
Molecular mechanisms of penile traction for penile rehabilitation in a bilateral cavernous nerve crush injury rat model
Highlight box
Key findings
• The molecular mechanism that enables penile traction to improve erectile function (EF) is mediated through an upregulation in eNOS expression, as demonstrated in a rat model of bilateral cavernous nerve injury (BCNI).
What is known, and what is new?
• Current literature describes an improved EF phenomenon among men using penile traction for Peyronie’s disease.
• This study demonstrated that penile traction has a potential protective effect on EF after a BCNI, possibly due to increased eNOS expression in the corpora cavernosa.
What is the implication, and what should change now?
• Penile traction is a noninvasive way to potentially aid the recovery of EF after radical prostatectomy.
• Further clinical evidence is required to support the efficacy of penile traction for patients with erectile dysfunction.
Introduction
Prostate cancer is the most common solid-organ malignancy in men. Fortunately, early detection and radical prostatectomy (RP) have allowed for 15-year, cancer-specific survival rates of 90% (1). However, this does not come without cost. RP is associated with high rates of penile shortening and erectile dysfunction (ED), ranging widely from 20–90% (2).
Post-RP ED is hypothesized to be due in part to cavernous nerve injury. Under normal physiology, cavernous nerve innervation facilitates release of nitric oxide (NO) during sexual stimulation and leads to increased blood flow to the corpora cavernosa. When the cavernous nerve is damaged, decreased blood and oxygen supply to the corpora results in the replacement of erectile tissue with fibrosis (3). After fully healed post-RP, the lack of residual healthy erectile tissue is commonly inadequate, leading to veno-occlusive dysfunction and an inability to achieve a full erection. This has come to be known as the “use it or lose it” phenomenon.
Penile rehabilitation is a common clinical practice intended to ameliorate penile morphologic and functional changes post-RP. It is based on the concept that improving blood flow to the penis will prevent fibrosis, allowing for healthy erections when nerve function recovers. Phosphodiesterase type 5 inhibitors are considered the first-line treatment option due to their safety profile, ease of use, and positive effect on erectile function (EF) (4). Other therapies include vacuum erectile devices, intraurethral alprostadil suppositories, intracavernosal vasoactive agent injections, low-intensity shock wave therapy, and, more recently, penile traction therapy (PTT) (2,3).
PTT has recently gained attention as a potential treatment for Peyronie’s disease (PD). Early studies show that PD patients treated with PTT have increased stretched penile length (SPL) and improved EF. Ziegelmann et al. postulated that the improved EF was due to the shear forces in penile traction upregulating endothelial nitric oxide synthase (eNOS) expression in endothelial cells of the corpora (5). Endothelial cells are known to release NO in response to both increases and decreases in sheer stresses (6). However, no study to date has specifically investigated the molecular mechanism facilitating improved EF in patients undergoing PTT for post-RP ED.
To explore the underlying beneficial mechanism of PTT, we applied PTT in a rat model of bilateral cavernous nerve crush injury (BCNI). The BCNI model simulates the nerve injury observed in RP and is commonly used to test potential treatments for penile changes observed after RP. We present this article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-53/rc).
Methods
Overall study design
Twenty-four male Sprague Dawley rats (Charles River, Wilmington, MA, USA), aged 11–13 weeks, were randomly and equally divided into three groups: Sham (cavernous nerve exposure, no nerve crush); Crush (treated with BCNI, no PTT); and Traction [treated with BCNI and PTT beginning 3 days after the operation, 30 minutes daily at 1 Newton (N) of force for 28 total days]. This sample size was chosen as it is close to other similar studies on BCNI and PTT (2,7). Experiments were performed according to our protocol (No. 910) approved by Tulane University’s Institutional Review Board (IRB) and Institutional Animal Care and Use Committee (IACUC), which complied with institutional guidelines for the care and use of animals. The protocol was prepared before the study without registration.
Nerve crush surgery
All surgical tools were autoclaved before each nonterminal surgery. Male Sprague Dawley rats were initially anesthetized with 5% isoflurane in an induction chamber. Once anesthetized, the rat was transferred to a thermoregulated surgical table, and a nose cone was attached to administer 2% isoflurane throughout the operation. The abdomen was shaved and prepped with an iodine-based solution, and a lower midline abdominal incision was made. The prostate gland was exposed, and cavernous nerves were visualized on the posterolateral surfaces. The major pelvic ganglion (MPG) was identified as a proximal reference point. In the Sham group, no further manipulation was performed. In the Crush and Traction groups, #7 Dumont microforceps were used to crush the cavernous nerve 2–3 mm distal to the MPG. The microforceps were applied for 30 seconds, 2 times, with a 1-minute interval between crushes. A successful crush was confirmed by observation of color change in the cavernous nerve. The abdomen was closed in 2 layers by using 3-0 absorbable sutures and skin clips. Humane endpoints included 20% weight loss, labored breathing, or immobility.
Traction therapy
Male Sprague Dawley rats in the Traction groups received PTT daily, starting on postoperative day 3 and lasting 28 days. Anesthesia was induced with 5% isoflurane in an induction chamber. Once anesthetized, the rat was transferred to a thermoregulated table, and a nose cone was attached to administer 2% isoflurane throughout the procedure. The rat was positioned supine. A hemostat was clamped to the prepuce and attached to a suspended pull scale. This is the same method used by Lin et al. in their study on vacuum therapy, traction therapy, and PD (7). When the prepuce was stretched, the underlying corpora were pulled with the skin, therein transferring the traction force. One N of traction was applied for 30 minutes daily.
Measuring SPL
Rats in all groups received weekly measurements of SPL. Anesthesia was induced with 5% isoflurane in an induction chamber. Once anesthetized, the rat was transferred to a thermoregulated table, and a nose cone was attached to administer 2% isoflurane throughout the procedure. The rat was positioned supine. A hemostat was clamped to one side of the prepuce and attached to a suspended pull scale to apply 0.5 N of traction. This left half of the glans exposed, allowing us to measure SPL. A caliper was used to measure the length of the penis from the pubic symphysis to the tip of the glands. Three measurements were taken and later averaged to ensure accuracy. The same researcher performed all measurements to minimize any deviation or error in the measurement procedure.
Erectile response
Four weeks post-BCNI, rats were anaesthetized by using 5% isoflurane in an induction chamber. Once anesthetized, the rat was transferred to a thermoregulated table, and a nose cone was attached to administer 2% isoflurane throughout the procedure. Continuous arterial pressure measurement was achieved by cannulating the carotid artery and attaching a pressure transducer connected to a computerized system for data acquisition.
The prostate gland was exposed, and cavernous nerves were identified on the posterolateral surfaces. The skin of the scrotum was then incised, and the penis was carefully degloved. The left ischiocavernous muscle was identified and incised to reveal the underlying tunica albuginea that surrounds the corpus cavernosa. A 21-gauge needle was inserted into the left corpus cavernosa and connected to a pressure transducer for acquisition of ICP. The left cavernous nerve was then reidentified, and a stainless-steel bipolar hook was used to encircle the nerve. The cavernous nerve was stimulated with a Grass stimulator at 2.5, 5, and 7.5 V to achieve a significant and consistent erectile response. Stimulations were performed for 50 seconds each, with at least 150 seconds of rest between stimulations.
The ratio of change in the intracavernosal pressure (ICP) to the mean arterial pressure (MAP) (ΔICP/MAP) at the peak ICP response was used to control the variations in systemic blood pressure. Stimulations and measurements were repeated for the right cavernous nerve. For rats in the Crush arm, stimulation occurred distal to the crush lesion. Continuous monitoring of breathing and arterial blood pressure was performed to assure depth of anesthesia during the surgery. The animals were euthanized via creation of bilateral pneumothorax and removal of the heart.
Tissue collection
After endpoint cavernosal response measurements, a mid-shaft penile segment was collected for analysis by immunohistochemistry (IHC) and trichrome staining. Tissue specimens were fixed in formaldehyde for 24–48 hours and followed by paraffin embedding. Sections were cut into 5 µm then mounted on slides and dried. Corpora cavernosa tissue was also harvested from the penile shaft for analysis by western blot. Tissue was flash frozen in liquid nitrogen and stored at −80 ℃.
Histology
Slides showing cross sections of the corpora cavernosa were deparaffinized and rehydrated for the following studies.
Gomori trichrome
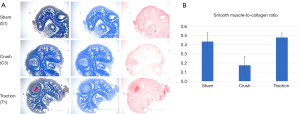
One slide per animal was stained with Gomori Trichrome by an automated staining system at the university’s pathology core facility. Image J was used to deconstruct slide images into their red, green, and blue components. Blue stain was used to measure collagen (COL) content in the corpora. Red stain was used to measure smooth muscle (SM) content. Values for the Sham, Crush, and Traction groups were averaged and compared.
IHC
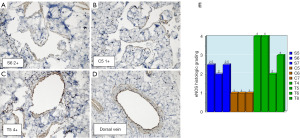
Slides of the corpora were immunohistochemically stained with eNOS (Abcam ab5589) according to the manufacturer’s instructions. A pathologist was blinded and asked to quantify the staining on the slides. Values for the Sham, Crush, and Traction groups were averaged and compared. Histologic grading of eNOS signal intensity, 1+ through 4+, was assigned, using each slide’s dorsal vein as the internal control. Dorsal vein eNOS expression was chosen as an internal control, as it is unaffected by the cavernous nerve crush due to innervation by branches of the pudendal nerve.
Western blot analysis
Corporal tissue was homogenized in radioimmunoprecipitation cell lysis buffer containing phosphatase and protease inhibitors. A Bradford assay was performed to determine protein concentration of each sample. A total of 15 µg of protein lysate for each sample was loaded and run on a sodium dodecyl sulfate-polyacrylamide gel. After the gel was finished running, protein was transferred to a polyvinylidene difluoride membrane and followed by blocking and incubation with primary antibodies. Corpora cavernosa blots were incubated with eNOS (Abcam ab5589), hypoxia-inducible factor-1alpha (HIF-1α) (Novus Biologicals NB100-105), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam ab9484) antibodies. A horseradish peroxidase-based secondary was used, and blots were visualized with a LAS-500 cooled CCD camera equipped with an enhanced chemiluminescence system for gel documentation. Image J was used to quantify band intensity. Band intensity was divided by total intensity expressed in all lanes to normalize each signal so that separate gels could be compared. Additionally, eNOS signal intensities were normalized to GAPDH signal intensity to account for any errors in well loading or protein quantification. Detailed methods, which have been followed in this study, have been described in previous studies (8,9).
Statistical analysis
Statistically significant differences between multiple groups were determined by using analysis of variance (ANOVA) with Tukey-Kramer post-hoc analysis. A Q Table (https://www2.stat.duke.edu/courses/Spring98/sta110c/qtable.html) was used to determine significance (α<0.05). An unpaired Student’s t-test (P) was used to determine statistically significant differences when appropriate, and P<0.05 was considered statistically significant. All statistical analyses were performed by using Microsoft Excel (Redmond, WA, USA).
Results
SPL
There was no significant preoperative difference in SPL between the three groups. At the end of the experiment, the Traction group had significantly greater SPL than the Crush group (30 vs. 27 mm; α<0.01) and Sham group (30 vs. 28 mm; α<0.05). There was no significant difference in SPL between the Crush and Sham group at the end of the experiment (Figure 1).

EF
Thirty days after surgery, EF was assessed by measuring the ΔICP/MAP during cavernous nerve stimulation at 2.5, 5, and 7.5 V. Animals that received a sham operation had significantly greater EF (ΔICP/MAP) compared to the Crush group at all voltages (32% vs. 17% at 2.5 V, 38% vs. 19% at 5 V, 39% vs. 23% at 7.5 V; α<0.05 for all). There was no significant difference in EF between the Crush and Traction groups (17% vs. 24% at 2.5 V, 19% vs. 24% at 5 V, 23% vs. 27% at 7.5 V; α>0.05 for all). Furthermore, rats that underwent PTT showed comparable EF to those in the Sham surgery group at all voltages (24% vs. 32% at 2.5 V, 24% vs. 38% at 5 V, 27% vs. 39% at 7.5 V; α>0.05 for all) (Figure 2). No voltage data was recorded for one of the traction animals due to technical difficulties, so n=7 for that group.

Western blots
eNOS
Western blot analysis was performed on corporal tissue, which revealed significantly greater eNOS expression in the Traction group relative to the Crush group (1.6 vs. 0.5; α<0.05). Sample S1 had to be excluded due to apparent contamination when creating the protein lysate. There was no significant difference in eNOS expression between the Sham and Traction groups (Figure 3A,3B).

HIF-1α
HIF-1α western blot (Figure 3C) was performed on all samples, but the blot containing animals 1–4 for the Sham, Crush, and Traction group appeared contaminated and was thereby excluded from analysis. Analysis of animals 5–8 from each group revealed significantly greater HIF-1α expression in the Traction group relative to the Crush group (2.1±1.4 vs. 0.3±0.2; α<0.05). There was no significant difference between the Sham and Traction group (1.1±0.5 vs. 2.1±1.4; α>0.05) or the Sham and Crush groups (1.1±0.5 vs. 0.3±0.2; α>0.05).
eNOS IHC
Slides stained with eNOS IHC are shown below (Figure 4). IHC was performed on several slides from each group (Sham n=3, Crush n=3, Traction n=4). Compared to the Crush group, the Traction group had noticeably increased expression of eNOS throughout the corpora. This confirms that the increased eNOS expression observed on western blot is due to increased expression in the corpora.
Trichrome staining
Trichrome staining was used to obtain SM-to-COL ratios for each group (Figure 5). Smaller SM-to-COL ratios indicate less SM and more fibrosis. The Sham and Traction groups had higher SM-to-COL ratios than the Crush group, but these differences were not statistically significant (Sham 0.44, Traction 0.48, Crush 0.35; P>0.05).
Discussion
Mankind has experimented with PTT for centuries. Historically, men would attach weights to their penis in the hopes of gaining penile length; however, these efforts have proven precarious, with penile swelling, nerve injury, and ED as adverse events (10). Recent experiments involving PD have reinvigorated research into PTT, with several studies reporting improved SPL and EF in patients receiving PTT (5). Seeking to identify a biologic pathway behind the improved EF seen in clinic patients, this study examined the effects of PTT in a rat BCNI model widely accepted as a model of RP-induced ED (10-12).
While PTT has not previously been tested in a rat model of BCNI, it has been studied in a rat model of PD. Lin et al. used a suspended tension gauge, which was attached to the prepuce of the penis via a clamp, to stretch rat penises for 20 minutes 3 times per day (7). Our experiment emulated this traction setup but only provided traction for 30 minutes once per day. The amount of force per area delivered by modern traction devices was calculated to be 4.5 Ns/cm2. To apply this to an average-sized rat penis, 0.61 N of force is needed. This was determined by finding the cross-sectional area of an average rat penis. It was noted that 1 N of force was well tolerated by the rat anatomy, which was employed to ascertain any adverse effects caused by traction. Traction was applied starting on postoperative day 3, as recommended by local veterinarians, to prevent animal distress. Traction was applied daily for 28 days, as 4 weeks correlates to the 2-year time point in humans, at which point EF is expected to have recovered to the extent possible if nerve-sparing RP was performed (2).
PTT in men with PD has been reported to increase SPL. A meta-analysis by Haney et al. examined more than 300 men with PD and compared SPL between those who did and did not use PTT. While there was no difference in SPL at baseline, the PTT group had 1.2 cm greater improvement in SPL at the completion of these studies (13). Our experimental findings are similar. While there was no preoperative difference in SPL between the three groups, the SPL of the Traction group was significantly greater by the completion of 28 days of PTT. This is promising, as many men fear penile shortening after RP and thus may defer on needed surgery.
There is also literature showing that PTT may improve sexual function after prostatectomy. Toussi et al. randomly assigned 82 men to either traction therapy or no traction therapy after prostatectomy. Compared to the control group, the men who performed traction therapy for 30 minutes once or twice a day had significantly better EF per the results of the International Index of Erectile Function (IIEF) questionnaire (IIEF-EF 0 vs. −6.5; P=0.03), intercourse satisfaction (IIEF-intercourse satisfaction +1 vs. −3.5; P<0.01), and overall satisfaction (IIEF-overall sexual satisfaction 0 vs. −3.5; P<0.01) (14). While the authors do not know the mechanism through which traction works, they postulate that eNOS may be involved in the mechanism. In terms of EF, our study demonstrated a significant reduction in EF (ΔICP/MAP) for rats in the Crush group when compared to the Sham group. The EF of rats in the Traction group was comparable to those in the Sham group, which represents this study’s control with retained EF.
Furthermore, western blot analysis of eNOS revealed that the Traction group had significantly greater expression than the Crush group. IHC later confirmed that the eNOS expression was located throughout the corpora, offering a potential mechanism of action. It is postulated that after cavernous nerve injury, there is a decrease of NO released in the corpora, which in turn leads to decreased blood flow, increased hypoxia, increased apoptosis, and eventual fibrosis (2). Our study implies that the addition of traction therapy post-RP can promote increased eNOS expression within the corpora, which could lead to increased blood flow and decreased hypoxia, decreased apoptosis, and decreased fibrosis. Gomori Trichrome was performed to analyze the SM-to-COL ratio in the corpora. We saw more SM in the Traction group than in the Crush group, which supports this theory of increased blood flow (SM is found in corporal vasculature). However, these findings were not significant, likely due to the large variation in amount of SM in the Crush group.
There are several limitations to our theory. Western blot analysis for HIF-1α was successfully performed on half of the samples from each group, as a surrogate measure of tissue hypoxia. The Traction group had significantly increased HIF-1α expression compared to the Crush group. If our theory of traction-induced blood flow were correct, we would expect HIF-1α to be decreased in the Traction group. A possible explanation for these unexpected findings is that 1 N is likely too much of a force when applying traction to a rat penis. We had hoped that by using more force than modern traction devices, we could increase the likelihood of observing an effect. However, this excessive force may have damaged the tissue and induced increased hypoxia and fibrosis in the corpora cavernosa.
In addition to limitations associated with preclinical and animal studies, another limitation to the study is the inconclusive effect that PTT had on EF. While it is possible to add animals to each group, it would not reveal useful information, as we have determined that the traction force used in this experiment was likely too excessive. Another limitation of the study is the method in which traction was administered to the corpora. One difficulty in using a rat model for penile traction is finding a way to apply a traction force to the corpora. Lin et al. achieved this by attaching a clamp to the prepuce and pulling on it (7). We modeled this approach and found that the corpora were pulled along with the skin overlying them. When performed at high forces, we noticed that the corpora were the limiting factor in how far the penis could be stretched. This indicates that the force applied to the prepuce was adequately applied to the corpora. With this said, we do not know the exact amount of force referred through the skin to the corpora, and the amount of referred force may vary between animals because of differences in skin elasticity or amount of skin around the penis.
Lastly, our study is limited by the lack of a “traction-only” group. Our analysis of HIF-1α revealed unexpectedly elevated levels of hypoxia in our traction group, but it is unclear whether this came from the nerve crush or the traction itself. A “traction-only” group would allow us to determine if these effects were caused by the traction therapy.
Conclusions
In conclusion, this study has demonstrated that PTT has the potential to increase SPL and increase corporal eNOS expression after RP. This increase in eNOS is a potential mechanism through which traction prevents deterioration of EF after RP. To our knowledge, this is the first study to demonstrate that PTT increases eNOS expression in the corporal cavernosa. Future authors are encouraged to determine the time and force of PTT needed for optimal penile rehabilitation.
Acknowledgments
Special thanks to Scott Bailey, PhD (Department of Urology, School of Medicine, Tulane University, New Orleans, LA, USA) for preparing and editing this manuscript.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-53/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-53/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-53/coif). BD reports has received a grant to fund this project from the Sexual Medicine Society of North America. WJGH is an advisor for Coloplast, Boston Scientific, and a speaker for Endo. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol (No. 910) has been reviewed and approved by Tulane University’s Institutional Review Board and Institutional Animal Care and Use Committee (IACUC), which complied with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 2001;28:555-65. [Crossref] [PubMed]
- Yuan J, Lin H, Li P, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol 2010;58:773-80. [Crossref] [PubMed]
- Bratu O, Oprea I, Marcu D, et al. Erectile dysfunction post-radical prostatectomy - a challenge for both patient and physician. J Med Life 2017;10:13-8. [PubMed]
- Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol 2008;18:613-20. [Crossref] [PubMed]
- Ziegelmann M, Savage J, Toussi A, et al. Outcomes of a Novel Penile Traction Device in Men with Peyronie's Disease: A Randomized, Single-Blind, Controlled Trial. J Urol 2019;202:599-610. [Crossref] [PubMed]
- Chatterjee S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front Physiol 2018;9:524. [Crossref] [PubMed]
- Lin H. Liu C, Wang R. Effect of Penile Traction and Vacuum Erectile Device for Peyronie's Disease in an Animal Model. J Sex Med 2017;14:1270-6. [Crossref] [PubMed]
- Greenberg JW, Kim H, Moustafa AA, et al. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci Rep 2021;11:10200. [Crossref] [PubMed]
- Greenberg JW, Kim H, Ahn M, et al. Combination of Tipifarnib and Sunitinib Overcomes Renal Cell Carcinoma Resistance to Tyrosine Kinase Inhibitors via Tumor-Derived Exosome and T Cell Modulation. Cancers (Basel) 2022;14:903. [Crossref] [PubMed]
- Cowper MG, Burkett CB, Le TV, et al. Penile Stretching as a Treatment for Peyronie's Disease: A Review. Sex Med Rev 2019;7:508-15. [Crossref] [PubMed]
- Haney NM, Talwar S, Akula PK, et al. Insulin-Like Growth Factor-1-Loaded Polymeric Poly(Lactic-Co-Glycolic) Acid Microspheres Improved Erectile Function in a Rat Model of Bilateral Cavernous Nerve Injury. J Sex Med 2019;16:383-93. [Crossref] [PubMed]
- Haney NM, Nguyen HMT, Honda M, et al. Bilateral Cavernous Nerve Crush Injury in the Rat Model: A Comparative Review of Pharmacologic Interventions. Sex Med Rev 2018;6:234-41. [Crossref] [PubMed]
- Haney NM, Kohn TP, Nichols PE, et al. The Effect of Adjunct Mechanical Traction on Penile Length in Men Undergoing Primary Treatment for Peyronie's Disease: A Systematic Review and Meta-analysis. Urology 2018;122:110-5. [Crossref] [PubMed]
- Toussi A, Ziegelmann M, Yang D, et al. Efficacy of a Novel Penile Traction Device in Improving Penile Length and Erectile Function Post Prostatectomy: Results from a Single-Center Randomized, Controlled Trial. J Urol 2021;206:416-26. [Crossref] [PubMed]


