
A pilot study of inflatable penile prosthesis placement in transgender neophallus using Tutoplast® pericardium graft sock technique
Highlight box
Surgical Highlights
• We detail our experience utilizing a human cadaveric pericardium graft in a proximal “sock” and distal “cap” technique in IPP placement in a transgender neophallus. Both patients report continued satisfaction with the prosthesis and ability to perform penetrative intercourse with the device activated at time of last follow-up and/or communication with patients, which is currently 14 months and 23 months, respectively.
What is conventional and what is novel/modified?
• While the utilization of a graft is quite common in transgender neophallus IPP placement to account for the anatomical differences versus those of cis-gender patients, there is no clear consensus on grafting technique.
• In this study we detail our experiences using a grafting approach with human cadaver pericardium graft (Tutoplast) in a proximal “sock” and distal “cap” technique in IPP placement in a transgender neophallus.
What is the implication, and what should change now?
• The lack of published literature on grafting techniques indicates a need for continued research in optimizing graft selection, placement, and overall IPP outcomes.
IntroductionOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
Gender-affirming surgery enables transgender individuals to align their physical appearance with their gender identity. There are several components to gender-affirming surgery including the construction of a phallus (neophallus) and scrotum, often the definitive step for individuals assigned male at birth (1). Following bottom surgery with neophallus creation in female-to-male gender reassignment, patients often desire placement of an inflatable penile prosthesis (IPP) for neophallus erectile function.
Traditionally, the IPP implants used in cis-males are also employed in genital gender affirming surgery (2). However, there are unique challenges in the transgender population, with one of the most significant being the lack of anatomical corpus cavernosum and crura for cylinder placement and anchoring. To account for this, multiple grafting approaches and materials have been utilized in IPP neophallus placement with the goal of facilitating cylinder anchoring and stability (1). Cylinder coverage with graft material has been shown to help reduce rates of percutaneous cylinder erosion in a neophallus and research is ongoing in this regard (3). Previous literature on neophallus IPP implantation and rates of complications highlight a need for continued research regarding mitigating infection and erosion in this cohort (2). Here we detail our experiences using a grafting approach with human cadaver pericardium graft in a “sock” and “cap” technique in IPP placement in a transgender neophallus. We present this article in accordance with the SUPER reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-837/rc).
Pre-operative preparations and requirementsOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
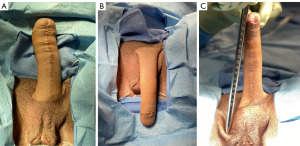
The pre-operative preparation for IPP placement in a neophallus generally aligns with that of our cis-gender IPP placements, but with unique considerations. At our institution, patients must be at least one-year post-phalloplasty prior to IPP placement. One of our patients had phalloplasty done via a radial forearm flap. The other originally underwent phalloplasty via a left radial forearm flap at an outside institution that failed with subsequent successful revision done via a right musculocutaneous latissimus dorsi flap, also at the outside institution. The patient’s original operative report for their phalloplasty is then subsequently reviewed by our team in order to confirm side of vascular pedicles and anastomosis, which will guide our approach to the opposite side for initial incision. We prefer utilizing the Titan Genesis pump (Coloplast Corp., Minneapolis, MN, USA) for placement in the neoscrotum and a single Coloplast Titan® cylinder for placement in the neophallus. We also ensure that a 6×6 cm human cadaver pericardium graft (Tutoplast®) is available. Pre-operative measurement of the neophallus from inferior pubic bone to glans is also conducted to estimate the required cylinder length (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of UTHealth – Houston (IRB # HSC-MS-19-0320). Written informed consent was obtained from the patients for publication of this surgical techniques report and all accompanying operative images. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step descriptionOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
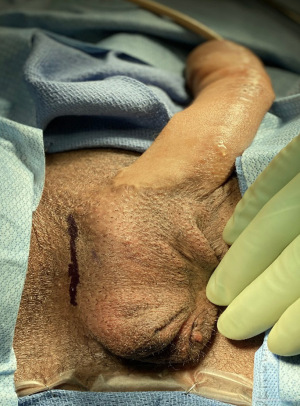
The procedure begins with our usual sterile preparation and set-up, which follows similarly to that of previously published surgical techniques in transgender IPP placements (4). This includes preoperative intravenous antibiotics, shaving of patient hair with clippers, placement of a 14 French foley catheter, and surgical site preparation with chlorhexidine gluconate (CHG) 2% and isopropyl alcohol 70% (ChloraPrep; Becton, Dickinson and Company; Franklin Lakes, NJ, USA). The device is soaked before implantation in a sterile solution containing a mixture of antibiotics including trimethoprim (Bactrim), gentamicin, and polymixin B (5). We maintain a “no-touch” technique during insertion of the device as well as limiting the time the device is exposed to air (6). IPP placement begins with a 3–4 cm vertical incision to the opposite side of the neophallus that has been previously used for vascular pedicles and anastomosis (Figure 2). This is more commonly known as a groin crease incision, made between the lateral edge of the scrotum and medial border of the thigh allowing for optimal access to anterior face of pubis (7). Of note, there have been various approaches reported in transgender IPP placement, with the infrapubic approach more commonly utilized in present-day then the parascrotal approach described here, which is not a novel approach and has been previously described as well (1,8-10). The decision regarding approach can vary due to several factors including patient surgical history, type of device, and surgeon preference (11). This incision is then carried down to the pubic bone, and once identified, three 0-Fibrewire stay-sutures are placed (Figure 3). Two stay-sutures are placed at the superior pubic symphysis on both sides, and a third one in the inferior portion of the pubic bone on the same side of the incision. Next, Brooks dilators (Coloplast Corp., Minneapolis, MN, USA) are used starting with a 9 mm dilator and progressed up to a 14 mm to dilate a tract up the dorsal penis. Care is taken to avoid the neourethra and to remain 1 cm proximal to the head of the penis. Particular care is also taken to ensure dilation is carried midline. Irrigation is then performed to ensure no injury to the neourethra.
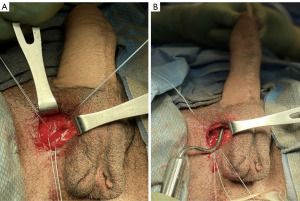
Following dilation, reservoir placement can be done either prior to or following cylinder placement. We utilized a commonly used submuscular placement of a 75 cc reservoir filled with 50 cc sterile of saline since we only used one cylinder.
Next, prior to placing the penile implant cylinder, Tutoplast® pericardium grafts were placed at both the proximal and distal ends of the cylinder forming a proximal “sock” and distal “cap” around the implant (Figure 4). The distal cap is used to minimize risk of distal cylinder erosion. We used a 6×6 cm graft for the “cap” and a similarly sized graft for the “sock”, which will then leave the body of the cylinder exposed. The implant is placed into the previously dilated track using a Furlow introducer (Figure 5). Additionally, with the differences in anatomy in a transgender neophallus versus a cis-gender IPP placement, we purposefully leave about 1 cm of neo-glans to prevent penile prosthesis protrusion which is a common complication in this cohort of patients (1,10,12). Using the three stay-sutures previously placed at the pubic bone, the proximal cylinder is anchored to the bone with sutures passing through it and the proximal Tutoplast® graft “cap” as well. The lower lateral suture provides stability and correct angle of the phallus when erect and the upper lateral and medial sutures are vital in maintaining length of the cylinder within the phallus (7). Care should be taken to avoid anchoring the cylinder to the adductor tendon.
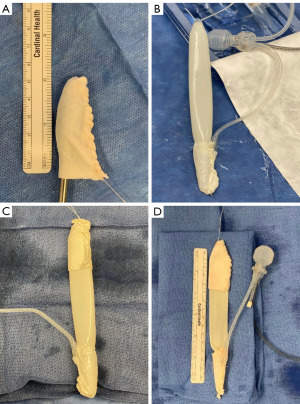
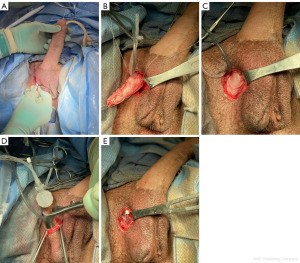
A dartos pocket is made in the neoscrotum and the pump is then placed (Figure 5). The tubing to the reservoir is then connected using the provided connector tools. Following this, the implant is cycled to check the function and position. The tissue overlying the incision is then closed in standard fashion with 3-0 Vicryl for dartos and 4-0 Monocryl for skin.
Postoperative considerations and tasksOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
Post-operatively, patients are managed per our standard IPP protocol. The implant is left 70–80% inflated until the 6–8 weeks post operative appointment. The patients are instructed not to engage in sexual intercourse or manipulate the pump during this time. Initial follow-up is scheduled at 6–8 weeks post operatively for device activation and teaching and then subsequent follow-up is at 6 months and then as needed (Figures 6,7). However, given that these patients were both geographically located outside of the general area of our institution, in-person follow-up times varied from our usual IPP protocol in conjunction with patient availability to travel to our institution.
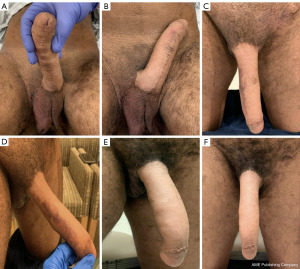
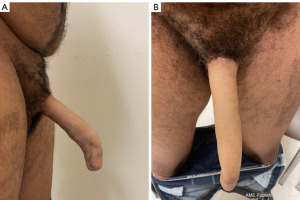
Patients had no complaints post-operatively, with cylinder and pump found to be in good position and functioning well at first post-operative visit 6–8 weeks following surgery. There was no difficulty with urinating through the neophallus. Both patients report continued satisfaction with the prosthesis as well as ability to perform penetrative intercourse with the device activated at time of last follow-up and/or communication with patients, which is currently 14 and 23 months, respectively. One patient noted occasional mild pain in neophallus that self-resolves and does not cause any concern and has been very pleased with the device and ease of use.
DiscussionOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
Consensus on the most appropriate surgical technique for neophallus IPP placement is lacking. This includes types of grafting material used as well as extent of cylinder coverage with graft. Much of the research regarding IPP placement in transgender neophalluses are single-center series describing surgical techniques versus comparison of methods and outcomes (1). Our goal in this pilot study is not to compare our experience here to other transgender IPP placements, but more so to publish what is to our understanding the first such description of Tutoplast® graft being used in this operation. Our approach is similar to others previously published utilizing the proximal and distal graft caps. Given the unique considerations required in transgender IPP placement versus cis-gender placement, research regarding various surgical approaches and techniques are vital. Trans-specific anatomical variations can lead to a high rate of post-implant complications, especially when compared to that of cis-gender IPP placement. An example of such anatomical variations includes the lack of anatomical corpus cavernosum and crura for cylinder placement and anchoring. This can create complications when proximal fixation is done via simple placement of the cylinder against the pubic bone and allowing a natural capsule to form, leading to instability during sexual intercourse. A recent systematic review of IPP complications in transgender surgery found overall complication rates of 36.2%, with inflatable device complications rates reaching 45.2%, respectively (8). Specific complications including cylinder malposition or migration, infection, or erosion also exist at high rates in this group (8).
We utilized Tutoplast®, a cadaveric pericardium allograft tissue, versus Dacron or another surgical mesh for this operation. Tutoplast® has a wide variety of documented clinical applications including extensive use in surgical correction of Peyronie’s Disease penile curvature with generally satisfactory results (13). Given the high rate of complications in transgender IPP placement, there is an increased risk of requiring device revision, with reports varying from 14% up to 80%, although a recent meta-analysis finds that number likely being closer to the lower-end of that range (8,10,14-16). The drawbacks of a grafting material such as Dacron or PTFE, albeit in Peyronie’s Disease research, include possible increased risk of fibrosis (17-19). In our experience, we have found that the level of fibrosis with Dacron makes IPP revision surgery more difficult, although it is important to note this is anecdotal. Tutoplast®, a processed human pericardium graft, is easily accessible to us at our institution and we have been satisfied with our results when utilizing it in cis-gender IPP placements. Thus, we sought to pilot utilizing this graft in our transgender IPP placements. While published research does show satisfactory results in utilization of other graft materials, the relative paucity of literature on this technique invites further study with other possible grafting approaches, which we sought to do here.
In selecting extent of cylinder coverage with grafting materials, we used a previously described distal and proximal “cap” and “sock” graft approach (12). In general, utilizing grafts at the base and tip of the cylinders as a means for preventing erosion is still in need of long-term follow-up and study. Solely proximal graft coverage with securement of the graft and cylinder to the pubis has been one reported approach, including with polyethelene terephthalate (Dacron) vascular graft and Hemashield Gold (Maquet, Rastatt, Germany), a similar polyester grafting material (1). A recent study utilizing allograft material at the distal end of the cylinder in neophallus placement appears to reduce erosion and infection rates with promising initial findings (20). Other approaches describe covering the entire cylinder to create a neotunica using polytetrafluoroethylene (GORE-TEX®, Gore Medical, Flagstaff, AZ, USA) (1). The latter approach has been described as providing neophallus alignment and proximal cylinder securement, but follow-up studies report possible constraint on the cylinder during inflation, leading to cylinder aneurysm and increased failure rates (12,21). However, in a retrospective analysis of IPP placements in a dedicated transgender surgery unit utilizing a similar approach of covering the entire cylinder with vascular graft, complication rates appear to be below that of a meta-analysis of aggregate complication rates in IPP placements in phalloplasty patients (4).
Increased risks of infection, inflammation and fibrosis with synthetic grafts are always concerning in prosthetic surgeries. This is particularly notable as a needed future avenue of study on this topic. It is important to understand ease of explantation with various grafting materials should need arise for removal and revision of device, which is unfortunately not an uncommon occurrence in transgender neophallus IPP placements (2). Traumatic removal of a device may induce damage to the patient’s flap, and possibly perfusion disorders in the flap.
In future studies a detailed look at various graft costs and comparison of cost passed on to patients is worthy of investigation as well as this is an essential aspect of patient access to such operations.
One limitation in this surgical technique paper presented here is that our follow-up was limited by the recency of these operations as well as limited sample size. The first patient underwent the procedure in mid-2021 and the second in early 2022. However, with follow-up now extending to almost two years post-IPP placement in our initial patient, the preliminary findings do appear promising. In the future we hope to have an increased sample size of patients that undergo placement via this technique and gathering longer-term data. Coupled with longer follow-up time periods, this will help elucidate whether this grafting technique be one that is safe, effective, and possibly more frequently adopted in transgender neophallus IPP placement.
An additional limitation includes objective assessment of satisfaction and outcomes in these patients. While validated surveys exists for assessment of sexual satisfaction following IPP placement, there does not appear to be any transgender specific questionnaires at this time (22). Both patients expressed satisfaction at follow-up visits with ability to perform penetrative intercourse and activate/deactivate device with no issues. Assessment of other metrics such as ability to urinate were also subjectively assessed, of which neither patient reported any concern. Future studies with an increased patient sample size will be useful in not only assessing outcomes and satisfaction more objectively but to also compare to these respective rates in cis-gender IPP patients.
Additionally, in comparison to cis-gender IPP results, the post-operative image in Figure 7 may appear to show results similar to that of a supersonic transporter deformity (SST), however, we purposefully left 1 cm of neo-glans distally to prevent device protrusion and the patient was extremely satisfied. As mentioned above, the patient was also able to perform penetrative intercourse without issue.
ConclusionsOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
A penile prosthesis device constructed specifically for transgender patients currently does not exist in the United States although developments and trials with trans phalloplasty prosthetics are ongoing (23). Therefore, grafting techniques, such as the one described here, which account for the unique anatomic differences in the transgender population continue remain a vital part of optimizing neophallus IPP outcomes and satisfaction among currently available options (24,25).
AcknowledgmentsOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-837/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-837/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-837/coif). RW is a consultant for Boston Scientific and Teleflex. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of UTHealth – Houston (IRB # HSC-MS-19-0320). Written informed consent was obtained from the patients for publication of this surgical techniques report and all accompanying operative images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Pre-operative preparations and requirements
- Step-by-step description
- Postoperative considerations and tasks
- Discussion
- Conclusions
- Acknowledgments
- Footnote
- References
- Kang A, Aizen JM, Cohen AJ, et al. Techniques and considerations of prosthetic surgery after phalloplasty in the transgender male. Transl Androl Urol 2019;8:273-82. [Crossref] [PubMed]
- Polchert M, Dick B, Raheem O. Narrative review of penile prosthetic implant technology and surgical results, including transgender patients. Transl Androl Urol 2021;10:2629-47. [Crossref] [PubMed]
- Segal RL, Massanyi EZ, Gupta AD, et al. Inflatable penile prosthesis technique and outcomes after radial forearm free flap neophalloplasty. Int J Impot Res 2015;27:49-53. [Crossref] [PubMed]
- Briles BL, Middleton RY, Celtik KE, et al. Penile Prosthesis Placement by a Dedicated Transgender Surgery Unit: A Retrospective Analysis of Complications. J Sex Med 2022;19:641-9. [Crossref]
- Wilson SK, Salem EA, Costerton W. Anti-infection dip suggestions for the Coloplast Titan Inflatable Penile Prosthesis in the era of the infection retardant coated implant. J Sex Med 2011;8:2647-54. [Crossref] [PubMed]
- Eid JF, Wilson SK, Cleves M, et al. Coated implants and "no touch" surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology 2012;79:1310-5. [Crossref] [PubMed]
- Blecher GA, Christopher N, Ralph DJ. Prosthetic Placement After Phalloplasty. Urol Clin North Am 2019;46:591-603. [Crossref] [PubMed]
- Rooker SA, Vyas KS, DiFilippo EC, et al. The Rise of the Neophallus: A Systematic Review of Penile Prosthetic Outcomes and Complications in Gender-Affirming Surgery. J Sex Med 2019;16:661-72. [Crossref] [PubMed]
- Morrison SD, Chen ML, Crane CN. An overview of female-to-male gender-confirming surgery. Nat Rev Urol 2017;14:486-500. [Crossref] [PubMed]
- Hoebeke PB, Decaestecker K, Beysens M, et al. Erectile implants in female-to-male transsexuals: our experience in 129 patients. Eur Urol 2010;57:334-40. [Crossref] [PubMed]
- Levine LA, Becher EF, Bella AJ, et al. Penile Prosthesis Surgery: Current Recommendations From the International Consultation on Sexual Medicine. J Sex Med 2016;13:489-518. [Crossref] [PubMed]
- Falcone M, Garaffa G, Gillo A, et al. Outcomes of inflatable penile prosthesis insertion in 247 patients completing female to male gender reassignment surgery. BJU Int 2018;121:139-44. [Crossref] [PubMed]
- Garcia-Gomez B, Ralph D, Levine L, et al. Grafts for Peyronie's disease: a comprehensive review. Andrology 2018;6:117-26. [Crossref] [PubMed]
- Neuville P, Morel-Journel N, Cabelguenne D, et al. First Outcomes of the ZSI 475 FtM, a Specific Prosthesis Designed for Phalloplasty. J Sex Med 2019;16:316-22. [Crossref] [PubMed]
- Cohen AJ, Bhanvadia RR, Pariser JJ, et al. Novel Technique for Proximal Bone Anchoring of Penile Prosthesis After Radial Forearm Free Flap Neophallus. Urology 2017;105:2-5. [Crossref] [PubMed]
- Hage JJ. Dynaflex prosthesis in total phalloplasty. Plast Reconstr Surg 1997;99:479-85. [Crossref] [PubMed]
- Yafi FA, Pinsky MR, Sangkum P, et al. Therapeutic advances in the treatment of Peyronie's disease. Andrology 2015;3:650-60. [Crossref] [PubMed]
- Levine LA, Burnett AL. Standard operating procedures for Peyronie's disease. J Sex Med 2013;10:230-44. [Crossref] [PubMed]
- Kadioglu A, Sanli O, Akman T, et al. Graft materials in Peyronie's disease surgery: a comprehensive review. J Sex Med 2007;4:581-95. [Crossref] [PubMed]
- Harris KT, Wu WJ, Manyevitch R, et al. Outcomes of inflatable penile prosthesis insertion using a neotunica allograft in neophalluses of patients on the bladder exstrophy-epispadias complex spectrum. J Pediatr Urol 2020;16:659.e1-6. [Crossref] [PubMed]
- Hoebeke P, de Cuypere G, Ceulemans P, et al. Obtaining rigidity in total phalloplasty: experience with 35 patients. J Urol 2003;169:221-3. [Crossref] [PubMed]
- Salter CA, Bach PV, Jenkins L, et al. Development and Validation of the Satisfaction Survey for Inflatable Penile Implant (SSIPI). J Sex Med 2021;18:1641-51. [Crossref]
- Preto M, Blecher G, Timpano M, et al. The Frontier of Penile Implants in Phalloplasty: Is the ZSI 475 FTM what we have been waiting for? Int J Impot Res 2020;33:779-83. [Crossref] [PubMed]
- Neuville P, Terrier JE, Paparel P, et al. 159 Preliminary study of a specific penile prosthesis conceived for phalloplasty, the ZSI 475 FtM. J Sex Med 2018;15:S197. [Crossref]
- Blewniewski M, Ostrowski I, Pottek T, et al. Safety and Efficacy Outcomes of ZSI 475 Penile Prosthesis. Urologia 2017;84:98-101. [Crossref] [PubMed]


