Testosterone replacement in the infertile man
Introduction
Hypogonadism defined as inadequate gonadal function manifested by deficiencies in gametogenesis or hormone secretion (1), is a common clinical condition affecting men’s fertility and sexual health. In the United States, almost 39% of men over the age of 45 years were found to have low serum testosterone (T) levels (2). While ageing has been considered the principal factor which contributes to the development of hypogonadism, below-normal serum T levels are being encountered more often in men seeking fertility. Such a trend may be secondary to changes in lifestyle and eating habits, or to elective delay of conception age. The Baltimore Longitudinal Study of Aging reported a 3–8% incidence of hypogonadism among men 20–45 years old (3). Furthermore, a 0.4% yearly decline in T was also detected in men of the same age group (4). The increase in paternal age at time of delivery has been documented in a study by Kovac et al., who particularly reported an upsurge in live births in men above 35 years of age (5).
Hypogonadism is more common among infertile patients. Low T or high luteinizing hormone (LH) levels were detected in about 30% of patients undergoing fertility evaluation (6). This association may be explained by the higher prevalence of genetic abnormalities, endocrinopathies and clinical varicocele among patients complaining of infertility. In a study by Tanrikut et al. (7), the serum T levels of 325 patients with varicocele were measured and compared to a control group of 510 vasectomy reversal patients. After adjusting for age, men with varicoceles had significantly lower T levels than the control group. Furthermore, microsurgical varicocele ligation resulted in a significant increase in serum T levels in more than two-thirds of the study participants. These findings suggest that varicocele is a significant risk factor for androgen deficiency and that surgical repair may be beneficial in restoring normal T levels.
Hypogonadism carries another clinical implication on male fertility. Exogenous T use has been on the rise over the past few decades in line with the significant advances in T delivery systems and the increase in public awareness of low T level and its associated symptoms in men (8-10). A 500% increase in physician prescription rates for T has been observed over the past 10 years (11). Despite this, men desiring to maintain their reproductive potential may not be fully aware of the risks of exogenous T therapy. In a recent survey of urologists, Ko et al. (12) observed that approximately 29% have used exogenous T to treat male infertility. The need for additional efforts to establish recommendations for managing hypogonadism in infertile men and raise physician awareness on the detrimental effects of supplemental T on sperm production were suggested.
Current clinical practice guidelines advocate hormone evaluation for infertile men when symptoms of hypogonadism exist or when a sperm concentration of less than 10 million/mL is encountered (13). Androgen replacement is generally indicated in men who are not trying to conceive and have persistently low serum T levels combined with clinical symptoms of hypogonadism. These include decreased libido, sexual dysfunction, fatigue, lack of concentration, decreased facial and body hair, decreased in lean body mass and increased fat mass.
In this review we aim to explore the different options available for the medical treatment of hypogonadism in men desiring to retain their fertility.
Hypothalamic pituitary gonadal axis
A brief understanding of the HPG axis is important in order to recognize the different therapeutic options utilized in androgen replacement in infertile men. Testosterone synthesis is regulated by the hypothalamus and pituitary gland (Figure 1). Gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus, passes through the hypothalamo-hypophyseal portal circulation to the anterior pituitary where it stimulates the production of the glycoprotein hormones, LH and follicle stimulating hormone (FSH). FSH and LH are consequently secreted into the circulation to carry stimulatory actions to the testes. FSH acts on Sertoli cells triggering spermatogenesis and hormone synthesis, primarily inhibin. LH binds to LH receptors on Leydig cells stimulating steroidogenesis and testosterone production. There is some evidence suggesting that FSH may stimulate Testosterone production by Leydig cells secondary to release of activating hormones from Sertoli cells (14). GnRH is secreted in a pulsatile manner leading to a similar response in LH and consequently testosterone synthesis giving rise to a circadian rhythm that is essential for human health and well-being.
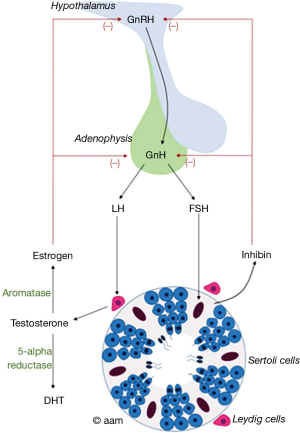
Testosterone is then aromatized to estradiol, which exerts a negative feedback on the hypothalamus and pituitary gland resulting in decreased production of GnRH, FSH and LH consequently maintaining testosterone in its optimal range. Inhibin also exerts negative feedback on the pituitary gland decreasing LH and FSH production (14).
Hypogonadism can be primary or secondary. Primary hypogonadism is suggested when a low serum T is associated with high FSH and LH levels. It results from disorders of the testes that lead to low testosterone production and impaired spermatogenesis (primary testicular failure) as seen in Klinefelter syndrome patients or after testicular insult from trauma, infection, surgery or chemo/radiotherapy. Secondary hypogonadism is however considered when both gonadotropins as well as serum T levels are low. It can be idiopathic or due to Kallman syndrome or secondary to pituitary or hypothalamic functional or nonfunctional growths.
Exogenous T replacement
Exogenous T has been increasingly used for both anabolic and androgenic purposes. Current estimates indicate the presence of up to 3 million anabolic steroid users in the United States (15). Internationally, a 3–4% life time prevalence risk of steroid abuse in men has been reported (16). This prevalence is estimated to be much higher among young gym attenders reaching up to 25% (17). Studies on high school seniors reveal a staggering 6.6% rate of exogenous T use among students mostly initiated before 16 years of age (18). Another survey among body builders confirmed T abuse at an early age where almost two thirds of abusers started T replacement before 23 years of age (19).
An understanding of the detrimental effects of T abuse is crucial and should be relayed to patients being evaluated for androgen replacement. Exogenous administration of T results in a negative feedback inhibition of the HPG axis which consequently causes a decrease in endogenous and intratesticular T levels. The latter, which is normally about 50–100 times higher than serum levels, is integral for optimal sperm production. Despite a normal or high serum T concentration in men receiving anabolic steroids, intratesticular T levels may be markedly inhibited resulting in oligozoospermia or azoospermia (20,21). Atrophy of the germinal epithelium together with spermatogenesis suppression and subsequent azoospermia after 10 weeks of therapy has been documented by a World Health Organization Task Force investigating exogenous T as a male contraceptive option (22). Recovery is expected after 6 months of treatment cessation, with almost half the patients returning to their baseline pre-treatment sperm density (22). However, up to 10% of patients with poor pre-treatment sperm production remain azoospermic after treatment cessation (23).
Consequently, exogenous T use should be discouraged in hypogonadal patients who either have infertility or wish to conceive in the future. Such patients should be offered alternative options that can raise their endogenous T instead of receiving synthetic forms of the hormone. More importantly, patients should be properly evaluated to look for correctable secondary causes of hypogonadism.
Therapeutic approaches
Several available treatment options can manipulate the HPG axis ultimately raising endogenous T levels. Most of these medications are not FDA approved for men. However, compelling evidence exist that confirms the efficiency and safety of such medications.
Clomiphene citrate (CC)
CC is a selective estrogen receptor modulator that competitively bind to estrogen receptors in the hypothalamus and pituitary gland. As a result, it prevents the inhibitory effect of estrogen on gonadotropin production thereby increasing LH and ultimately T production by the testes. CC is given at a dose of 25 mg every day or 50 mg every other day. Potential side effects include facial flushing, excessive sweating, gynecomastia and breast tenderness, weight gain, hypertension, cataracts, and acne.
In an attempt to investigate the efficacy of CC as a method of androgen replacement, Guay et al. initially treated 21 older men who had secondary hypogonadism with 50 mg CC twice daily for 7 days and demonstrated normalization of serum T levels at such a short term (24). Following that, they performed a double blind placebo controlled cross over study on 17 symptomatic men with a mean age of 62 years. Treatment was given for 8 weeks and erectile function was assessed by nocturnal penile tumescence testing (NPT) and validated sexual questionnaires. Despite normalization of serum T levels, no improvements in NPT or sexual questionnaires were detected in the whole group initially. However, after adjusting for patient age in a post hoc analysis, statistically significant improvements in sexual function were demonstrated in younger men aligning with a higher likelihood of organic ED in older men that may be refractory to hormonal manipulation. In another observational study by the same group (25), 173 hypogonadal men with self-reported ED were treated with CC (50 mg) 3 times a week for 4 months. In addition to confirming the increase in LH, FSH and free testosterone levels, they reported an improvement in sexual function in 75% of studied men. Taylor and Levine compared CC efficacy to T gel replacement therapy (26), with an average post-treatment testosterone of 573 and 553 ng/dL in the CC and T gel groups, respectively. Further support of the efficacy of CC in hypogonadal symptom relief comes from a retrospective comparative study by Ramasamy et al. (27), where CC was compared to T injections and T gels. While post-treatment T levels were highest with injections (1,104 ng/dL) in comparison to CC (504 ng/dL) or T gels (412 ng/dL), no significant differences were reported on the degree of symptomatic improvement between the treatment groups. Another study verified the safety and efficacy of long-term CC use (28). Eighty-six men with hypogonadism were treated with 25–50 mg CC for a mean duration of 19 months resulting in an average testosterone level of 550 ng/dL with significant improvements in libido and energy (28).
Existing studies on the influence of CC on sperm production confirm favorable outcomes. Patankar et al. treated patients with oligospermia (including severe oligospermia) with only 3 months of CC and demonstrated a statistically significant improvement in semen parameters (29). In another clinical trial, 101 oligospermic men were randomized to either receive treatment with CC 50 mg daily (n=56) or receive no treatment (n=45). Significant improvements in sperm density and motility were observed in the treated group. Seven pregnancies were reported in the treated group, while no pregnancies were observed in the control group (30). Wang et al. randomized 46 men with idiopathic oligospermia to 6 months of treatment with a placebo, CC (25 or 50 mg/day), mesterolone (100 mg/day), or pentoxifylline (1,200 mg/day) or 4 months of treatment with testosterone enanthate (100 or 250 mg every other week). Patients treated with placebo, mesterolone, pentoxifylline, and testosterone did not develop any significant improvement in mean sperm concentration or pregnancy. Patients receiving CC at both dosages showed a significant increase in mean sperm concentration and pregnancy rates (36.4% with 25 mg and 22.2% with 50 mg) (31).
Recent interest in CC isomers has emerged. Enclomiphene citrate (EC) is a more potent, shorter acting isomer of CC. In a phase IIB study, 12 men previously treated with 1% T gel for at least 6 months, were randomized to 25 mg EC or 1% T gel and results were compared at 3 and 6 months. Before therapy, T levels were comparable between both groups and averaged 165 ng/dL. After therapy, an increase in T to >540 ng/dL was observed in both groups. Unlike T gel, EC increased sperm counts in all men at 3 and 6 months’ interval (32). Another study comparing different doses of EC to 1% T gel or placebo, confirmed a comparable efficacy between EC and 1% T gel on serum T elevation (33).
Human chorionic gonadotropin (hCG)
hCG is an LH analog purified from the urine of pregnant women or through recombinant technology (34). Discovered in 1932, hCG has been used in women to stimulate follicular maturation. In men, it is administered subcutaneously or intramuscularly 2 to 3 times per week at doses of 2,000 to 3,000 international units (IU). It stimulates Leydig cell production of endogenous T and is capable of initiating spermatogenesis in men with hypogonadotropic hypogonadism (35). This effect, however, is often not sufficient to induce spermatogenesis necessitating the concurrent use of FSH. In one case series, 13 azoospermic men with hypogonadotropic hypogonadism were initially treated with a combination of hCG and human menopausal gonadotropin (hMG) to stimulate spermatogenesis. hMG was then withdrawn and patients continued treatment with hCG alone for a period ranging from 3 to 24 months. After 12 months of treatment, sperm counts significantly decreased in all patients with one of them becoming azoospermic (36). hCG administered in lower doses also maintained a favorable testicular response. In a randomized trial by Roth et al., experimental gonadotropin deficiency was induced in 37 normal men with GnRH antagonists. Patients were then randomized to receive either several low doses of hCG or T gel daily. Testicular fluid obtained by percutaneous aspiration was assessed at baseline and after 10 days of treatment. Results indicate that intratesticular T levels increased in patients receiving far lower doses of hCG that what is therapeutically prescribed (37).
hCG has been investigated in late onset hypogonadism (LOH) despite a prevalent understanding that LOH is secondary to deterioration of the HPG axis. Liu et al. conducted a randomized double-blind placebo controlled trial on 40 healthy, ambulatory, and community-dwelling men with an average age of 67 years (38). After administration of 5,000 IU of hCG twice weekly for 3 months, serum T levels increased from a baseline of 320 to 778 ng/dL. Despite this improvement, a paradoxical reduction in testicular volume was detected among the study subjects which was unfortunately not correlated with reports of semen parameters (38). Moreover, the study’s primary endpoint was a 20% increase in muscle strength, which was not achieved after treatment. Further studies are still required in this subset of patients before considering hCG as an alternative to T therapy in men with LOH.
hCG and testosterone
The combination of lower doses of hCG with ongoing T replacement has been assessed in few studies. This regimen’s ability to maintain intratesticular T levels and spermatogenesis favors it as an alternative approach for men who desire fertility preservation while on T replacement. In 26 men receiving low doses of hCG (500 IU every other day) combined with either transdermal or intramuscular T for a mean duration of 6.2 months, no significant differences in pre-treatment semen parameters were observed even after 1 year from initiating the protocol (39). Coviello et al. assessed intratesticular T levels from 29 men who were randomized to receive 200 mg T enanthate weekly in combination with either saline placebo or 125, 250, or 500 IU hCG every other day for 3 weeks. Intratesticular T levels increased linearly with increasing hCG confirming that relatively low dose hCG maintains testicular function in healthy men with endogenous gonadotropin suppression (40).
Aromatase inhibitors (AIs) (anastrozole and letrozole)
Aromatase is an enzyme that belongs to the cytochrome P-450 family. It is present in the testis, liver, brain, and adipose tissue and is responsible for converting T to estradiol. AIs block this conversion thereby minimizing the negative effects of estradiol on gonadotropin secretion and intratesticular T production. The subsequent increase in levels of LH, FSH and T consequently improve spermatogenesis (41). Such medications are most commonly indicated in the treatment of male infertility in obese patients or when the testosterone to estradiol ratio is less than ten. AIs are classified into steroidal or non-steroidal. Anastrozole (1 mg) and letrozole (2.5 mg) are third generation AIs that are highly specific and well-tolerated.
AIs have been largely evaluated for the management of age-related hypogonadism. In a recent study by Dias et al. (42), AIs consistently increased serum T levels and, more importantly, improved patients’ bone mineral density (BMD). Fear of a presumed negative effect on BMD emerged initially. It was mainly based on the clinical features of male aromatase deficiency syndrome, a condition caused by a mutation in the CYP19 and characterized by elevated T, LH, FSH, absent estradiol levels, osteopenia, failure of epiphyseal plate closure and tall stature (43). Unlike male deficiency syndrome, serum estradiol is not totally eliminated from the body with AIs. Studies reveal up to 65% reduction in serum estradiol levels in men taking AI’s (44). This preserves the beneficial effects of estrogen on BMD, which together with the witnessed increase in serum T, maintains or improve BMD measures.
Few studies have addressed AI effects on serum T levels and on BMD among elderly men. While all studies have shown significant improvements in T levels after treatment, beneficial effects on bone health were not unanimous. Leder et al. randomized 37 elderly men (age 62–74 years) into three groups; anastrozole 1 mg once daily, anastrozole 1 mg twice weekly, and placebo (45). Treatment was given for 12 weeks and patients were followed with a serum hematocrit level, medical outcome score health survey, international index of erectile function, and American Urological Association symptom index questionnaires. They reported an increase in serum bioavailable and total testosterone levels in the treatment groups to the youthful normal range (207 and 570 ng/dL for group 1; 178 and 520 ng/dL for group 2, respectively), without a significant change on any of the follow up tools (45). Another randomized double blind placebo controlled trial investigated 69 men more than 60 years of age with borderline or low T levels and symptomatic hypogonadism. Compared with the placebo group, patients treated with anastrozole 1 mg once daily showed significant improvements in serum T level, however, a significant decrease in their spine BMD was also reported (46). Adverse effects are generally minimal; while a slight insignificant increase in PSA level has been reported in one study, no major effects on serum hematocrit, liver function or urinary symptoms were noted (45).
Conclusions
The prevalence rate of hypogonadism in men seeking fertility is increasing. Efforts to raise the public as well as physician awareness about the detrimental effects of exogenous T on HPG axis and sperm production are mandatory. Several alternative medications have been successfully utilized for hormone replacement with documented improvement in hypogonadal symptoms. Patients electing to utilize such medications should be monitored for T related consequences as well as drug specific side effects.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Petak SM, Nankin HR, Spark RF, et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients--2002 update. Endocr Pract 2002;8:440-56. [PubMed]
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762-9. [Crossref] [PubMed]
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724-31. [Crossref] [PubMed]
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737-45. [Crossref] [PubMed]
- Kovac JR, Addai J, Smith RP, et al. The effects of advanced paternal age on fertility. Asian J Androl 2013;15:723-8. [Crossref] [PubMed]
- Handelsman DJ. Testicular dysfunction in systemic disease. Endocrinol Metab Clin North Am 1994;23:839-56. [PubMed]
- Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int 2011;108:1480-4. [Crossref] [PubMed]
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. [Crossref] [PubMed]
- Gan EH, Pattman S, H S, Pearce S, et al. A UK epidemic of testosterone prescribing, 2001-2010. Clin Endocrinol (Oxf) 2013;79:564-70. [Crossref] [PubMed]
- Handelsman DJ. Pharmacoepidemiology of testosterone prescribing in Australia, 1992-2010. Med J Aust 2012;196:642-5. [Crossref] [PubMed]
- Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med Wkly 2012;142:w13539. [PubMed]
- Ko EY, Siddiqi K, Brannigan RE, et al. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol 2012;187:973-8. [Crossref] [PubMed]
- American Urological Association. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- McQuaid JW, Tanrikut C. Physiology of Testosterone Production. In: Mulhall JP, Hsiao W. editors. Men's Sexual Health and Fertility. New York: Springer, 2014.
- Pope HG Jr, Wood RI, Rogol A, et al. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev 2014;35:341-75. [Crossref] [PubMed]
- Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. J Androl 2012;33:515-28. [Crossref] [PubMed]
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc 2006;38:644-51. [Crossref] [PubMed]
- Buckley WE, Yesalis CE 3rd, Friedl KE, et al. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA 1988;260:3441-5. [Crossref] [PubMed]
- Westerman ME, Charchenko CM, Ziegelmann MJ, et al. Heavy Testosterone Use Among Bodybuilders: An Uncommon Cohort of Illicit Substance Users. Mayo Clin Proc 2016;91:175-82. [Crossref] [PubMed]
- McLachlan RI, O'Donnell L, Meachem SJ, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl 2002;23:149-62. [PubMed]
- Weinbauer GF, Nieschlag E. Gonadotrophin-releasing hormone analogue-induced manipulation of testicular function in the monkey. Hum Reprod 1993;8 Suppl 2:45-50. [Crossref] [PubMed]
- World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 1996;65:821-9. [Crossref] [PubMed]
- Gu Y, Liang X, Wu W, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009;94:1910-5. [Crossref] [PubMed]
- Guay AT, Bansal S, Hodge MB. Possible hypothalamic impotence. Male counterpart to hypothalamic amenorrhea? Urology 1991;38:317-22. [Crossref] [PubMed]
- Guay AT, Jacobson J, Perez JB, et al. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res 2003;15:156-65. [Crossref] [PubMed]
- Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med 2010;7:269-76. [Crossref] [PubMed]
- Ramasamy R, Scovell JM, Kovac JR, et al. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy. J Urol 2014;192:875-9. [Crossref] [PubMed]
- Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int 2012;110:573-8. [Crossref] [PubMed]
- Patankar SS, Kaore SB, Sawane MV, et al. Effect of clomiphene citrate on sperm density in male partners of infertile couples. Indian J Physiol Pharmacol 2007;51:195-8. [PubMed]
- Mićić S, Dotlić R. Evaluation of sperm parameters in clinical trial with clomiphene citrate of oligospermic men. J Urol 1985;133:221-2. [PubMed]
- Wang C, Chan CW, Wong KK, et al. Comparison of the effectiveness of placebo, clomiphene citrate, mesterolone, pentoxifylline, and testosterone rebound therapy for the treatment of idiopathic oligospermia. Fertil Steril 1983;40:358-65. [Crossref] [PubMed]
- Kaminetsky J, Werner M, Fontenot G, et al. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med 2013;10:1628-35. [Crossref] [PubMed]
- Wiehle R, Cunningham GR, Pitteloud N, et al. Testosterone restoration by enclomiphene citrate in men with secondary hypogonadism: pharmacodynamics and pharmacokinetics. BJU Int 2013. [Epub ahead of print]. [Crossref] [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Birmingham, Alabama. Gonadotropin preparations: past, present, and future perspectives. Fertil Steril 2008;90:S13-20. [Crossref] [PubMed]
- Turek PJ, Williams RH, Gilbaugh JH 3rd, et al. The reversibility of anabolic steroid-induced azoospermia. J Urol 1995;153:1628-30. [Crossref] [PubMed]
- Depenbusch M, von Eckardstein S, Simoni M, et al. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol 2002;147:617-24. [Crossref] [PubMed]
- Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab 2010;95:3806-13. [Crossref] [PubMed]
- Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab 2002;87:3125-35. [Crossref] [PubMed]
- Hsieh TC, Pastuszak AW, Hwang K, et al. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 2013;189:647-50. [Crossref] [PubMed]
- Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab 2005;90:2595-602. [Crossref] [PubMed]
- Raven G, de Jong FH, Kaufman JM, et al. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006;91:3324-8. [Crossref] [PubMed]
- Dias JP, Melvin D, Simonsick EM, et al. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology 2016;4:33-40. [Crossref] [PubMed]
- Morishima A, Grumbach MM, Simpson ER, et al. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80:3689-98. [PubMed]
- de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol 2011;9:93. [Crossref] [PubMed]
- Leder BZ, Rohrer JL, Rubin SD, et al. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab 2004;89:1174-80. [Crossref] [PubMed]
- Burnett-Bowie SA, McKay EA, Lee H, et al. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 2009;94:4785-92. [Crossref] [PubMed]


