
Gemcitabine as first-line therapy for high-grade non-muscle invasive bladder cancer: results from a tertiary center in the contemporary BCG-shortage era
Highlight box
Key findings
• Intravesical gemcitabine is safe and effective for high risk NMIBC.
What is known and what is new?
• BCG shortage has posed a major challenge in the treatment of NMIBC.
• Various intravesical agents have been reported. Gemcitabine appears to be an attractive option given its accessibility and prior reports in BCG resistant cases.
What is the implication, and what should change now?
• Gemcitabine can be safely applied to those who has high risk NMIBC when BCG is not available. Future randomized clinical trial is required to compare gemcitabine with other intravesical agents.
Introduction
Urothelial carcinoma (UC) of the bladder is the sixth most common cancer in the United States. In 2020, 81,400 new cases of bladder cancer were estimated with approximately 18,000 dying of this disease (1). Non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of new cases with many recurring after initial treatment. NMIBC represents a heterogeneous group with common treatment goal directed at reducing recurrence and progression of disease. Because of the propensity for tumor recurrence, patients with NMIBC require frequent, diligent follow-up including regular clinic visits with urine analyses, urine markers, and repeat cystoscopies making bladder UC one of the most expensive cancers to survey and treat in developed nations (2).
Patients with American Urological Association (AUA) intermediate or high-risk NMIBC are advised to receive intravesical therapy after transurethral resection (3). These subgroups include those with tumors classified as high-grade and/or carcinoma in situ. In addition, patients with large lesions, multifocal tumors, lamina propria invasion, or recurrence within 2 years have been shown to be at increased risk (4). For intermediate and high-risk NMIBC, intravesical immunotherapy in the form of Bacillus Calmette-Guérin (BCG) has been considered the first-line treatment for decades even though response rates can be suboptimal with up to 40% of patients failing initial treatment (5,6).
Unfortunately, the recent nationwide shortage of BCG has limited the receipt of intravesical therapy for many patients in the United States (7). This shortage has forced urologists to ration doses and prioritize BCG for select, high-risk cases (7,8). Given these limitations, alternative regimens have been recommended by the AUA and other societies. One of the more well-known treatments, gemcitabine, has been broadly studied in the BCG-resistant setting with acceptable durable responses (9-11); nonetheless, there is scarcity of data in the upfront setting. Here we present a single institution experience of intravesical gemcitabine for patients with intermediate or high-risk NMIBC in the contemporary era of limited BCG supply. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-772/rc).
Methods
Patient population
We performed a retrospective review of NMIBC patients treated at our institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of University of Arizona (Protocol# 2102500773) and informed consent was taken from all the patients. During the study period, there was a shortage of BCG supply and therefore none of the patients included in the analysis received BCG. A flow scheme is presented in Figure S1. Eligible patients were those with intermediate or high-risk non-muscle-invasive UC of the bladder and no previous BCG treatment or those with a high-grade recurrence after 12 months from last dose of BCG since these patients are generally treated in the same way as BCG-naïve patients (12). Exclusion criteria were those with atypical variant histology and those with less than 3 months follow-up or incomplete follow-up.
Treatment and follow-up
Induction consisted of gemcitabine intravesical installation once a week at a dose of 2,000 mg/50 mL for 6 consecutive weeks typically 4 to 6 weeks after transurethral resection of bladder tumor (TURBT). At each instillation, urethral catheters were unclamped after 2 hours or sooner if patients experienced significant discomfort. All instillations were performed at our Cancer Center by a dedicated team who provided follow-up visits and recorded adverse events. All patients were required to undergo quarterly surveillance cystoscopy every 3 months for 2 years and semi-annually thereafter. Patients who had no tumor after induction were recommended to receive maintenance treatments every 4 weeks for a total of 40 weeks (10 treatments) (11).
Endpoints
The primary efficacy endpoint was complete response (CR) rate, defined as the percentage of patients with CR at the 3-month visit from initial chemotherapy instillation. Response was determined based on visual assessment by cystoscopy and urine cytology. A post-induction biopsy was not routinely performed unless any lesions were detected. Any suspicious lesion was biopsied per clinician discretion. If the biopsy was negative for cancer, the case was considered CR, and if the biopsy was positive, the case was considered non-CR. If cystoscopy indicated no remaining tumors and urine cytology (bladder barbotage) was negative, the patient was considered CR. If a patient did not have a recurrence, the patient was censored at the date of the last adequate disease assessment. Adverse events were recorded and summarized descriptively by preferred term and maximal severity.
Statistical analysis
Duration of CR was estimated using the Kaplan-Meier method using SPSS® Statistics 22.0 (IBM, Armonk, NY, USA).
Results
Clinicodemographic characteristics
A total of 33 consecutive patients treated from March 2019 to October 2021 met inclusion criteria and included 29 males and 4 females. Six of the patients were lost to follow up. Approximately 85% had not received any prior BCG therapy while 15% were considered BCG-exposed (12). Median age was 75 years (range, 38–89) and median follow-up from first to last clinic visit was 21.4 months (4.1–39.4). All tumors were high-grade in histology. Tumor stages were cTa in 39.4%, cT1 in 54.5%, and cTis in 6.1% of patients. The average size of tumors in cT1 group was 2.3 cm (0.5–6.5). The majority (90.9%) were classified as high-risk per American Urological Association/Society of Urologic Oncology guidelines (Table 1) (3).
Table 1
| Patient demographics | N (%) |
|---|---|
| Age (year), median [range] | 75 [38–89] |
| Gender | |
| Male | 29 (87.9) |
| Female | 4 (12.1) |
| Previous BCG treatment | 5 (15.2) |
| Clinical tumor stage | |
| Ta | 13 (39.4) |
| T1 | 18 (54.5) |
| CIS | 2 (6.1) |
| Tumor grade (high-grade) | 33 (100.0) |
| Presence of concomitant CIS | 6 (18.2) |
| Ta + CIS | 2 (6.1) |
| T1 + CIS | 4 (12.1) |
| Presence of lymphovascular invasion (T1 + LVI) | 2 (6.1) |
| Focality | |
| Solitary | 14 (42.4) |
| Multifocal | 19 (57.6) |
| Previous upper tract UC | 3 (9.1) |
| AUA risk groups | |
| Intermediate | 3 (9.1) |
| High | 30 (90.9) |
AUA, American Urological Association; BCG, Bacillus Calmette-Guérin; CIS, carcinoma-in-situ; UC, urothelial carcinoma.
Disease outcomes
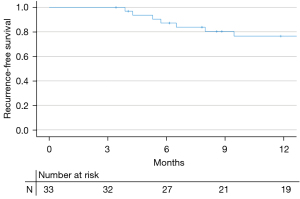
A total of 12 recurrences occurred over the study period. The 3-month CR was 84.8% (28/33). Four recurrences were high-grade NMIBC. There was one progression to invasive disease treated with cystectomy. All four patients with high-grade recurrence had HG multifocal disease at initial presentation. Three out of four (75%) had early recurrence at 3 months. Half of them had concomitant CIS. Among patients who achieved CR at 3 months, approximately 86.9% (20/23) remained disease-free at 6 months. At the time of analysis, 9 patients completed maintenance therapy while 4 patients stopped maintenance due to recurrence. Due to the rise of the COVID-19 pandemic, many patients were not able to pursue maintenance therapy. The 6-month and 12-month recurrence-free survival (RFS) were 87.2% and 76.5%, respectively. The estimated median RFS was not reached (Figure 1).
Adverse events
Approximately 78.8% of patients were able to complete all 6 cycles with 60.6% reporting acceptable tolerability without experiencing any side effects. Dysuria (18.2%), Fatigue/myalgia (15.2%), and urgency/frequency (6.1%) were the more common side-effects (Table 2).
Table 2
| Symptoms | N (%) |
|---|---|
| Dysuria | 6 (18.2) |
| Myalgia/fatigue | 5 (15.2) |
| Frequency/urgency | 2 (6.1) |
| Urinary tract infection | 1 (3.0) |
| Hematuria/pain | 1 (3.0) |
Discussion
Intravesical therapy is commonly used in NMIBC as it leads to a decline in tumor recurrence risk. BCG, a live attenuated strain of Mycobacterium bovis, is currently the only agent approved by the US Food and Drug Administration (FDA) for primary therapy of carcinoma in situ. It is also used in high-grade papillary UC and has been shown to reduce progression and delay cystectomy (13). Unfortunately, there has been a global shortage of TICE® BCG which has prompted advisories on using alternative agents, such as gemcitabine, during this extended supply shortage (14). However, the literature regarding these alternative intravesical therapies in the context of the ongoing BCG shortage is scarce. In this study, we report a contemporary experience of mostly, high-risk NMIBC patients treated with gemcitabine as adjuvant therapy after TURBT showing acceptable oncologic outcomes with good tolerability at short-term follow-up.
Several studies have investigated the optimal gemcitabine dosage for intravesical therapy. In our study we used 2,000 mg for each instillation given at a weekly basis. In a phase I clinical trial (15), Dalbagni et al. investigated 4 different dose levels in 18 BCG-refractory patients. It was concluded that gemcitabine was well tolerated, and a dose of 2,000 mg twice weekly was appropriate for phase II trial. In a small study including 9 patients with NMIBC (16), 2,000 mg gemcitabine was administered intravesically once a week in the four weeks before transurethral resection of superficial bladder cancer and in the four successive weeks with acceptable tolerability. Interestingly, this study also measured the plasma level of gemcitabine to assess systemic absorption. It was found that peak plasma concentrations of gemcitabine never exceeded 1,000 ng/mL before TUR and 350 ng/mL after TUR and rapidly declined. The 2-gram weekly dosing seemed to become a widely adopted regimen among urology societies and has been later used in multiple studies (17-19).
In our cohort, approximately 79% of patients were able to complete a full induction course. This is consistent with prior studies. When gemcitabine was compared to standard dose and reduced dose BCG (18), patients were able to hold full-dose BCG, reduced-dose BCG, and gemcitabine for the protocol-specified duration 87%, 95%, and 71% of the time (P<0.05). Morabito et al. reported that 53 patients out of 61 (86.9%) completed the weekly gemcitabine for 8 weeks (20). In another study, however, tolerability was better for gemcitabine, whereas the BCG-group experienced the need for delayed treatment or withdrawal in 12.5% of cases (21).
Several studies have assessed the efficacy of gemcitabine in the adjuvant setting with weekly or biweekly dosing. A RCT including 109 patients with recurrent NMIBC (55 treated with mitomycin C and 54 treated with gemcitabine), 39 (72%) of 54 patients remained free of recurrence in the gemcitabine arm versus 33 (60%) of 55 in the mitomycin C arm at a median follow-up of 36 months (22). This is similar to our findings as nearly 70% of patients remained disease free with a median follow up of 21.4 months. Porena et al. reported a study including 64 high-risk NMIBC patients who were randomized to gemcitabine or BCG treatment (21). At a mean follow-up of 44 months, the recurrence rate was 28.1% in BCG group and 53.1% in gemcitabine group (P=0.037). No patients developed disease progression. The authors concluded that gemcitabine may be useful for patients intolerant to or otherwise unable to receive BCG. Recently, McElree et al. reported a series of 107 patients with high risk NMIBC receiving sequential gemcitabine and docetaxel (23). The RFS was 89%, 85% and 82% at 6, 12 and 24 months, respectively. Adding docetaxel seemed to improve the RFS when compared to gemcitabine alone. Nadofaragene firadenovec (a novel agent delivering interferon alfa-2b cDNA into the bladder epithelium) has been recently approved by the FDA based on a multicenter RCT for BCG-unresponsive NMIBC (24). Complete response within 3 months of the first dose was seen in 55 (53.4%) of 103 patients with CIS (with or without a high-grade Ta or T1 tumor). More prospective studies are needed to compare different agents and regimens in high risk NMIBC.
Gemcitabine has been widely investigated in the salvage therapy setting. In our cohort, 20% (1/5) of BCG-exposed patients experienced recurrence at 3 months after gemcitabine treatment. Similarly, in a multicenter prospective randomized trial, Di Lorenzo et al. reported the recurrence rate decreased from 88% to 52% for BCG-refractory disease after gemcitabine induction (10). In a retrospective study including 69 patients with BCG-failure, 39.1% of patients achieved CR after an induction course of gemcitabine (25). The SWOG S0353 trial showed moderate efficacy at intermediate follow-up with a 21% 2-year CR rate for patients after at least 2 prior courses of BCG (11). Together these data suggest that gemcitabine is a reasonable and well-established second-line treatment option for patients who have failed BCG. It is noteworthy that patients who have adverse risk factors, including lamina propria invasion, large tumor size, concomitant CIS, presence of LVI are associated with cancer progression and worse survival (26). In the absence of adequate intravesical therapy, radical cystectomy should be offered for those willing or fit for surgery.
In the era of limited supply of BCG, there is a critical need for first-line alternatives in intermediate and high-risk NMIBC. Our results showed acceptable oncologic and safety data at short-term follow-up. We acknowledge the limitations inherent to a retrospective study including the nonrandomized design and the non-uniformity of patient and tumor characteristics. In addition, the small number of patients does not allow for any definitive conclusions. Only a large, prospective study would clarify any oncologic non-inferiority of gemcitabine to BCG. Nevertheless, our study showed gemcitabine, a well-tolerated and readily-available agent in many urology practices, reduced recurrences when used in the adjuvant setting for patients with newly diagnosed or occasionally recurrent high-grade non–muscle-invasive UC who were unable to access BCG due to ongoing supply shortages.
Conclusions
Adjuvant gemcitabine showed adequate oncologic and safety efficacy in a contemporary cohort of patients with mostly high-risk NMIBC who were unable to receive BCG therapy due to current supply shortages. Prospective studies are needed to further investigate gemcitabine for select NMIBC patients who are unable to access or tolerate BCG.
Acknowledgments
Funding: This study was supported by Department of Urology Grant from the Dr. George W Drach Endowment.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-772/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-772/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-772/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-772/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of University of Arizona (Protocol# 2102500773) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hou JC, Alhalabi F, Lemack GE, et al. Outcome of transvaginal mesh and tape removed for pain only. J Urol 2014;192:856-60. [Crossref] [PubMed]
- Leal J, Luengo-Fernandez R, Sullivan R, et al. Economic Burden of Bladder Cancer Across the European Union. Eur Urol 2016;69:438-47. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Hussain MH, Wood DP, Bajorin DF, et al. Bladder cancer: narrowing the gap between evidence and practice. J Clin Oncol 2009;27:5680-4. [Crossref] [PubMed]
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124-9. [Crossref] [PubMed]
- Packiam VT, Johnson SC, Steinberg GD. Non-muscle-invasive bladder cancer: Intravesical treatments beyond Bacille Calmette-Guérin. Cancer 2017;123:390-400. [Crossref] [PubMed]
- Cernuschi T, Malvolti S, Nickels E, et al. Bacillus Calmette-Guérin (BCG) vaccine: A global assessment of demand and supply balance. Vaccine 2018;36:498-506. [Crossref] [PubMed]
- Packiam VT, Werntz RP, Steinberg GD. Current Clinical Trials in Non-muscle-Invasive Bladder Cancer: Heightened Need in an Era of Chronic BCG Shortage. Curr Urol Rep 2019;20:84. [Crossref] [PubMed]
- Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin-refractory transitional cell carcinoma of the bladder. J Clin Oncol 2006;24:2729-34. [Crossref] [PubMed]
- Di Lorenzo G, Perdonà S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010;116:1893-900. [Crossref] [PubMed]
- Skinner EC, Goldman B, Sakr WA, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol 2013;190:1200-4. [Crossref] [PubMed]
- Roumiguié M, Kamat AM, Bivalacqua TJ, et al. International Bladder Cancer Group Consensus Statement on Clinical Trial Design for Patients with Bacillus Calmette-Guérin-exposed High-risk Non-muscle-invasive Bladder Cancer. Eur Urol 2022;82:34-46. [Crossref] [PubMed]
- Herr HW, Laudone VP, Badalament RA, et al. Bacillus Calmette-Guérin therapy alters the progression of superficial bladder cancer. J Clin Oncol 1988;6:1450-5. [Crossref] [PubMed]
- Member Communication and Recommendations [Webpage]. American Urological Association; 2020. Available online: https://www.auanet.org/about-us/bcg-shortage-info#supply.
- Dalbagni G, Russo P, Sheinfeld J, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guérin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol 2002;20:3193-8. [Crossref] [PubMed]
- Mattioli F, Curotto A, Manfredi V, et al. Intravesical gemcitabine in superficial bladder cancer: a phase II safety, efficacy and pharmacokinetic study. Anticancer Res 2005;25:2493-6. [PubMed]
- Gontero P, Casetta G, Maso G, et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC). Eur Urol 2004;46:339-43. [Crossref] [PubMed]
- Kuperus JM, Busman RD, Kuipers SK, et al. Comparison of Side Effects and Tolerability Between Intravesical Bacillus Calmette-Guerin, Reduced-Dose BCG and Gemcitabine for Non-Muscle Invasive Bladder Cancer. Urology 2021;156:191-8. [Crossref] [PubMed]
- Hurle R, Casale P, Morenghi E, et al. Intravesical gemcitabine as bladder-preserving treatment for BCG unresponsive non-muscle-invasive bladder cancer. Results from a single-arm, open-label study. BJUI Compass 2020;1:126-32. [Crossref] [PubMed]
- Morabito F, Rossi R, Graziano ME, et al. Multicenter study on the use of gemcitabine to prevent recurrence of multiple-recurring superficial bladder tumors following intravesical antiblastic agents and/or BCG: evaluation of tolerance. Arch Ital Urol Androl 2006;78:1-4. [PubMed]
- Porena M, Del Zingaro M, Lazzeri M, et al. Bacillus Calmette-Guérin versus gemcitabine for intravesical therapy in high-risk superficial bladder cancer: a randomised prospective study. Urol Int 2010;84:23-7. [Crossref] [PubMed]
- Addeo R, Caraglia M, Bellini S, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2010;28:543-8. [Crossref] [PubMed]
- McElree IM, Steinberg RL, Martin AC, et al. Sequential Intravesical Gemcitabine and Docetaxel for bacillus Calmette-Guérin-Naïve High-Risk Nonmuscle-Invasive Bladder Cancer. J Urol 2022;208:589-99. [Crossref] [PubMed]
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol 2021;22:107-17. [Crossref] [PubMed]
- Sternberg IA, Dalbagni G, Chen LY, et al. Intravesical gemcitabine for high risk, nonmuscle invasive bladder cancer after bacillus Calmette-Guérin treatment failure. J Urol 2013;190:1686-91. [Crossref] [PubMed]
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 2015;33:643-50. [Crossref] [PubMed]


