
A systematic review evaluating the effectiveness of exercise training on physical condition in prostate cancer patients undergoing androgen deprivation therapy
Highlight box
Key findings
• Moderate-quality evidence indicated that exercise training may improve body composition [lower body fat mass (BFM) and body fat rate (BFR)], muscular strength, and quality of life (QoL) in prostate cancer (PCa) patients undergoing androgen deprivation therapy (ADT). Meanwhile, low-quality evidence demonstrated that exercise training could improve such symptoms as fatigue, depression, sexual function, and cardiometabolic changes.
What is known and what is new?
• Exercise training is helpful in preventing the negative effects of ADT and improving QoL in men with PCa. In addition, exercise training improves muscular strength, body composition, and physical performance in PCa patients receiving ADT.
• This study assessed the evidence level of the systematic evaluation of the effect of exercise on QoL of PCa patients receiving ADT. No evidence of high quality was found.
What is the implication, and what should change now?
• Exercise training can be used to manage adverse effects in PCa patients treated with ADT.
Introduction
Androgen deprivation therapy (ADT) is an effective prostate cancer (PCa) treatment strategy that can curb the development or progression of the disease (1). However, ADT may cause hypogonadism and other adverse effects such as obesity, sarcopenia, metabolic syndrome, cardiovascular diseases, osteoporosis, sexual function failure, diabetes mellitus, and gynecomastia (2-11). Apart from these well-established complications, there is data showing that between 5–50% of PCa patients receiving ADT exhibit some form of cognitive dysfunction (12,13). Recent papers suggest that ADT may be a risk factor of major depression disorder and suicidal behavior (14).
Several studies have demonstrated that exercise training is helpful in preventing the negative effects of ADT on physical health, as well as in improving quality of life (QoL) in men with PCa (15-17). Oncology exercise guidelines recommend all cancer patients engage in 20–30 minutes of moderate-intensity, aerobic, and resistance exercise 3–5 times per week (18). Resistance exercise training (RET) and aerobic exercise training (AET) are practicable and well-tolerated therapies for patients with PCa to improve their physical condition, including body composition, muscular strength, cardiorespiratory fitness, physical function, and fatigue (19).
Notably, how exercise training improves the physical condition of PCa patients undergoing ADT has become a focus of research (20). PCa cells are predisposed to oxidative stress, which is required for the aggressive phenotype (21). Oxidative stress can promote the occurrence and progression of PCa by activating androgen receptor (AR) signaling, and PCa patients undergoing ADT produce a large amount of reactive oxygen species (ROS) (22). Excessive generation of ROS disrupts the mechanisms of cancer suppression, leading to cell damage and death. Moreover, numerous data demonstrate that PCa is associated with the development of oxidative stress (23). Simultaneously, ADT-induced osteoporosis, cardiovascular diseases, and body composition changes are also correlated with oxidative stress (24). Exercise training is a fundamental form of therapy for treating diseases. Antioxidant indicators increase after exercise training, while prooxidant indicators tend to decline, regardless of exercise volume, intensity, type, or population. Thus, physical activity has an antioxidant effect (25), and regular AET enhances the brain’s antioxidant capacity (26).
There is a growing body of systematic reviews/meta-analyses (SRs/MAs) on exercise training for PCa patients undergoing ADT. For evidence users, there are many systematic reviews that need to be retrieved and read for different clinical issues of the same disease, which is time-consuming and laborious. On the other hand, summarizing the existing SRs/MAs, can comprehensively present the high-quality research results about a specific subject, save time, and obtain a higher level of evidence resources, which is timelier and more feasible to solve clinical problems. The quality of the methodology and evidence has not been evaluated, and whether the results can offer reliable evidence for clinicians is still under debate. Hence, the purpose of this study was to assess the methodological quality, risk of bias, reporting quality, and evidence quality of available SRs/MAs on exercise training and physical condition for PCa patients receiving ADT. We present this article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-272/rc).
Methods
Eligibility criteria
Type of studies
The present study summarized available SRs/MAs on exercise training for PCa patients undergoing ADT. We excluded meeting summaries, case reports, SR/MA protocols, comment updates, studies with inadequate data, duplicate records, and network MAs of the effects of various exercise training programs.
Type of participants
Participants were diagnosed with PCa and underwent ADT. No restrictions were imposed on age, race, course of disease, and pathological type.
Type of interventions
PCa patients undergoing ADT were assigned to a treatment group and control group. The treatment group received exercise training in addition to routine care. No restrictions were placed on the exercise type, frequency, and intervention time.
Type of comparison
The control group only received routine treatment (usual or standard care) with no exercise training.
Type of outcomes
The primary outcome measure was body composition. The secondary outcomes included mood, QoL, physical function, cardiometabolic changes, bone mineral density (BMD), and sexual function.
Search strategy
A comprehensive search in Web of Science, Embase, PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Databases, VIP Journals Database, and Chinese Biomedical Databases (CBM) was conducted for SRs/MAs on exercise training for PCa patients undergoing ADT. The retrieval was as of April 25, 2022 with the language restrictions of Chinese and English. Both subject headings and free words were used in the search. The search terms included prostatic neoplasms, prostate neoplasm, prostate cancer, androgen deprivation therapy, exercise, physical activity, meta-analysis, and systematic review. The search strategy is summarized in Table S1 in the supplementary materials.
Data extraction and management
Three reviewers (MJ, ZM, and PZ) participated in literature screening, data extraction, and quality evaluation of the included SRs/MAs. Article screening and data extraction were done by 2 reviewers (XZ and DC) independently.
The searched studies were imported into Endnote X9 for management. After removing duplicate records, we screened the titles and abstracts to exclude irrelevant studies. Based on a full-text review, 8 SRs/MAs with 51 outcomes were included in this study. Two reviewers independently extracted data and cross-checked the results. Extracted data included the author, year of publication, quality assessment methods, duration of intervention, intervention measures, number of patients, number of included studies, outcome indicators, data analysis methods (whether sensitivity analysis and subgroup analysis were used), and conclusions. Any disagreements were discussed with a third researcher (YY) for a final consensus.
Assessment of methodological quality
A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR-2) was adopted to assess the methodological quality of the included SRs/MAs (27). This scale comprises 16 items, 7 of which are critical items that can affect the validity of a review. The evaluation items are answered as yes, partial yes, or no. Based on methodological flaws in the critical items, the overall confidence in the results of a review is rated as critically low, low, moderate, or high. A study with no flaws or only 1 noncritical weakness was rated as high quality. If a review contained more than 1 weakness but no critical defects, it was classified as moderate quality. A review with 1 critical flaw was graded as low quality. A review with more than 1 critical defect was considered critically low quality.
Assessment of risk of bias
The risk of bias of the included SRs/MAs was assessed using Risk of Bias in Systematic Reviews (ROBIS) (28). The risk of bias assessment involves 3 phases: evaluating relevance, determining concerns about the review process, and grading the overall risk of bias. In addition, phase 2 covers 4 domains: research inclusion criteria, literature selection, data extraction and study assessment, and synthesis and findings. The overall risk of bias in the included studies was graded as low, unclear, or high.
Assessment of reporting quality
The PRISMA statement was used to evaluate the reporting quality of the included studies (29). PRISMA consists of a 27-item checklist involving the title, abstract, introduction, methods, results, and discussion sections. Each item received a response of either yes (full report), partial report, or no (no report) according to the integrity of information in the SRs/MAs.
Quality assessment of evidence
The quality of evidence in the included literature was assessed using the Grades of Recommendations, Assessment, Development and Evaluation (GRADE) system (30). This system describes 5 quality-compromising factors: limitations, indirectness, imprecision, inconsistency, and publication bias. There are also 3 confidence-increasing factors in the GRADE system: large effect quantity, dose-effect relationship, and negative bias. Two researchers (MJ and PZ) thoroughly evaluated the quality of evidence in the included studies and classified them as high, moderate, low, or very low quality. If there were no compromising factors, the evidence would be graded as high quality. If 1 compromising factor was found, the evidence was rated as moderate quality. When there were compromising factors, the quality of evidence was considered low. When there were 3 or more compromising factors, the evidence was rated as very low quality.
Data synthesis
Dichotomous data are presented as risk ratio (RR) or odds ratio (OR), and continuous data are reported as standard mean difference (SMD) or weighted mean difference (WMD) with 95% confidence intervals (CIs). The study selection process, the characteristics and results of the included SRs/MAs, and the results of the evaluation tools are summarized in tables or figures.
Results
Study selection
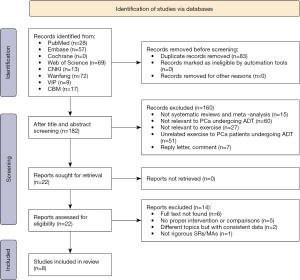
We retrieved 265 articles from the databases and imported them into Endnote for management. After removing 83 duplicates, the titles and abstracts were screened, and 160 articles were excluded. Based on a full-text review, 14 studies were further excluded. Finally, 8 articles (31-38) were included in the present study. The study selection process is shown in Figure 1.
Study characteristics
The included studies were published between 2015 and 2022. Five articles were from China, 2 from Australia, and 1 from Denmark. The 8 eligible studies involved 7,483 patients and 100 trials in total, but only 92 randomized controlled trials with 7,146 patients were finally included in the present study.
The intervention in the treatment group was exercise training (RET or AET) plus routine care, while the intervention in the control group was only usual or standard care. The intervention duration was more than 3 months. Most eligible SRs/MAs used the Cochrane risk of bias tool to assess the quality of their original studies, but 1 MA (32) adopted the Physiotherapy Evidence Database (PEDro) quality assessment scale. The effect of exercise training on body composition in PCa patients undergoing ADT was reported in 6 SRs/MAs (31-35,38), changes in physical function were reported in 3 SRs/MAs (31,33,36), improvement in fatigue was reported in 3 SRs/MAs (31,37,38), and patients’ QoL was reported in 4 SRs/MAs (35-38). Meanwhile, some SRs/MAs reported on BMD and sexual health. Additionally, 3 SRs/MAs performed sensitivity and subgroup analyses (36-38). All 8 eligible SRs/MAs showed that exercise training improved physical conditions in PCa patients undergoing ADT. Three SRs/MAs (32,33,35) proposed that larger studies were needed to verify their results due to their small sample sizes. The basic characteristics of the included SRs/MAs are presented in Table 1.
Table 1
| Author [year] | Country | No. of included studies [sample size] | Type of included studies | Intervention | Control | Intervention duration (months) | Outcome | Quality assessment tool | Data analysis methods | Sensitivity/subgroup analysis | Results summary | Financial support |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yunfeng G, et al. [2017] | China | 15 trials [1,135], 14 RCTs [1,063] | RCT [14] + CT [1] | RET/AET | Usual care | 4–12 | ①②③④⑤⑥ | Cochrane Risk of Bias Tool | MA | No/yes | Effective | No mention |
| Bigaran A, et al. [2021] | Australia | 18 trials [1,204], 11 RCTs [2 groups, 939] in this overview | RCT (14, 11 RCTs consist of 2 groups, 3 RCTs consist of 3 groups) + NRS [4] | Exercise | Usual care | 3–13 | ①③⑦⑧⑨ | Cochrane Risk of Bias Tool; PEDro quality assessment scale | SR/MA | No/yes | Effective (limited evidence) | No mention |
| Chen Z, et al. [2019] | China | 7 [468] | RCT | Supervised Exercise | Usual care | 3–12 | ①② | Cochrane Risk of Bias Tool | SR/MA | No/yes | Effective (limited evidence) | No mention |
| Shao W, et al. [2022] | China | 12 [715] | RCT | Exercise; AET/RET/impact exercise | Usual care | 3–12 | ①⑤ | Cochrane risk of bias tool | SR/MA | No/yes | Effective | No support |
| Teleni L, et al. [2016] | Australia | 9 [738] | RCT [7] | AET/RET | Standard care or no treatment | 3–6 | ①③⑧⑩ | Cochrane Risk of Bias Tool | MA | No/yes | Effective (limited evidence) | Yes |
| Ussing A, et al. [2022] | Denmark | 18 [1,477] | RCT | Supervised exercise | No exercise therapy | 3–12 | ②⑩⑪ | Cochrane Risk of Bias tool; GRADE | SR/MA | Yes/yes | Effective | Yes |
| Yang B, et al. [2017] | China | 10 [841] | RCT | Exercise | Usual care | 3–6 | ④⑩ | Cochrane Risk of Bias tool | MA | Yes/yes | Effective | No mention |
| Ying M, et al. [2018] | China | 1 [905] | RCT | Exercise | Usual care | 3–6 | ①④⑩⑪ | Cochrane Risk of Bias tool | MA | Yes/yes | Effective | No support |
Outcomes: ①, body composition; ②, physical function; ③, cardiometabolic changes; ④, fatigue; ⑤, BMD; ⑥, sexual health; ⑦, exercise capacity; ⑧, blood pressure; ⑨, inflammatory markers; ⑩, quality of life; and ⑪, depression. SR, systematic review; MA, meta-analysis; RCT, randomized controlled trial; CT, controlled trial; NRS, non-randomized studies; AET, aerobic exercise training; RET, resistance exercise training; PEDro quality assessment scale: Physiotherapy Evidence Database quality assessment scale; GRADE, Grades of Recommendations, Assessment, Development and Evaluation; BMD, bone mineral density.
Methodological quality of the included SRs/MAs
The methodological quality of the included SRs/MAs was assessed using the AMSTAR-2 tool. Due to various methodological defects in critical (2,7,13,15) and noncritical items (10,12,14,16), 3 SRs/MAs (32,35,36) were of moderate methodological quality, 4 SRs/MAs (31,34,37,38) were of very low methodological quality, and 1 SR/MA (33) was of low methodological quality. The methodological quality of the included SRs/MAs is shown in Table S2.
Risk of bias of the included SRs/MAs
The risk of bias in the included SRs/MAs was evaluated utilizing the ROBIS tool. The assessment results showed that all SRs/MAs had a low risk in phase 1 of the ROBIS scale. In terms of the 4 domains in phase 2, all eligible SRs/MAs had a low risk in domain 1, 6 SRs/MAs (31-36) had a low risk of bias in domain 2, 5 SRs/MAs (32,34-37) had a low risk of bias in domain 3, and 4 SRs/MAs (32,35,36,38) had a low risk in domain 4. Furthermore, 5 SRs/MAs (31,34,36-38) were rated as having a low overall risk of bias in phase 3. The risk of bias assessment of the included SRs/MAs is presented in Table S3.
Reporting quality of the included SRs/MAs
The reporting quality of the included SRs/MAs was assessed using the PRISMA tool. Five studies (32-36) received a yes (full report) to item 5 (protocol and registration), 6 studies (31,33-37) received a yes (full report) to item 12 (risk of bias), and 6 studies (32,34-38) received a yes (full report) to item 23 (additional analysis). The reporting quality is presented in Table S4.
Efficacy of exercise for PCa patients undergoing ADT
The eligible SRs/MAs involved 13 types of outcome measures. The effect of exercise training on body composition in PCa patients on ADT was analyzed in 6 SRs/MAs (31-35,38), fatigue was reported in 3 SRs/MAs (31,37,38), QoL was discussed in 4 SRs/MAs (35-38), depression was reported in 2 SRs/MAs (36,38), and physical function was reported in 3 SRs/MAs (31,33,36). Simultaneously, cardiometabolic changes, BMD, sexual health, exercise capacity, blood pressure, and inflammatory markers were also discussed. The outcome measures in the included SRs/MAs are summarized in Table S5.
Body composition
Body composition comprises body mass index (BMI), lean body mass (LBM), body fat rate (BFR), and body fat mass (BFM). Six SRs/MAs (31-35,38) reported on body composition. Nonetheless, only 1 of them (38) comprehensively investigated the effect of exercise interventions on body composition in PCa patients undergoing ADT, showing that an exercise-dominated lifestyle significantly improved body composition in PCa patients on ADT (SMD =−0.1, 95% CI: −0.19, −0.01, I2=0%, P=0.03). The remaining 5 papers (31-35) analyzed their outcome indicators statistically.
BMI was reported in 2 SRs/MAs (31,38). Yunfeng et al. (31) analyzed the BMI index with a cutoff of 6 months and found that exercise training improved BMI (SMD =−0.33, 95% CI: −0.55, −0.12, I2=38%, P= 0.002, <6 months; SMD =−0.59, 95% CI: −1.01, 0.17, I2=25%, P= 0.006, >6 months). In contrast, Ying et al. (38) found no significant improvement in BMI (SMD =−0.11, 95% CI: −0.32, 0.10, I2=9%, P=0.30).
LBM was discussed in 6 SRs/MAs (31-35,38). Three SRs/MAs (31,33,38) revealed that exercise training did not significantly improve LBM in PCa patients undergoing ADT [Yunfeng et al. (31): SMD =−0.08, 95% CI: −0.20, 0.30, I2=0%, P= 0.57; Chen et al. (33): MD=−0.49 kg, 95% CI: −0.76, 1.74, I2=0%, P=0.44; Ying et al. (38): SMD =−0.01, 95% CI: −0.24, 0.22, I2=0%, P=0.91]. However, 2 SRs/MAs (32,34) found that exercise training significantly improved LBM in PCa patients receiving ADT [Bigaran et al. (32): MD =0.70 kg, 95% CI: 0.39, 1.01, I2=0%, P<0.0001; Shao et al. (34): MD =0.88, 95% CI: 0.40, 1.36; I2=0%, P=0.0003]. One MA (34) showed that RET alone did not significantly improve LBM compared to usual care (MD =1.43, 95% CI: −0.29, 3.14, I2=58%, P=0.10), but RET combined with other exercises such as AET improved LBM (MD =0.86, 95% CI: 0.16, 1.56, I2=0%, P<0.05). In addition, its subgroup analysis showed that exercise training improved LBM regardless of intervention intensity (8–12 repetition maximum or 6–12 repetition maximum), exercise duration (>6 or ≤6 months), immediate exercise after ADT, or delayed exercise after ADT (MD =2.61, 95% CI: 0.89, 4.32, I2=0%, P<0.01; MD =0.83, 95% CI: 0.12, 1.55, I2=0%, P<0.05; MD =0.75, 95% CI: 0.23, 1.28, I2=0%, P<0.01; MD =1.60, 95% CI: 0.37, 2.83, I2=0%, P<0.05; MD =0.93, 95% CI: 0.18, 1.67, I2=0%, P<0.05; MD =1.02, 95% CI: 0.08, 1.96, I2=0%, P<0.05). Another SR/MA (35) did not conduct heterogeneity analysis and showed that exercise did not significantly improve LBM (MD =−0.20, 95% CI: −1.72, 1.32).
BFM was described in 5 SRs/MAs (31,32,34,35,38). Two of them (31,38) suggested that exercise training did not significantly lower BFM in PCa patients undergoing ADT (P>0.05). One MA (32) found that exercise training improved whole-BFM and trunk fat mass (MD =−0.67 kg, 95% CI: −1.08, −0.27, I2=51%, P=0.001; MD =−0.49 kg, 95% CI: −0.87, −0.12, I2=51%, P=0.01). Another SR (34) suggested that exercise training improved BFM (MD =−0.60, 95% CI: −1.10, −0.10, I2=0%, P=0.02). Moreover, RET combined with other exercises such as AET (MD =−0.21, 95% CI: −0.85, 0.44, I2=0%, P=0.53) showed greater improvement in LBM than RET alone (MD =−1.19, 95% CI: −1.99, −0.40, I2=0%, P<0.01). It also revealed that high frequency (6–12 repetition maximum) training (MD =−1.15, 95% CI: −1.97, −0.34, I2=0%, P<0.01) and immediate exercise after ADT (MD =−1.37, 95% CI: −2.25, −0.49, I2=0%, P<0.01) had more beneficial effects on BFM. Another study (35) did not perform heterogeneity analysis, and its results revealed that exercise improved BFM (MD =−0.61, 95% CI: −2.48, 1.26).
BFR was reported in 5 SRs/MAs (31,32,34,35,38), all of which revealed that exercise training was beneficial to BFR [Yunfeng et al. (31): SMD =−0.22, 95% CI: −0.42, −0.01, I2=0%, P= 0.04; Bigaran et al. (32): MD =−0.79, 95% CI: −1.16, −0.42, I2=59%, P<0.0001; Shao et al. (34): MD =−0.93, 95% CI: −1.39, −0.47, I2=15%, P<0.0001; Teleni et al. (35): MD =−0.71, 95% CI: −1.96, 0.55; Ying et al. (38): SMD =−0.21, 95% CI: −0.40, 0.03, I2=0%, P=0.03]. One SR/MA (34) performed subgroup analysis and found that RET with 8–12 repetition maximum, prolonged exercise duration, and immediate exercise after ADT significantly improved BFR (P<0.05), but delayed exercise after ADT did not improve BFR (MD =−0.97, 95% CI: −1.97, 0.04, I2=35%, P=0.06).
Fatigue
Two SRs/MAs (37,38) demonstrated that exercise training significantly alleviated fatigue (Yang et al.: SMD =−0.32, 95% CI: −0.45, −0.18, I2=35%, P<0.00001; Ying et al.: SMD =0.17, 95% CI: 0.00, 0.34, I2=0%, P=0.05). Another MA (31) found that exercise training for more than 6 months significantly improved fatigue (SMD =−9.3, 95% CI: −16.22, −2.39, I2=49%, P=0.003), while exercise training for fewer than 6 months did not improve fatigue (SMD =0.84, 95% CI: −1.43, 3.10, I2=51%, P=0.85). Its subgroup analysis showed no difference in the effects of AET and RET on fatigue (SMD =0.09, 95% CI: −0.27, 0.44, I2=51%, P= 0.63).
QoL
Four SRs/MAs (35-38) analyzed the effects of exercise training on QoL in PCa patients undergoing ADT. One of them (36) revealed that exercise training improved disease-specific QoL in PCa patients undergoing ADT (SMD =0.43, 95% CI: 0.29, 0.58, I2=11%, P<0.00001). Its subgroup analysis showed that AET/RET significantly improved disease-specific QoL (P=0.00001), but football training did not improve disease-specific QoL (P=0.64). Meanwhile, Ying et al. (38) found that simple exercise training significantly improved patients’ QoL (SMD =0.17, 95% CI: 0.00, 0.34, I2=0%, P=0.05), while exercise training combined with dietary advice did not improve patients’ QoL (SMD =0.45, 95% CI: −0.17, 1.08, I2=80%, P=0.15).
Depression
Depression was reported in 2 SRs/MAs (39,40). One article (36) showed that exercise training mitigated depression (SMD =−0.23, 95% CI: −0.54, 0.08). In contrast, the other (38) revealed that there was no statistical difference in the improvement of depression between the exercise training group and usual care group (MD =−0.18, 95% CI: −0.67, 0.31, I2=46%, P=0.47). However, the results had a high risk of bias due to the small sample sizes in the 2 reviews.
Physical function
Physical function was reported in 3 studies (40-42). Physical function consists of chest press, leg press, and VO2 peak. One MA (31) found that exercise training significantly improved physical function (P<0.05), including leg press (SMD =0.78, 95% CI: 0.57, 0.99, I2=0%, P<0.00001), chest press (SMD =0.71, 95% CI: 0.50, 0.92, I2=0%, P<0.00001), and VO2 peak regardless of intervention duration (SMD =0.35, 95% CI: 0.04, 0.66, I2=0%, P=0.03, <6 months; SMD =0.59, 95% CI: 0.16, 1.03, I2=0%, P=0.007, >6 months). However, its subgroup analysis showed no statistically significant difference in the improvement of VO2 peak between the AET and RET groups (SMD =−0.12, 95% CI: −0.44, 0.21, I2=0%, P= 0.63). Chen et al. (33) found that exercise training significantly improved leg press and chest press (P<0.0001). Meanwhile, Ussing et al. (36) found that exercise training significantly improved VO2 peak (MD =1.76, 95% CI: 0.82, 2.69), muscle strength (SMD =0.47, 95% CI: 0.28, 0.65), walking performance (SMD =−0.41, 95% CI: −0.60, −0.22, I2=29%, P<0.0001), and sit-to-stand performance (SMD =0.35, 95% CI: 0.14, 0.56).
Other outcomes
Cardiometabolic changes were reported in 1 MA (31), which showed that exercise training significantly improved total serum cholesterol (SMD =0.35, 95% CI: 0.1, 0.61, I2=0%, P= 0.007), but there was no significant improvement in triglycerides, high-density lipoprotein (HDL), and fasting glucose (SMD =0.27, 95% CI: −0.5, 1.03, I2=87%, P= 0.5; SMD =0.21, 95% CI: −0.13, 0.55, I2=0%, P= 0.08; SMD =−0.30, 95% CI: −0.64, 0.04, I2=0%, P= 0.30). Fasting blood glucose was reported in 1 review (32), which revealed that exercise training improved fasting blood glucose (MD =−0.38 mmol/L, 95% CI: −0.65, −0.11, I2=0%, P=0.006).
BMD change was discussed in 2 SRs/MAs (43,44). One MA (31) found no significant difference in BMD change between the exercise training group and usual care group (SMD =−0.03, 95% CI: −0.07, 0.01, I2=0%, P= 0.12). The other (34) indicated that exercise training did not significantly inhibit the loss of whole-body BMD, lumbar BMD, total hip BMD, and femoral neck BMD (MD =−0.00, 95% CI: −0.01, 0.01, I2=0%, P=0.74; MD =0.00, 95% CI: −0.00, 0.01, I2=0%, P=0.16; MD =0.00, 95% CI: −0.00, 0.01, I2=0%, P=0.09; MD =0.00, 95% CI: −0.00, 0.00, I2=0%, P=0.74).
Notably, 1 MA (31) concluded that exercise training improved sexual health in PCa patients undergoing ADT (SMD =0.66, 95% CI: 0.35, 0.97, I2=2%, P<0.00001). Another study (32) reported that exercise training had a beneficial effect on 400-m-walk performance (MD =−10.11 s, 95% CI: −14.34, −5.88, I2=0%, P<0.00001), but had no significant effect on 6-min-walk performance (MD =52.57, 95% CI: −3.03, 108.16, I2=0%, P=0.06).
Blood pressure was reported in 2 SRs/MAs (32,35). One of them (32) revealed that exercise training significantly improved diastolic blood pressure (MD =−2.22 mmHg, 95% CI: −3.82, −0.61, I2=0%, P=0.007). In contrast, the other (35) reported that exercise did not significantly improve systolic blood pressure (MD =1.72, 95% CI: −2.47, 5.90). C-reactive protein level was reported in one MA (32), which indicated that exercise training could reduce the level of C-reactive protein (mg/L) (MD =−1.16 mg/L, 95% CI: −2.11, −0.20, I2=47%, P=0.02).
Evidence quality of the included SRs/MAs
The GRADE system was adopted to evaluate the evidence quality of the 51 outcomes extracted from the 8 eligible SRs/MAs. Based on the evidence quality assessment, 8 outcomes had moderate evidence quality (15.69%), 23 had low evidence quality (45.10%), and 20 had very low evidence quality (39.22%). There was no high-quality evidence. The most important factor for the compromised quality of evidence was the publication bias (40/51, 78.43%) caused by a lack of funnel plots or a limited number of original studies. The secondary factors were imprecision (35/51, 68.63%) and risk of bias (33/51, 64.71%). The evidence quality of the included SRs/MAs is shown in Table 2.
Table 2
| Outcomes | References | Studies [participants] | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| Body composition | ||||||||
| BMI | Yunfeng G, et al. [2017] | |||||||
| <6 months | 5 [346] | −1① | 0 | 0 | −1④ | 0 | Low | |
| >6 months | 2 [91] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| Ying M, et al. [2018] | 6 [452] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| LBM | Yunfeng G, et al. [2017] | 4 [196] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Bigaran A, et al. [2021] | 5 [372] | 0 | 0 | 0 | −1④ | 0 | Moderate | |
| Chen Z, et al. [2019] | 7 [490] | −1① | 0 | 0 | 0 | 0 | Moderate | |
| Shao W, et al. [2022] | 9 [562] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| Teleni L, et al. [2016] | 4 [335] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Ying M, et al. [2018] | 5 [292] | −1① | 0 | 0 | −1④ | −1⑤ | Very low | |
| The percentage fat mass | Yunfeng G, et al. [2017] | 5 [398] | −1① | 0 | 0 | −1④ | −1⑥ | Very Low |
| Bigaran A, et al. [2021] | 4 [275] | 0 | −1② | 0 | −1④ | 0 | Low | |
| Shao W, et al. [2022] | 8 [428] | −1① | 0 | 0 | 0 | 0 | Moderate | |
| Teleni L, et al. [2016] | 4 [335] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Ying M, et al. [2018] | 5 [393] | −1① | 0 | 0 | −1④ | −1⑤ | Very low | |
| BFM | Bigaran A, et al. [2021] | 5 [372] | 0 | −1② | 0 | −1④ | 0 | Low |
| Shao W, et al. [2022] | 9 [549] | −1① | 0 | 0 | 0 | 0 | Moderate | |
| Physical function | ||||||||
| Leg press | Yunfeng G, et al. [2017] | 5 [417] | −1① | 0 | 0 | 0 | −1⑥ | Low |
| Chen Z, et al. [2019] | 4 [235] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| Chest press | Yunfeng G, et al. [2017] | 6 [428] | −1① | 0 | 0 | 0 | 0 | Moderate |
| Chen Z, et al. [2019] | 5 [335] | −1① | 0 | 0 | −1④ | 0 | Low | |
| VO2 peak | Yunfeng G, et al. [2017] | |||||||
| <6 months | 3 [202] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| >6 months | 2 [105] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| Ussing A, et al. [2022] | 6 [406] | 0 | −1② | 0 | −1④ | −1⑤ | Very low | |
| Cardiometabolic changes | ||||||||
| Total serum cholesterol | Yunfeng G, et al. [2017] | 4 [238] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Triglyceride | Yunfeng G, et al. [2017] | 4 [238] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Teleni L, et al. [2016] | 3 [300] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| HDL | Yunfeng G, et al. [2017] | 3 [138] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Fasting glucose | Yunfeng G, et al. [2017] | 4 [238] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Bigaran A, et al. [2021] | 3 [217] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Teleni L, et al. [2016] | 3 [300] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Fatigue | Yunfeng G, et al. [2017] | |||||||
| <6 months | 5 [433] | −1① | −1② | 0 | 0 | 0 | Low | |
| >6 months | 3 [321] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| Yang B, et al. [2017] | 9 [784] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| Ying M, et al. [2018] | 9 [737] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| Depression | Ying M, et al. [2018] | 2 [163] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| BMD | Yunfeng G, et al. [2017] | 3 [171] | −1① | 0 | 0 | −1④ | −1⑥ | Very low |
| Shao W, et al. [2022] | ||||||||
| The whole-body BMD | 4 [329] | −1① | 0 | 0 | −1④ | −1⑥ | Very low | |
| The lumbar BMD | 7 [426] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| The total hip BMD | 6 [406] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| The femoral neck BMD | 5 [259] | −1① | 0 | 0 | −1④ | −1⑤ | Very low | |
| Sexual health | Yunfeng G, et al. [2017] | 3 [220] | 0 | 0 | 0 | −1④ | −1⑥ | Low |
| QoL | Yang B, et al. [2017] | 10 [841] | −1① | 0 | 0 | 0 | −1⑤ | Low |
| Ying M, et al. [2018] | 6 [554] | −1① | 0 | 0 | 0 | −1⑤ | Low | |
| Health-related QoL | ||||||||
| Teleni L, et al. [2016] | 5 [427] | 0 | 0 | 0 | 0 | −1⑤ | Moderate | |
| Ussing A, et al. [2022] | 4 [246] | 0 | −1② | 0 | −1④ | −1⑥ | Very low | |
| Disease-specific QoL | ||||||||
| Teleni L, et al. [2016] | 3 [271] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Ussing A, et al. [2022] | 12 [894] | 0 | 0 | 0 | 0 | −1⑤ | Moderate | |
| Exercise capacity | Bigaran A, et al. [2021] | |||||||
| The 400-m-walk test, s | 3 [222] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| 6-min walk test, m | 3 [180] | 0 | 0 | 0 | −1④ | −1⑥ | Low | |
| Diastolic blood pressure, mmHg | Bigaran A, et al. [2021] | 5 [357] | 0 | 0 | 0 | −1④ | 0 | Moderate |
| C-reactive protein, mg/L | Bigaran A, et al. [2021] | 3 [217] | 0 | 0 | −1③ | −1④ | −1⑥ | Very low |
①, most data are extracted from the studies at a moderate or high risk of bias, which have serious limitations in concealment, randomization, allocation and blinding; ②, few confidence intervals overlap, or the I2 values are relatively large (medium and high heterogeneity); ③, the population, intervention measures, and measurement outcomes in original studies are quite different, or the intervention measures cannot be directly compared; ④, the sample size is small; the confidence intervals are wide (the sample size of continuous variables <400, the sample size of binary variables <300); or the 95% CI crosses the invalid line; ⑤, the funnel diagram is asymmetry; ⑥, few studies are included, and the results are positive and may result in publication bias. BMI, body mass index; LBM, lean body mass; BFM, body fat mass; VO2, oxygen consumption; HDL, high-density lipoprotein; BMD, bone mineral density; QoL, quality of life.
Discussion
Summary of main findings
The current overview summarized available SRs/MAs on exercise training for PCa patients undergoing ADT to elucidate the effectiveness of exercise therapy. The quality of the included SRs/MAs was assessed. All eligible studies were published in the past 5 years, except 1 (35) published in 2016, 2 studies (31,37) published in 2017. The relative novelty of the articles suggests that using exercise training as an intervention method for PCa patients, especially those undergoing ADT treatment, has gained traction from clinical workers in recent years.
The methodological quality of the 8 included SRs/MAs was evaluated using the AMSTAR-2 tool. Three of the studies (31,34,35) were rated as moderate quality, 4 (30,33,36,37) were graded as very low quality, and 1 (32) was considered low quality. According to the ROBIS tool, the primary causes for a high risk of bias were an insufficient literature search, inappropriate study selection, unsuitable data collection and synthesis methods, and an inadequate discussion on the risk of bias. The high risk of bias may have made the current evidence less reliable. Based on the PRISMA checklist, we found that protocol and registration, risk of bias, additional analyses, and funding sources were not properly reported in the included reviews. The defects mentioned above may have affected the clarity and transparency of the included SRs/MAs.
Six articles (30-34,37) analyzed changes in body composition in PCa patients undergoing ADT, showing that exercise training could improve BMI (30), LBM (31,33,34), BFM (31,33,34), and BFR (30,31,33,34,37). Three articles (30,36,37) found that exercise training could significantly alleviate fatigue symptoms. The duration of exercise regime appears important, and Yunfeng et al. (31) highlighted that a longer-term exercise regime beyond 6 months significantly improve fatigue. Four articles (34-37) reported that exercise training could improve the QoL of patients. One article (35) showed that exercise training could relieve depression, while another (37) reported that exercise training did not improve depression. Three articles (30,32,35) suggested that exercise training significantly improved physical function. In addition, exercise training was also reported to improve physical changes (30,31), physical health (30), exercise capacity (31), blood pressure (31), and C-reactive protein (31). In short, exercise training could improve body composition (BMI, LBM, BFM, and BFR), physical function, QoL, and other factors. These findings are in line with earlier research. Exercise has been reported to bring psychological and physical benefits to PCa patients undergoing ADT, including relieving fatigue, reducing depression, enhancing cardiopulmonary function, and increasing muscle strength (45,46). These benefits may be correlated with the fact that exercise training can enhance the antioxidation system and reduce oxidative stress. Several studies have reported that oxidative stress and prooxidant–antioxidant imbalance play a key role in the development of PCa, which may be associated with increased ROS and damaged antioxidation systems (23,41,43,47-49). PCa patients undergoing ADT may produce a large amount of ROS, including superoxide anion (O2−), hydroxyl radical (OH−), and hydrogen peroxide (H2O2), destroying the balance between prooxidant and antioxidant enzymes and aggravating oxidative stress. Routine exercise can regulate redox signaling and enhance antioxidant defense, thus curbing the onset and development of PCa (39,40,42,44,50).
However, there were differences in the effect of exercise training on BMI, LBM, fatigue, depression, and cardiometabolic indexes. The main factors for the different results are as follows: (I) the number of included studies was small, and the sample size was insufficient; (II) because PCa patients are generally older, the influence of basic diseases such as diabetes, hypertension, and hyperlipidemia may have caused different results; and (III) the type, intensity, and duration of exercise training, as well as probability in PCa patients on ADT were inconsistent.
In addition, the differences in the outcomes are due to the low-quality methodologies and evidence in the included SRs/MAs. The AMSTAR-2 tool was used for the methodological quality assessment, and the included SRs/MAs were rated as either low or very low quality. The main reasons for the low quality were as follows: (I) some SRs/MAs did not list the excluded studies in detail or describe the rationality of the exclusion, which might have affected the credibility and transparency of the results; (II) except for 1 study, none of the included SRs/MAs investigated publication bias, and no funnel diagram (51) was drawn due to there being less than 10 included randomized controlled trials (RCTs); and (III) some studies failed to identify the funding sources or conflicts of interest, making it difficult to assess their impact on the results.
Eight cases of moderate-quality evidence (8/51, 15.69%) suggested that exercise training might help PCa patients on ADT prevent adverse events. Exercise training could reduce BFM and BFR in body composition, increase muscle strength, and improve QoL. Subgroup analysis found that AET and RET had better performance in improving adverse reactions. The credibility of other outcomes was impaired as a result of several limitations, most notably a lack of description of the randomization, allocation concealment, and blind methods in original RCTs, as well as a potential risk of selective reporting. In a word, the quality of evidence included in this overview was low.
Implications for future study
Our overview is the first to comprehensively focus on exercise training in PCa patients receiving ADT. The methodological quality of original studies was highly correlated with the overall quality of SRs/MAs. Therefore, a significant number of multicenter, large-scale RCTs on the effects of exercise training for PCa patients on ADT are required to increase the quality of evidence. In this overview, there was no unified standard for evaluating the efficacy of exercise training. Thus, future research should be based on the efficacy evaluation indicators that have been recognized in the guidelines, recommended by expert consensus, or accepted by experts in the industry, such as body composition, QoL, and BMD. In addition, longer follow-up is required in future research to investigate the long-term effect of exercise training on PCa men undergoing ADT. Also, some areas appear to be under-investigated in regard to a potential effect of physical exercise on non-cancer health aspects in those patients, mainly the sexual and mental health.
Additionally, given the observed relatively high rate of methodological concerns, we believe that SRs/MAs should be registered in advance. Protocols should be published to enhance the objectivity and authenticity of research, improve the level of documentary evidence, and reduce the risk of bias. AMSTAR-2, PRISMA, and ROBIS should be utilized to reduce subjective bias and improve research quality. Subgroup and sensitivity analyses should be conducted when there is high heterogeneity in the data. Moreover, funding sources and conflicts of interest in original studies should be described in detail to avoid the impact of researchers on the objective evaluation of evidence quality.
Limitations
This overview has some limitations. Firstly, inconsistent evaluation results in the methodological quality assessment may have been due to the low quality of the included literature, low credibility of evidence, and the different opinions of the researchers. Secondly, the languages of the included SRs/MAs were mainly Chinese and English. Lack of articles in other languages may have resulted in omission of included studies. Thirdly, the importance of exercise in advanced cancer has always been overlooked, and the few limited studies that attempt to evaluate this aspect could not committed to a homogenous clinical outcome, which was highlighted in the SR/MA. Moreover, this overview did not analyze the type, intensity, duration of exercise training, and stage of PCa patients undergoing ADT. Lastly, there was a risk of publication bias for a limited number of RCTs included in some SRs/MAs, and no funnel charts were drawn.
Conclusions
Exercise training is a potential adjunctive therapeutic strategy for PCa patients undergoing ADT. Nevertheless, due to a lack of high-quality evidence in this overview, more well-designed and large-scale studies are required to support our findings with more robust evidence.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81973866) and the Sichuan Provincial Administration of Traditional Chinese Medicine (No. 2021ZD016).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-272/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-272/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-272/coif). HK received support for attending a conference from Janssen Pharmaceuticals (2022), participated in an advisory board meeting: Ferring Pharmaceuticals (2021), Janssen Pharmaceuticals (2022) and received honorarium from Recordati for a presentation (2021). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014;65:467-79. [Crossref] [PubMed]
- Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;339:1036-42. [Crossref] [PubMed]
- Rosario DJ, Davey P, Green J, et al. The role of gonadotrophin-releasing hormone antagonists in the treatment of patients with advanced hormone-dependent prostate cancer in the UK. World J Urol 2016;34:1601-9. [Crossref] [PubMed]
- Albertsen P. Androgen deprivation in prostate cancer--step by step. N Engl J Med 2009;360:2572-4. [Crossref] [PubMed]
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238-44. [Crossref] [PubMed]
- Herr HW, O'Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol 2000;163:1743-6. [Crossref] [PubMed]
- Edmunds K, Tuffaha H, Galvão DA, et al. Incidence of the adverse effects of androgen deprivation therapy for prostate cancer: a systematic literature review. Support Care Cancer 2020;28:2079-93. [Crossref] [PubMed]
- Siebert AL, Lapping-Carr L, Morgans AK. Neuropsychiatric Impact of Androgen Deprivation Therapy in Patients with Prostate Cancer: Current Evidence and Recommendations for the Clinician. Eur Urol Focus 2020;6:1170-9. [Crossref] [PubMed]
- Kim DK, Lee HS, Park JY, et al. Androgen-deprivation therapy and the risk of newly developed fractures in patients with prostate cancer: a nationwide cohort study in Korea. Sci Rep 2021;11:10057. [Crossref] [PubMed]
- Challa AA, Calaway AC, Cullen J, et al. Cardiovascular Toxicities of Androgen Deprivation Therapy. Curr Treat Options Oncol 2021;22:47. [Crossref] [PubMed]
- DE Nunzio C. Androgen deprivation therapy and cardiovascular risk in prostate cancer. Minerva Urol Nephrol 2022;74:508-17. [PubMed]
- Reiss AB, Saeedullah U, Grossfeld DJ, et al. Prostate cancer treatment and the relationship of androgen deprivation therapy to cognitive function. Clin Transl Oncol 2022;24:733-41. [Crossref] [PubMed]
- Lonergan PE, Washington SL 3rd, Cowan JE, et al. Androgen Deprivation Therapy and the Risk of Dementia after Treatment for Prostate Cancer. J Urol 2022;207:832-40. [Crossref] [PubMed]
- Izard JP, Siemens DR. Androgen Deprivation Therapy and Mental Health: Impact on Depression and Cognition. Eur Urol Focus 2020;6:1162-4. [Crossref] [PubMed]
- Cormie P, Zopf EM. Exercise medicine for the management of androgen deprivation therapy-related side effects in prostate cancer. Urol Oncol 2020;38:62-70. [Crossref] [PubMed]
- Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003;21:1653-9. [Crossref] [PubMed]
- Galvão DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340-7. [Crossref] [PubMed]
- Buffart LM, Galvão DA, Brug J, et al. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 2014;40:327-40. [Crossref] [PubMed]
- Campos C, Sotomayor P, Jerez D, et al. Exercise and prostate cancer: From basic science to clinical applications. Prostate 2018;78:639-45. [Crossref] [PubMed]
- Gillessen S, Armstrong A, Attard G, et al. Management of Patients with Advanced Prostate Cancer: Report from the Advanced Prostate Cancer Consensus Conference 2021. Eur Urol 2022;82:115-41. [Crossref] [PubMed]
- Kumar B, Koul S, Khandrika L, et al. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 2008;68:1777-85. [Crossref] [PubMed]
- Jakubczyk K, Dec K, Kałduńska J, et al. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski 2020;48:124-7. [PubMed]
- Kalinina EV, Gavriliuk LA, Pokrovsky VS. Oxidative Stress and Redox-Dependent Signaling in Prostate Cancer. Biochemistry (Mosc) 2022;87:413-24. [Crossref] [PubMed]
- Gomes MJ, Martinez PF, Pagan LU, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget 2017;8:20428-40. [Crossref] [PubMed]
- de Sousa CV, Sales MM, Rosa TS, et al. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med 2017;47:277-93. [Crossref] [PubMed]
- Camiletti-Moirón D, Aparicio VA, Aranda P, et al. Does exercise reduce brain oxidative stress? A systematic review. Scand J Med Sci Sports 2013;23:e202-12. [Crossref] [PubMed]
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [Crossref] [PubMed]
- Whiting P, Savović J, Higgins JP, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Yunfeng G, Weiyang H, Xueyang H, et al. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: An update meta-analysis. Medicine (Baltimore) 2017;96:e7368. [Crossref] [PubMed]
- Bigaran A, Zopf E, Gardner J, et al. The effect of exercise training on cardiometabolic health in men with prostate cancer receiving androgen deprivation therapy: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2021;24:35-48. [Crossref] [PubMed]
- Chen Z, Zhang Y, Lu C, et al. Supervised Physical Training Enhances Muscle Strength but Not Muscle Mass in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Systematic Review and Meta-Analysis. Front Physiol 2019;10:843. [Crossref] [PubMed]
- Shao W, Zhang H, Qi H, et al. The effects of exercise on body composition of prostate cancer patients receiving androgen deprivation therapy: An update systematic review and meta-analysis. PLoS One 2022;17:e0263918. [Crossref] [PubMed]
- Teleni L, Chan RJ, Chan A, et al. Exercise improves quality of life in androgen deprivation therapy-treated prostate cancer: systematic review of randomised controlled trials. Endocr Relat Cancer 2016;23:101-12. [Crossref] [PubMed]
- Ussing A, Mikkelsen MK, Villumsen BR, et al. Supervised exercise therapy compared with no exercise therapy to reverse debilitating effects of androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2022;25:491-506. [Crossref] [PubMed]
- Yang B, Wang J. Effects of Exercise on Cancer-related Fatigue and Quality of Life in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Meta-analysis of Randomized Clinical Trials. Chin Med Sci J 2017;32:13-21. [Crossref] [PubMed]
- Ying M, Zhao R, Jiang D, et al. Lifestyle interventions to alleviate side effects on prostate cancer patients receiving androgen deprivation therapy: a meta-analysis. Jpn J Clin Oncol 2018;48:827-34. [Crossref] [PubMed]
- Rebillard A, Lefeuvre-Orfila L, Gueritat J, et al. Prostate cancer and physical activity: adaptive response to oxidative stress. Free Radic Biol Med 2013;60:115-24. [Crossref] [PubMed]
- Peres A, Branchini G, Marmett B, et al. Potential Anticarcinogenic Effects From Plasma of Older Adults After Exercise Training: An Exploratory Study. Front Physiol 2022;13:855133. [Crossref] [PubMed]
- Pavan ICB, Basei FL, Severino MB, et al. NEK6 Regulates Redox Balance and DNA Damage Response in DU-145 Prostate Cancer Cells. Cells 2023;12:256. [Crossref] [PubMed]
- Zheng Q, Cui G, Chen J, et al. Regular Exercise Enhances the Immune Response Against Microbial Antigens Through Up-Regulation of Toll-like Receptor Signaling Pathways. Cell Physiol Biochem 2015;37:735-46. [Crossref] [PubMed]
- Rago V, Di Agostino S. Novel Insights into the Role of the Antioxidants in Prostate Pathology. Antioxidants (Basel) 2023;12:289. [Crossref] [PubMed]
- Nomikos NN, Nikolaidis PT, Sousa CV, et al. Exercise, Telomeres, and Cancer: "The Exercise-Telomere Hypothesis". Front Physiol 2018;9:1798. [Crossref] [PubMed]
- Bourke L, Smith D, Steed L, et al. Exercise for Men with Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016;69:693-703. [Crossref] [PubMed]
- Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 2014;32:335-46. [Crossref] [PubMed]
- Khandrika L, Kumar B, Koul S, et al. Oxidative stress in prostate cancer. Cancer Lett 2009;282:125-36. [Crossref] [PubMed]
- Ripple MO, Henry WF, Rago RP, et al. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst 1997;89:40-8. [Crossref] [PubMed]
- Costanzo-Garvey DL, Case AJ, Watson GF, et al. Prostate cancer addiction to oxidative stress defines sensitivity to anti-tumor neutrophils. Clin Exp Metastasis 2022;39:641-59. [Crossref] [PubMed]
- Brown M, Rébillard A, Hart NH, et al. Modulating Tumour Hypoxia in Prostate Cancer Through Exercise: The Impact of Redox Signalling on Radiosensitivity. Sports Med Open 2022;8:48. [Crossref] [PubMed]
- Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.


