
Complex ureteral stricture from chronic schistosomiasis requiring ileal ureter interposition: a case report
Highlight box
Key findings
• Schistosoma infection is an uncommon but likely underrecognized cause of ureteral stricture disease- especially amongst immigrants from endemic areas.
What is known and what is new?
• Ureteral schistosomiasis has been implicated in urologic disease, most notably a risk factor for squamous cell bladder cancer.
• This manuscript highlights upper tract manifestation of this disease which is less widely recognized.
What is the implication, and what should change now?
• Providers should have a heightened awareness of this condition, especially amongst patients from areas where schistosomal infection is common, to help guide treatment.
Introduction
Schistosomiasis, caused by the parasitic helminth Schistosoma hematobium, has a variety of clinical manifestations. From a urologic perspective, the most notorious remains squamous cell carcinoma of the bladder. In addition to malignancy, S. hematobium can also result in calcifications and granulomatas of the urinary tract (1). This parasite causes significant morbidity in endemic areas, such as sub-Saharan Africa, but is uncommonly encountered in the United States (US).
Herein, we present a case of a 40-year-old Kenyan immigrant found to have ureteral calcifications, mimicking nephrolithiasis. At the time of attempted ureteroscopic stone treatment, multiple ureteral strictures were encountered, and the patient ultimately underwent ureterectomy and ileal interposition with final pathology revealing schistosomiasis. We present this case in accordance with the CARE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-792/rc).
Case presentation
A 40-year-old male with a history of type 2 diabetes and no urologic history initially presented to the emergency department with left-sided flank pain. The patient had immigrated to the US from Africa 20 years prior and has not returned since. He grew up in Somalia and Kenya and had no significant medical history from his childhood. Laboratory results were notable for a normal white blood cell count and no eosinophilia. Computed tomography imaging was suspicious for three mid-ureteral calcifications measuring up to 8 mm (160 HU), moderate hydronephrosis, and mild urinary bladder wall thickening. Medical expulsive therapy was initiated, and he was referred to urology.
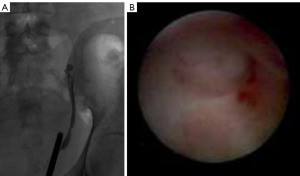
After one month, the patient’s symptoms persisted and he was taken to the operating room for ureteroscopy. A wire was unable to be advanced into the renal pelvis and retrograde pyelography revealed no passage of contrast proximal to the iliac vessels (Figure 1). Ureteroscopy confirmed a blind-ending ureter at this location with normal appearing ureteral mucosa distal to the obstruction (Figure 1).
The patient then underwent percutaneous nephrostomy tube placement. Urine samples were taken from the renal pelvis and sent for culture, including acid-fast bacilli and mycobacterium tuberculosis, which were ultimately negative. Antegrade pyelogram and ureteroscopy revealed an additional more proximal stricture with a pinpoint opening (Figure 2). The stricture was approximately 3 cm in length, and contrast ultimately terminated at the level of the iliac vessels at the previously seen blind end. Intraoperative measurements of stricture length were approximated based on calibration of radiopaque forceps with known measurements.
Given there were two separate strictures at different locations within the ureter of unclear etiology, we opted to perform ureterectomy with ileal interposition. The total length of the diseased segment was >3 cm, located proximal to the iliac vessels in the mid-ureter, which we deemed too proximal for reimplantation with psoas hitch or Boari flap. Augmentation techniques using buccal mucosal grafts were not an option as the distal stricture did not contain an identifiable lumen to augment. Ureteroureterostomy was considered, but given two sites of stricture, we were concerned about the ability to obtain a tension-free anastomosis. Additionally, given the unclear etiology of his stricture without prior history of stone disease, injury, or instrumentation—we had a moderately high suspicion of a progressive pathology which could potentially recur at another site within the ureter. All of these factors influenced our decision to proceed with ileal ureter interposition. The ureter was identified and resected just distal to the ureteropelvic junction. The specimen was grossly notable for two palpable, firm nodules consistent with the areas of stricture. The resected ureter was sent for pathologic analysis, which demonstrated schistosomiasis with calcification and giant cell reaction.
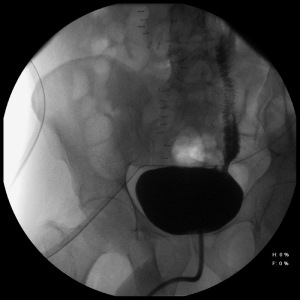
The patient’s hospital course was unremarkable. Infectious disease recommended 3,000 mg of praziquantel with a second dose two weeks later. Liver ultrasound was performed to evaluate for systemic schistosomiasis and was free of any abnormalities. Cystogram once week postoperatively demonstrated a patent left ileal ureter without leak (Figure 3). His stent was removed five weeks postoperatively and the patient is doing well.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Schistosoma is a genus of five species of parasitic trematodes, and can cause disease in humans, most commonly in the liver and genitourinary system. Schistosoma infection is rarely found in the US, but disease burden in Africa, the Middle East, and South America is considerable, with about 70% of cases in sub-Saharan Africa (2). Infection is transmitted through direct invasion of worms present in infected water or ingestion of larva found in infected raw foods. Freshwater snails serve as the intermediary host, and adult worms can live in humans for up to 30 years (2). Genitourinary schistosomiasis is caused by S. hematobium. Chronic infection results from pairs of the flatworms living in the venous system of the lower urinary tract, depositing eggs in surrounding structures. Approximately 10% of people infected with schistosomiasis will develop a urologic complication (1).
Squamous cell carcinoma of the bladder is perhaps the most well-known urologic complication of schistosomiasis. Bladder squamous cell carcinoma is often aggressive, with mortality higher than urothelial cell carcinoma (3). Chemotherapy is generally less effective than for urothelial carcinoma so treatment involves upfront radical cystectomy, with significantly decreased mortality with early stage radical cystectomy (4). Despite aggressive treatment, 5-year disease-free survival after diagnosis of schistosomiasis-related squamous cell carcinoma treated with radical cystectomy is approximately 50% (1). Although early intervention is beneficial, there are no clear guidelines for bladder cancer surveillance in cases of known genitourinary schistosomiasis. After discussion with the patient and evaluation of these risks and benefits, the patient presented here will be surveilled with annual cystoscopy.
Ureteral schistosomiasis is extraordinarily rare and has been reported in only a handful of case reports. In one case, a recent immigrant was found to have linear calcifications along the bladder and ureter after presenting with abdominal pain, and diagnosis was confirmed with positive serology (3). In another described case, a Somalian immigrant presented with dysuria and abdominal pain and was found to have distal ureteral narrowing with no calcifications and hydronephrosis. The patient was treated with praziquantel, and after an episode of recurrence of symptoms one month after treatment began, a six-month pyelogram demonstrated absence of ureteral stenosis (5). An additional case of a Zimbabwean immigrant who presented with hydronephrosis and a distal ureteral stricture was diagnosed with schistosomiasis through biopsy of the stricture. The patient failed conservative management with multiple laser treatments and stents, and ultimately required a Boari flap ureteral reimplant (6).
The strictures identified here were initially presumed to be routine nephrolithiasis, owing to their heavy calcifications and round appearance. The case presented here is remarkable for the length, complete obliteration and multifocality of the ureteral structure. Given the unclear diagnosis, we opted for ureterectomy and ileal interposition to definitively treat the stricture in case of a progressive or malignant condition. Most likely, the patient acquired schistosomiasis during his time living in Somalia or Kenya, so this case would represent sequelae of a long-term, chronic infection.
The sub-Saharan immigrant population in the US has increased over 50% from 2010–2018, and awareness of the possible chronic urologic sequelae these patients may face is imperative for optimal care (7). Returning travelers and veterans returning from foreign areas are another population at risk of schistosoma infection. In a non-endemic setting such as the US, earlier detection in suspected cases through serologic testing would be optimal (8). Microscopic analysis of stool and urine samples may also be useful to quantify parasite burden but are poorly sensitive (5). Detection in suspected cases may direct treatment to include schistosomal-eradication efforts in conjunction with standard urologic care to improve outcomes and prevent further complications.
Conclusions
In conclusion, providers who work with populations at risk of schistosomal infection should be aware of possible urologic sequalae and consider this diagnosis for ureteral calcifications that do not respond to medical expulsive therapy or ureteral strictures of unexplained etiology. Once a diagnosis of schistosomiasis has been confirmed patients infected will require systemic treatment to eradicate the helminths to prevent additional urologic pathology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-792/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-792/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-792/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khalaf I, Shokeir A, Shalaby M. Urologic complications of genitourinary schistosomiasis. World J Urol 2012;30:31-8. [Crossref] [PubMed]
- Vennervard B. Schistosomiasis and other trematode infections In: Jameson J, Fauci AS, Kasper DL, et al. Harrison's Principles of Internal Medicine. 20 edition. McGraw Hill; 2018. Accessed April 02, 2022.
- Lee HJ, Sung WS. Calcification of the urinary bladder and ureter in schistosomiasis. Kidney Res Clin Pract 2018;37:304-5. [Crossref] [PubMed]
- Scosyrev E, Yao J, Messing E. Urothelial carcinoma versus squamous cell carcinoma of bladder: is survival different with stage adjustment? Urology 2009;73:822-7. [Crossref] [PubMed]
- Neal PM. Schistosomiasis--an unusual cause of ureteral obstruction: a case history and perspective. Clin Med Res 2004;2:216-27. [Crossref] [PubMed]
- Pal PO, Smith RD, Allen S, et al. Schistosomiasis-A Disobedient Ureter, a Disobedient Diagnosis. J Endourol Case Rep 2017;3:114-8. [Crossref] [PubMed]
- Echeverria-Estrada C, Batalova J. Sub-Saharan Africal immigrants in the United States. US Census Data. Available online: https://www.migrationpolicy.org/article/sub-saharan-african-immigrants-united-states-2018. Accessed May 14, 2022.
- Kehinde EO, Anim JT, Hira PR. Parasites of urological importance. Urol Int 2008;81:1-13. [Crossref] [PubMed]



