is the necessity for IM injection. The Endocrine Society Clinical Practice Guidelines for testosterone therapy recommend serum testosterone levels should be measured one week after receiving a dose of TC or TE, targeting a therapeutic level of 400 to 700 ng/dL (3). For TU, levels should be measured prior to each subsequent injection (3).
TC
TC is available both branded as Depo-Testosterone® (Pharmacia and Upjohn Company, New York, NY, USA) and as a generic (41). Both options are supplied in 100 mg/mL (10 mL) and 200 mg/mL (1 and 10 mL) concentrations, prepared in cottonseed oil. The USA FDA recommended starting dose for male hypogonadism is 50 to 400 mg IM every 2 to 4 weeks (41). The Endocrine Society Clinical Practice Guidelines for testosterone therapy suggest an alternative of either 75 to 100 mg IM weekly or 150 to 200 mg IM every 2 weeks (3).
A PK study evaluated serum levels of testosterone periodically for 14 days after administration of TC 200 mg IM in 11 hypogonadal men (42). The mean Cmax was supratherapeutic (1,112±297 ng/dL) and occurred between days four and five post-injection. After day 5, testosterone levels declined and by day 14 the mean Cavg approached 400 ng/dL. These large fluctuations in serum testosterone over a 2-week period illustrate the less than ideal kinetics of TC IM injections.
The fluctuation in serum testosterone levels can result in mood swings or changes in libido, which is a formulation specific IM adverse effect that should be closely monitored. Other common adverse effects with TC use are local inflammation and pain at the site of injection, also due to IM administration (41). As cottonseed oil is the formulation vehicle, TC use is contraindicated in anyone with a known hypersensitivity to testosterone synthesized from soy.
TE
TE is generically available in 200 mg/mL (5 mL vial) concentration, prepared in sesame oil (43). The recommend starting dose for TE is the same as TC per the USA FDA and Endocrine Society guidelines (3,43). TE associated adverse effects are also driven by its IM administration and mimic those of TC.
The effect of varying doses of TE on serum testosterone was evaluated in 23 males with primary hypogonadism (44). Subjects received IM injections of TE based on one of the following regimens: 100 mg weekly, 200 mg every 2 weeks, 300 mg every 3 weeks, or 400 mg every 4 weeks. Serum testosterone was measured weekly during the initial treatment period of 12 weeks. After receiving the last dose of the treatment period, testosterone levels were then measured more frequently. For the 100 mg group, the average Cmax peaked above 1,200 ng/dL 24 hours after the last dose and declined to slightly above 600 ng/dL after 1 week. In the 200 mg group, the average Cmax was also greater than 1,200 ng/dL and occurred 48 hours after the last dose. The level plateaued around the lower therapeutic limit after 2 weeks. The 300 and 400 mg groups similarly had an average Cmax above 1,200 ng/dL within 36–48 hours. For both groups, levels plateaued below the therapeutic range (300 ng/dL by week 3 for the 300 mg group and week 4 for the 400 mg group). Based on these results, TE dosed at 100 mg once weekly or 200 mg every 2 weeks maintains serum testosterone within therapeutic range by the end of the dosing regimen. As TC and TE have different oil vehicles, they are rated AO by the USA FDA, meaning they are not therapeutically equivalent (45).
TU
The formulation of TU contains a concentration of 250 mg/mL supplied in 3 mL vials, prepared in refined castor oil and is branded as Aveed® (Endo Pharmaceuticals, Malvern, PA, USA) (46). The recommended dosing strategy is 750 mg given IM in the gluteus medius, followed by 750 mg 4 weeks later, then 750 mg every 10 weeks thereafter. Dosage titration is not recommended.
An open-label study was performed to examine serum testosterone levels after treatment with IM TU using the approved dosing strategy for a total of nine injections (47). Testosterone levels were measured at baseline and at days 4, 7, 11, 14, 21, 28, 42, 56, and 70 after the third injection and 4, 7, 11, 14, 21, 42, and 70 after the fourth injection. A total of 130 hypogonadal males received treatment, but levels were available for 117. Overall, levels were similar after the third and fourth injections, with a mean Cmax of 813 ng/dL reached by day seven and a mean Cmin between 323 to 339 ng/dL by week 10 after each injection. The PK profile of TU does not demonstrate supratherapeutic peaks, and trough levels are seen later after each injection when compared to TE and TC (47).
After each injection a healthcare provider must observe the patient for 30 minutes due to the serious adverse reactions of pulmonary oil microembolism (POME) and anaphylaxis. POME can occur during or after any injection throughout the course of therapy and includes symptoms such as the urge to cough, shortness of breath, throat tightening, chest pain, dizziness, and syncope (46). There is a USA FDA Boxed Warning for the risk of POME and anaphylaxis; as a result, TU is only available through a restricted use program (Aveed® REMS Program). Other adverse effects reported with greater than 3% incidence during TU clinical trials included acne, injection site pain, and increased PSA (47).
Conclusions
When considering all available routes of delivery, concentrations, and branded or generic choices, there are currently over 30 different testosterone preparations to consider when choosing one for a patient. The decision on the best product choice should include patient preference, PKs, treatment burden, cost and insurance coverage. Products may also need to be switched throughout TRT based upon patient response, preference, and adverse effects. In all circumstances, the decisions should be an open dialogue between the patient and clinician to allow for the most successful TRT regimen.
Acknowledgements
The authors would like to graciously thank Joseph Kanasz, BFA, from the Cleveland Clinic Center for Medical Arts and Photography for creation of Figures 1-2 in this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- United States Food and Drug Administration [Internet]. Silver Spring: United States Food and Drug Administration; 2016 [updated 2005 March 3; cited 2016 Apr 25]. Available online: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161874.htm
- Manni A, Pardridge WM, Cefalu W, et al. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab 1985;61:705-10. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920-6. [Crossref] [PubMed]
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. [Crossref] [PubMed]
- Saad F, Aversa A, Isidori AM, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol 2011;165:675-85. [Crossref] [PubMed]
- Osterberg EC, Bernie AM, Ramasamy R. Risks of testosterone replacement therapy in men. Indian J Urol 2014;30:2-7. [Crossref] [PubMed]
- Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol 2004;77:257-67. [Crossref] [PubMed]
- Delorimier AA, Gordan GS, Lowe RC, et al. Methyltestosterone, related steroids, and liver function. Arch Intern Med 1965;116:289-94. [Crossref] [PubMed]
- Daggett PR, Wheeler MJ, Nabarro JD. Oral testosterone, a reappraisal. Horm Res 1978;9:121-9. [Crossref] [PubMed]
- Bagatell CJ, Bremner WJ. Androgen and progestagen effects on plasma lipids. Prog Cardiovasc Dis 1995;38:255-71. [Crossref] [PubMed]
- Nieschlag E, Behre HM, Bouchard P, et al. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update 2004;10:409-19. [Crossref] [PubMed]
- Yoshida EM, Erb SR, Scudamore CH, et al. Severe cholestasis and jaundice secondary to an esterified testosterone, a non-C17 alkylated anabolic steroid. J Clin Gastroenterol 1994;18:268-70. [Crossref] [PubMed]
- Striant [package insert]. Chesterbrook (PA): Actient Pharmaceuticals LLC; May 2015.
- Dinsmore WW, Wyllie MG. The long-term efficacy and safety of a testosterone mucoadhesive buccal tablet in testosterone-deficient men. BJU Int 2012;110:162-9. [Crossref] [PubMed]
- Wang C, Swerdloff R, Kipnes M, et al. New testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab 2004;89:3821-9. [Crossref] [PubMed]
- Korbonits M, Slawik M, Cullen D, et al. A comparison of a novel testosterone bioadhesive buccal system, striant, with a testosterone adhesive patch in hypogonadal males. J Clin Endocrinol Metab 2004;89:2039-43. [Crossref] [PubMed]
- Natesto™ [package insert]. Malvern (PA): Endo Pharmaceuticals; May 2015.
- Mattern C, Hoffmann C, Morley JE, et al. Testosterone supplementation for hypogonadal men by the nasal route. Aging Male 2008;11:171-8. [Crossref] [PubMed]
- Deansley R, Parkes AS. Further experiments on the administration of hormones by the subcutaneous implantation of tablets. Lancet 1938;2:606-9. [Crossref]
- Behre HM, Wang CC, Handelsman DJ, et al. Pharmacology of testosterone preparations. Available online: https://www.researchgate.net/publication/264848721_Pharmacology_of_testosterone_preparations
- Testopel [package insert]. Malvern (PA): Auxilium Pharmaceuticals Inc.; May 2015.
- Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 1990;71:216-22. [Crossref] [PubMed]
- Androderm [package insert]. Parsippany (NJ): Actavis; May 2015.
- Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999;84:3469-78. [PubMed]
- Meikle AW, Mazer NA, Moellmer JF, et al. Enhanced transdermal delivery of testosterone across nonscrotal skin produces physiological concentrations of testosterone and its metabolites in hypogonadal men. J Clin Endocrinol Metab 1992;74:623-8. [PubMed]
- Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs 2012;72:1591-603. [Crossref] [PubMed]
- AndroGel, 1% [package insert]. North Chicago (IL): AbbVie; May 2015.
- AndroGel, 1.62% [package insert]. North Chicago (IL): AbbVie; May 2015.
- Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 2000;85:4500-10. [PubMed]
- Fortesta [package insert]. Malvern (PA): Endo Pharmaceuticals; May 2015.
- Dobs AS, McGettigan J, Norwood P, et al. A novel testosterone 2% gel for the treatment of hypogonadal males. J Androl 2012;33:601-7. [Crossref] [PubMed]
- Testim [package insert]. Malvern (PA): Auxilium Pharmaceuticals Inc.; September 2009.
- Marbury T, Hamill E, Bachand R, et al. Evaluation of the pharmacokinetic profiles of the new testosterone topical gel formulation, Testim, compared to AndroGel. Biopharm Drug Dispos 2003;24:115-20. [Crossref] [PubMed]
- Vogelxo [package insert]. Maple Grove (MN): Upsher-Smith Laboratories; May 2015.
- Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003;88:2673-81. [Crossref] [PubMed]
- Axiron [package insert]. Indianapolis (IN): Eli Lilly; May 2015.
- Wang C, Ilani N, Arver S, et al. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin Endocrinol (Oxf) 2011;75:836-43. [Crossref] [PubMed]
- Byrne M, Nieschlag E. Testosterone replacement therapy in male hypogonadism. J Endocrinol Invest 2003;26:481-9. [Crossref] [PubMed]
- Edelstein D, Basaria S. Testosterone undecanoate in the treatment of male hypogonadism. Expert Opin Pharmacother 2010;11:2095-106. [Crossref] [PubMed]
- Depo-Testosterone© [package insert]. New York (NY): Pharmacia and Upjohn Company; April 2015.
- Nankin HR. Hormone kinetics after intramuscular testosterone cypionate. Fertil Steril 1987;47:1004-9. [Crossref] [PubMed]
- Testosterone enanthate [package insert]. Eatontown (NJ): West-Ward Pharmaceuticals; November 2015.
- Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab 1980;51:1335-9. [Crossref] [PubMed]
- United States Food and Drug Administration Orange Book [Internet]. Silver Spring: United States Food and Drug Administration; 2016 [updated 2016 April; cited 2016 May 13]. Available online: http://www.accessdata.fda.gov/scripts/cder/ob/docs/tempai.cfm
- Aveed© [package insert]. Malvern (PA): Endo Pharmaceuticals; May 2015.
- Wang C, Harnett M, Dobs AS, et al. Pharmacokinetics and safety of long-acting testosterone undecanoate injections in hypogonadal men: an 84-week phase III clinical trial. J Androl 2010;31:457-65. [Crossref] [PubMed]