
Immediate gemcitabine bladder instillation following bladder closure during robotic-assisted radical nephroureterectomy: a multi-institutional report of feasibility and initial outcomes
Highlight box
Key findings
• Of patients undergoing radical nephroureterectomy (RNU) who received 2 g intravesical gemcitabine immediately following bladder cuff closure or non-gemcitabine intravesical chemotherapies at the beginning of the procedure, there was no difference in bladder recurrence-free survival (bRFS).
• No adverse events attributable to the use of gemcitabine occured within 30 days postoperatively.
What is known and what is new?
• Use of intravesical MMC reduces bladder recurrence after RNU. Intravesical gemcitabine reduces recurrence following transurethral resection of bladder tumors, but its efficacy in reducing bladder recurrence after RNU is unknown.
• This study shows that gemcitabine instilled immediately following bladder cuff during RNU closure is safe and has comparable bRFS compared to established chemotherapy agents.
What is the implication, and what should change now?
• Intravesical gemcitabine immediately after bladder neck closure is safe and effective.
• This lower-cost streamlined approach to perioperative intravesical chemotherapy could increase adherence among urologists performing RNU.
Introduction
Radical nephroureterectomy (RNU) is the standard treatment for high-risk upper tract urothelial carcinoma (UTUC) (1,2). Recurrence within the bladder after RNU is common, with rates ranging from 22% to 47% (3-5). While the mechanism of bladder recurrence following RNU is likely multifactorial, genomic characterization of UTUC and subsequent bladder recurrence suggests a common clonal origin, lending credence to the theory of downstream seeding (6).
Multiple strategies exist to reduce bladder recurrence after RNU, including avoiding entry into the urinary tract, removal of the kidney and ureter en bloc with a bladder cuff, and early clipping of the ureter to avoid tumor seeding into the bladder (7,8). Perioperative instillation of intravesical chemotherapy has proven to be an effective strategy to reduce bladder recurrence. Based on two prospective trials demonstrating a reduction in bladder recurrence, the National Comprehensive Cancer Network and European Association of Urology UTUC guidelines recommend the use of a single dose of intravesical chemotherapy after RNU to reduce bladder recurrence, with the favored regimens being Mitomycin C (MMC) or pirarubicin at the time of catheter removal (1,2).
Despite the proven efficacy of MMC and other chemotherapeutic agents in reducing bladder recurrence following RNU, utilization is only 51% (9). This may be partly due cost and limited availability of MMC, but primarily due to concerns about the irritative effects of MMC instilled following a recent cystorrhaphy. In 2018, a large randomized controlled trial demonstrated intravesical gemcitabine to reduce bladder recurrence in patients with non-muscle-invasive bladder cancer (NMIBC) following transurethral resection of bladder tumors (TURBT) (10). Given these findings, we evaluated the use of single intraoperative gemcitabine instillation immediately following bladder cuff closure during RNU. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-112/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). And Individual consent for this retrospective analysis was waived. After approval from the institutional review boards of University of Iowa Hospitals & Clinics and Hackensack University Medical Center, we retrospectively reviewed the records of patients at two tertiary referral centers (University of Iowa Hospitals & Clinics and Hackensack University Medical Center) from 2016 to 2020 who underwent robotic-assisted RNU and received perioperative intravesical gemcitabine (2 g immediately after bladder cuff closure for one hour) or non-gemcitabine chemotherapies [40 mg mitomycin C (MMC) or 50 mg doxorubicin for one hour following foley catheter placement at the beginning of the procedure and drained before bladder opening]. Informed consent was obtained on all patients prior to surgery and included consent for intravesical chemotherapy. Prior to RNU, 92% (22/24) of patients in the gemcitabine and 84% (26/31) of patients in the non-gemcitabine cohorts underwent diagnostic ureteroscopy and biopsy of suspicious lesions. Ureteroscopy and/or biopsies were omitted if preoperative imaging or retrograde pyelograms were concerning for UTUC, with either a positive cytology or poorly to non-functional kidney. No patients underwent percutaneous biopsy, and all patients underwent cystoscopy prior to RNU and were confirmed to be free of any bladder tumors. Only patients with pure UC were included. Patients who did not receive intravesical chemotherapy or had no surveillance cystoscopy following RNU were excluded. Routine follow-up after RNU included physical examination, routine labs, selective use of urine cytology, cystoscopy, and imaging studies. Patients were typically evaluated at 3, 6, and 12 months following RNU, and every 6–12 months. Complications were graded according to the Clavien-Dindo classification.
All patients underwent transperitoneal RNU as previously described with the use of the da Vinci Xi platform (Intuitive Surgical Inc., Sunnyvale, CA) (11). RNU in the gemcitabine and non-gemcitabine cohorts were performed by one of two surgeons or one of three surgeons, respectively. During renal mobilization, the ureter was ligated immediately with a Hem-o-lok clip (Teleflex, Morrisville, NC) to reduce tumor seeding. Additional clips if there was known tumor distal to the initial clip. The ureterectomy was performed with an extravesical bladder cuff excision, noting to remove the entire intramural ureter and ureteral orifice. The cystotomy was closed in two layers with absorbable barbed suture in a running fashion. A leak test was performed with a minimum of 180 mL of saline to ensure a watertight cystorrhaphy. All patients were deemed to have a watertight closure and received gemcitabine. Using standard precautions and sterile technique, 2 g of gemcitabine in 100 cc normal saline was instilled via sterile IV extension tubing connected to the foley catheter. The catheter was then clamped for one hour. In the non-gemcitabine group, either MMC (40 mg in 40 mL sterile water) or doxorubicin (50 mg in 50 mL saline) was instilled for one hour at the beginning of the case and drained before bladder opening. This method has been previously reported (12). Outpatient cystogram was not routinely performed prior to catheter removal.
Statistical analysis
Chi-squared or Fisher’s exact tests were used to compare categorical variables, whereas t-tests were used to compare continuous variables between gemcitabine and non-gemcitabine cohorts. The primary end point was bladder recurrence-free survival (bRFS) and was estimated using the Kaplan-Meier method. A log-rank test was used to evaluate differences in survival between cohorts. All tests were two-sided and assessed for significance at the 5% level using R v4.0.2 (http://www.r-project.org).
Results
We identified 55 patients with UTUC who received intraoperative intravesical chemotherapy while undergoing RNU, of whom 24 received gemcitabine and 31 received non-gemcitabine chemotherapies. Demographics, clinical information, and tumor characteristics are presented in Table 1. Median age at time of RNU was 74.0 (IQR 68–80.3) years old. Forty percent (22/55) of patients had prior or concurrent bladder cancer. Of patients receiving non-gemcitabine chemotherapy, 71% (22/31) received MMC and 29% (9/31) received doxorubicin. A similar proportion of patients in each cohort had high grade disease on final pathology, 76% versus 74% (P=1.0). Overall, 36% (20/55) of patients were found to have pathologic ≥ pT2 disease. Among the entire cohort, rates of ≥ pT2 disease after RNU were not statistically different between patients who received neoadjuvant chemotherapy (47%) versus those who did not (34%, P=0.34). While more patients (42%) in the non-gemcitabine cohort were ≥ pT2 compared the gemcitabine cohort (29%), this was not statistically different. Concomitant carcinoma in situ was not routinely reported by pathology and therefore was unable to be measured.
Table 1
| Covariate | Gemcitabine (N=24) | Non-gemcitabine (N=31) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 75 [70–80] | 74 [68–80] | 0.89 |
| Gender | |||
| Female | 6 (25%) | 13 (42%) | 0.26 |
| Male | 18 (75%) | 18 (58%) | |
| Race | |||
| Black | 1 (4.2%) | 0 (0%) | 0.72 |
| Asian | 0 (0%) | 1 (3.2%) | |
| Hispanic | 1 (4.2%) | 0 (0%) | |
| White | 22 (92%) | 30 (97%) | |
| Type 2 diabetes | |||
| No | 17 (71%) | 25 (81%) | 0.53 |
| Yes | 7 (29%) | 6 (19%) | |
| Smoking history | |||
| Current | 1 (4.2%) | 6 (21%) | 0.2 |
| Former | 14 (58%) | 16 (55%) | |
| Never | 9 (38%) | 7 (24%) | |
| Missing | 0 | 2 | |
| History of bladder cancer | |||
| No | 14 (58%) | 19 (61%) | 1.00 |
| Yes | 10 (42%) | 12 (39%) | |
| Neoadjuvant chemotherapy | |||
| No | 16 (67%) | 24 (77%) | 0.54 |
| Yes | 8 (33%) | 7 (23%) | |
| Adjuvant chemotherapy | |||
| No | 23 (96%) | 23 (74%) | 0.02* |
| Yes | 1 (4%) | 8 (26%) | |
| Side | |||
| R | 11 (46%) | 12 (39%) | 0.77 |
| L | 13 (54%) | 19 (61%) | |
| Preoperative grade | |||
| HG | 18 (82%) | 15 (58%) | 0.12 |
| LG | 4 (18%) | 11 (42%) | |
| No biopsy | 2 | 5 | |
| Hydronephrosis | |||
| Non-mild | 17 (71%) | 28 (90%) | 0.08 |
| Mod-severe | 7 (29%) | 3 (10%) | |
| Location | |||
| Renal | 12 (57%) | 16 (52%) | 0.79 |
| Ureter/both | 9 (43%) | 15 (48%) | |
| Not reported | 3 | 0 | |
| Focality | |||
| Unifocal | 14 (67%) | 22 (71%) | 0.76 |
| Multifocal | 7 (33%) | 9 (29%) | |
| Not reported | 3 | 0 | |
| Pathologic T stage | |||
| <T2 | 17 (71%) | 18 (58%) | 0.42 |
| ≥T2 | 7 (29%) | 13 (42%) | |
| Pathologic grade | |||
| HG | 16 (76%) | 23 (74%) | 1.00 |
| LG | 5 (24%) | 8 (26%) | |
| Not reported | 3 | 0 | |
| Nodal status | |||
| N0 | 12 (50%) | 15 (48%) | 0.46 |
| N1 | 2 (8.3%) | 0 (0%) | |
| N2 | 1 (4.2%) | 2 (6.5%) | |
| Nx | 9 (38%) | 14 (45%) | |
| BMI (kg/m2), median [IQR] | 27.7 [26.2–31.4] | 29.2 [24.4–34.8] | 0.92 |
| Tumor size (cm), median [IQR] | 2.6 [1.6–4.3] | 3.0 [1.9–5.3] | 0.17 |
| Follow-up (months), median [IQR] | 12 [6.4–20.0] | 20 [12.2–30.5] | # |
*, values are statistically significant (P<0.05). #, P values are not provided for length of follow up. The significance of the difference in survival between cohorts is performed in the Figures 1-3. These numbers are for descriptive purposes. HG, high grade; LG, low grade; BMI, body mass index; IQR, interquartile range.
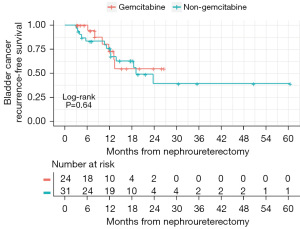
Median follow-up in the gemcitabine and non-gemcitabine cohorts was 11.9 (IQR 6.4–20.0) and 19.6 (IQR 12.2–30.5) months, respectively. Thirty-five percent (19/55) of patients experienced a bladder cancer recurrence which was diagnosed by surveillance cystoscopy and subsequent TURBT (Table S1). There was no difference in bRFS between the two cohorts (P=0.64) (Figure 1). By 12 months post-surgery, 25% of patients had experienced bladder recurrence. The 12-month bRFS survival was 73% for gemcitabine, 76% for non-gemcitabine, and 76% overall. Overall survival also did not differ between patient cohorts (P=0.71) (Figure 2). By 12 months after surgery, 9% of patients had died. Median survival time was 35.3 months overall, 35.3 months for the non-gemcitabine group, and 32.9 for the gemcitabine group. The estimated 12-month overall survival rate was 93% for gemcitabine, 90% for non-gemcitabine, and 91% overall. Cancer-specific survival did not differ between gemcitabine patient cohorts (P=0.21) (Figure 3). By 12 months after surgery, 4% of patients had died due to cancer. Median cancer-specific survival time was 35.3 months for the non-gemcitabine group but was inestimable for the overall and gemcitabine curves. The estimated 12-month cancer-specific survival rate was 100% for gemcitabine, 93% for non-gemcitabine, and 96% overall.


The median length of stay was 2 days (IQR 2–4) and no patients were readmitted within 30 days of surgery in the gemcitabine cohort. The median length of stay was 3 days (IQR 2–4) and three patients (8%) were readmitted within 30 days of surgery in the non-gemcitabine cohort. Median foley catheter duration was 10 days (IQR 10–35) in the gemcitabine group with one patient requiring prolonged catheterization (35 days) due to a history of urinary retention and 12 days (IQR 10–40) in the non-gemcitabine group, with one prolonged catheterization (40 days) due to a bladder leak noted on cystogram. Complications are noted in Table 2. The gemcitabine group had an overall complication rate of 4/24 (17%) compared to 4/31 (13%) in the non-gemcitabine group. Half (2 of 4) of the complications in each group were high grade. No adverse events specifically attributable to gemcitabine chemotherapy were noted.
Table 2
| Patient | Chemotherapy | Complication | Clavien-Dindo grade | LOS (days) |
|---|---|---|---|---|
| 1 | Gemcitabine | Delirium | 1 | 4 |
| 2 | Gemcitabine | UTI | 1 | 2 |
| 3 | Gemcitabine | PE | 4 | 10 |
| 4 | Gemcitabine | Chyle leak | 3 | 8 |
| 5 | Mitomycin C | SBO | 3 | 1 |
| 6 | Doxorubicin | Chyle leak | 3 | 2 |
| 7 | Mitomycin C | Bladder leak | 1 | 1 |
| 8 | Mitomycin C | Ileus | 1 | 4 |
LOS, length of stay; UTI, urinary tract infection; PE, pulmonary embolism; SBO, small bowel obstruction.
Discussion
Guidelines recommend a single dose of intravesical chemotherapy after RNU for UTUC to reduce the risk of bladder recurrence (1,2). The ODMIT-C trial utilized a single dose of MMC at the time of catheter removal and found an 11% absolute risk reduction and relative reduction of 40% for bladder recurrence, with a number needed to treat of nine in order to prevent one bladder tumor (13). The THP Monotherapy study group trial used pirarubicin instilled 48 hours postoperatively and found a reduction of 25% (14). A subsequent meta-analysis also demonstrated a benefit of postoperative intravesical chemotherapy, with an HR of 0.51 for bladder recurrence (95% CI: 0.32–0.82). After 12 months follow-up, this would result in 127 fewer bladder cancer recurrences (95% CI: 44–182) per 1,000 participants (15). Despite the efficacy of perioperative instillation of intravesical chemotherapy based on level one evidence, routine utilization of these agents has not been embraced by the majority of urologists (9). Common concerns include uncertainty regarding the risk of adverse events of these intravesical agents, high cost, and office infrastructure required during instillation at the time of catheter removal (9,15). Consequently, only 51% of urological oncologists in the United States reported administering peri-operative intravesical chemotherapy (9). We assessed the safety and efficacy of immediate intraoperative intravesical gemcitabine instillation after cystotomy closure, as this approach may mitigate typical concerns associated with perioperative intravesical chemotherapy following RNU.
Messing et al. reported the use of intravesical gemcitabine for reduction of bladder recurrence following TURBT in the setting of NMIBC (10). Participants were randomized to receive intravesical instillation of gemcitabine or saline for 1 hour immediately following TURBT. Gemcitabine demonstrated decreased 4-year estimated disease recurrence rate compared to placebo (35% versus 47%, P<0.001). While hypotonic water or saline irrigation may flush out circulating tumor cells and cause cell osmolysis, it is likely that the direct cytotoxic effect of intravesical chemotherapy is more efficacious. Freifeld et al. (16) reported on the intraoperative use of gemcitabine in a retrospective study analyzing the impact of installation timing on bladder recurrence following RNU. In this study, patients received either intraoperative intravesical MMC or gemcitabine which was either drained before managing the bladder cuff, or postoperative MMC. Of the patients who received intraoperative intravesical chemotherapy, 68% received MMC and 32% received gemcitabine. There was no difference in 12-month bRFS between the those who received intraoperative chemotherapy 82% and postoperative chemotherapy (72%). Though trending toward a benefit of utilizing MMC over gemcitabine, there was no statistically significant difference in bRFS between gemcitabine and MMC at 12 months, with a HR of 2.67 (95% CI: 0.853–8.357; P=0.092) (16). Although the timing of bladder instillation was not immediately after closure, this study supports our finding of similar efficacy between gemcitabine and non-gemcitabine intravesical chemotherapy. In our cohort, estimated 12 months bRFS rates was 73% for gemcitabine and 76% for the non-gemcitabine group (P=0.64). This demonstrated that gemcitabine efficacy was similar to established chemotherapy mitomycin C (MMC) and doxorubicin with short-term (12 months) follow-up. Tumor-specific factors which increase the risk for bladder recurrence after nephroureterectomy include multifocality, ureteral tumor location, and ≥ pT2 disease (4). While there was no significant difference in pathologic stage between cohorts in this study, more patients were pT2 in the non-gemcitabine group, and this could possibly impact results in a larger cohort. We were unable to compare outcomes directly to patients who underwent RNU without any intravesical chemotherapy during this timeframe as the use of intravesical therapy is our standard practice, however the rates of bladder recurrence in the first year after RNU in patients who didn’t receive intravesical therapy were 27% and 32% in the ODMIT-C and THP trials, respectively (13,14). There are currently two Phase II trials accruing patients to assess intravesical gemcitabine versus placebo for UTUC bladder recurrence NCT03062059 and GEMINI (NCT04398368).
Similar to intravesical chemotherapy use following TURBT for NMIBC, MMC is the primary perioperative intravesical chemotherapy utilized in the United States following RNU (9,17). Reported toxicities of MMC range from low grade (dysuria, transient hematuria) to high grade (prolonged chemical cystitis, dystrophic bladder calcifications, perivesical inflammation and fibrosis) (18,19). Major complications have been reported to occur in 5.2% of patients after TURBT with MMC instillation compared to 0.9% after TURBT alone (18). The safety profile of intravesical gemcitabine has been demonstrated in several trials in the setting of TURBT. In the Messing trial, there was no significant difference in Clavien-Dindo 1–3 complications between Gemcitabine and saline groups and no Clavien-Dindo 4–5 events were demonstrated during the study (10). Similarly, in a prospective trial of 120 patients with BCG unresponsive NMIBC randomized to salvage intravesical regimens of monthly maintenance treatments of gemcitabine or MMC, gemcitabine demonstrated better tolerability than MMC with regards to chemical cystitis (21.1% in MMC and 5.5% in gemcitabine arm, P=0.013) and dysuria/frequency (20.0% in MMC and 9.2% in gemcitabine arm, P=0.023) (20). This improved safety profile was demonstrated in our gemcitabine cohort, reflected by low median length of stay of 2 days, no readmissions within 30 days, and overall complication rate of 17%, none of which were related to intravesical gemcitabine.
There is currently no consensus regarding the optimal timing of instillation of postoperative chemotherapy. The ODMIT-C trial required instillation of MMC at time of foley catheter removal, at least one week following RNU (13). In the pirarubicin trial instillation was performed within 48 hours of RNU (14). Based on the tumor seeding theory and TURBT studies, the earliest possible instillation of intravesical chemotherapy could result in the greatest reduction of recurrences since tumor cells implantation may occur within 24 hours (21). Immediate post-TURBT intravesical chemotherapy prevents tumor cell implantation secondary to the traumatic agitation of the TURBT (22). Noennig et al. compared MMC instilled intraoperatively to postoperatively on post-operative day 1 or later and found that patients who received intraoperative MMC had a lower 1-year rate of bladder recurrence (HR =0.113, 95% CI: 0.28–0.63, P=0.01), supporting the practice of intraoperative instillation to avoid tumor seeding (23). Freifeld et al. found no difference in recurrence rates between intraoperative and postoperative intravesical chemotherapy groups [12-month bRFS rates were 82% and 72% respectively (log rank P=0.365)]; however, the intraoperative group in their study had higher rates of high-grade and muscle invasive disease (16).
The lower cost of gemcitabine compared to MMC and the simplified logistics of intraoperative instillation of gemcitabine compared to outpatient intravesical chemotherapy administration at the time of catheter removal may also increase perioperative intravesical chemotherapy utilization. Compared with MMC, gemcitabine is considerably less expensive (average sales price for 2 g of gemcitabine is $55.70 and for 40 mg of MMC is $1,062.72) (10). As up to 10% of primary urothelial cell cancers are considered UTUC, 75% of which are considered high risk, the preferable use of gemcitabine over MMC during RNU could result in considerable cost savings. There have also been recent concerns of MMC shortage stemming from production hurdles. The additional obstacles of dedicating time during a post-operative visit, need for specialized nursing or healthcare provider for administration, varying discomfort experienced by a conscious patient during intravesical chemotherapy administration, and coordination with pharmacy can be overcome with intraoperative administration of gemcitabine immediately after bladder cuff closure.
Our study has several notable limitations. The retrospective nature of the study may have introduced unmeasured bias into patient selection and follow-up. Additionally, due to the retrospective study, heterogenous patient population, and low recurrence rates, the study may have been underpowered to detect a difference in bladder recurrence rates. Patients were not prospectively assigned to high or low risk categories per EAU guidelines. This was addressed to a degree, however, by comparing the proportion of patients with high grade UTUC on biopsy or cytology and preoperative hydronephrosis. There was heterogeneity in the non-gemcitabine group. Two different intravesical chemotherapy were used, as doxorubicin was utilized when MMC was in national short supply. However, use of doxorubicin, an analogue of the anthracycline pirarubicin, for the prevention of bladder recurrence has previously been utilized (12). Additionally, non-gemcitabine chemotherapy agents were instilled at the beginning of the case whereas gemcitabine was instilled after bladder cuff closure, and such differences could confound the results. Five different surgeons were included at two different centers, and while the nephroureterectomy technique described did not differ significantly between groups, un-captured differences could have confounded the recurrence risk. Post-operative complication data may not have been completely captured if patients presented to an outside facility, and retrospective assessment of complications could have resulted in measurement bias with systematic underreporting of low-grade complications. Lastly, the data is limited by the small sample size with only 19 recurrences between groups, thus compromising an assessment of comparative effectiveness. Nevertheless, our aim was to report the safety and feasibility of a more affordable chemotherapy option, with a more streamlined workflow, in an effort to improve surgeon adherence to instillation of intravesical chemotherapy during RNU.
Conclusions
In patients undergoing RNU, intravesical gemcitabine instilled immediately following bladder cuff closure has similar rates of bRFS compared to non-gemcitabine chemotherapy. This lower-cost, more streamlined approach to perioperative intravesical chemotherapy could increase adherence among urologists performing RNU for UTUC.
Acknowledgments
The abstract of this article has been published on the Scientific Program of 38th World Congress of Endourology Program Book.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-112/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-112/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-112/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-112/coif). Michael Stifelman is on the surgical advisory board for Intuitive Surgery and receives educational grants from Ethicon. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review boards of University of Iowa Hospitals & Clinics and Hackensack University Medical Center and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:329-54. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol 2021;79:62-79. [Crossref] [PubMed]
- Cho YH, Seo YH, Chung SJ, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J Urol 2014;55:453-9. [Crossref] [PubMed]
- Seisen T, Granger B, Colin P, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol 2015;67:1122-33. [Crossref] [PubMed]
- Lee CH, Ku JY, Jeong CW, et al. Predictors for Intravesical Recurrence Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A National Multicenter Analysis. Clin Genitourin Cancer 2017;15:e1055-61. [Crossref] [PubMed]
- Audenet F, Isharwal S, Cha EK, et al. Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma. Clin Cancer Res 2019;25:967-76. [Crossref] [PubMed]
- Yamashita S, Ito A, Mitsuzuka K, et al. Efficacy of early ureteral ligation on prevention of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: a prospective single-arm multicenter clinical trial. Jpn J Clin Oncol 2017;47:870-5. [Crossref] [PubMed]
- Braun AE, Srivastava A, Maffucci F, et al. Controversies in management of the bladder cuff at nephroureterectomy. Transl Androl Urol 2020;9:1868-80. [Crossref] [PubMed]
- Lu DD, Boorjian SA, Raman JD. Intravesical chemotherapy use after radical nephroureterectomy: A national survey of urologic oncologists. Urol Oncol 2017;35:113.e1-7. [Crossref] [PubMed]
- Messing EM, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA 2018;319:1880-8. [Crossref] [PubMed]
- Argun OB, Mourmouris P, Tufek I, et al. Radical Nephroureterectomy Without Patient or Port Repositioning Using the Da Vinci Xi Robotic System: Initial Experience. Urology 2016;92:136-9. [Crossref] [PubMed]
- Moriarty MA, Uhlman MA, Bing MT, et al. Evaluating the safety of intraoperative instillation of intravesical chemotherapy at the time of nephroureterectomy. BMC Urol 2015;15:45. [Crossref] [PubMed]
- O'Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 2011;60:703-10. [Crossref] [PubMed]
- Ito A, Shintaku I, Satoh M, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol 2013;31:1422-7. [Crossref] [PubMed]
- Hwang EC, Sathianathen NJ, Jung JH, et al. Single-dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database Syst Rev 2019;5:CD013160. [Crossref] [PubMed]
- Freifeld Y, Ghandour R, Singla N, et al. Intraoperative prophylactic intravesical chemotherapy to reduce bladder recurrence following radical nephroureterectomy. Urol Oncol 2020;38:737.e11-6. [Crossref] [PubMed]
- Dobé TR, Califano G, von Rundstedt FC, et al. Postoperative Chemotherapy Bladder Instillation After Radical Nephroureterectomy: Results of a European Survey from the Young Academic Urologist Urothelial Cancer Group. Eur Urol Open Sci 2020;22:45-50. [Crossref] [PubMed]
- Filson CP, Montgomery JS, Dailey SM, et al. Complications associated with single-dose, perioperative mitomycin-C for patients undergoing bladder tumor resection. Urol Oncol 2014;32:40.e1-8. [Crossref] [PubMed]
- Mathes J, Todenhöfer T. Managing Toxicity of Intravesical Therapy. Eur Urol Focus 2018;4:464-7. [Crossref] [PubMed]
- Addeo R, Caraglia M, Bellini S, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2010;28:543-8. [Crossref] [PubMed]
- Erman A, Kamenšek U, Dragin Jerman U, et al. How Cancer Cells Invade Bladder Epithelium and Form Tumors: The Mouse Bladder Tumor Model as a Model of Tumor Recurrence in Patients. Int J Mol Sci 2021;22:6328. [Crossref] [PubMed]
- See WA, Rohlf DP, Crist SA. In vitro particulate adherence to fibronectin: correlation with in vivo particulate adherence to sites of bladder injury. J Urol 1992;147:1416-23. [Crossref] [PubMed]
- Noennig B, Bozorgmehri S, Terry R, et al. Evaluation of Intraoperative Versus Postoperative Adjuvant Mitomycin C with Nephroureterectomy for Urothelial Carcinoma of the Upper Urinary Tract. Bladder Cancer 2018;4:389-94. [Crossref] [PubMed]


