
A study of the histopathology of collagen fleece (TachoSil) patching of tunica albuginea in the rat penis and a literature review of penile graft materials in experimental animals
Highlight box
Key findings
• TachoSil patching of penile tunica albuginea (TA) defect forms a distinctive barrier against inflammation, protecting the underlying corpus cavernosum (CC). However, no regeneration of the tunica defect is observed at 2 months in experiments in rats.
What is known and what is new?
• TachoSil patch is used in patients to bridge gaps in TA during surgery for Peyronie’s disease with or without penile prosthesis implantation. However, the histopathological changes in the defect and the underlying CC are unknown.
• Unlike other reported materials, a complete regeneration of TA is not seen. However, TachoSil protects the underlying CC from inflammation.
What is the implication, and what should change now?
• Using TachoSil to cover gaps in TA during penile surgery has the advantage of protecting the underlying CC. However, a better understanding of the mechanism TachoSil sustains erection without a penile prosthesis is needed.
Introduction
A penile tunica albuginea (TA) defect may arise during penile prosthesis surgery or reconstruction of the penis in patients with Peyronie’s disease (PD). Different free grafts may cover the defect in a patient with and without erectile dysfunction resulting in variable degrees of success (1-3). Of-the-shelf grafts are attractive because they are readily available and avoid donor site morbidity. These graft materials’ most significant clinical outcome benchmarks are maintained erection, avoidance of recurrence curvature, and avoidance of shortening due to graft fibrosis. In experimental animals, the fate of the graft varied from degeneration, inflammation, and fibrosis, on one side of the spectrum to complete regeneration of TA-like tissue replacement on the other (Table 1). An alternate graft is a collagen fleece covered with thrombin and fibrinogen reported in conjunction with surgical treatment of PD (31,32). TachoSil has gained popularity for patients with good erection or who need a penile prosthesis (1,33,33-38). The use of TachoSil had distinct advantages of shelf availability, self-adhesion property, hemostatic effect, no need for suturing, shortening of the procedure time and avoidance of needle puncture of the penile prosthesis (39). The application was adapted for plaque incision or partial or complete excision with and without prosthesis implantation.
Table 1
| Study | Graft | Setting | Animal | Duration | Histology of graft | Erection | Cavernosography |
|---|---|---|---|---|---|---|---|
| Leungwattanakij (4), 2003 | Cadaveric pericardium | Cadaveric pericardium vs. vein | Rat | 4 months | Penile fibrosis is significantly higher with pericardium than with vein or control | NA | NA |
| Leungwattanakij (5), 2003 | Cadaveric pericardium | Cadaveric pericardium vs. dermis, vein, or Gore-Tex | Rat | 6 months | Minimal fibrosis for all grafts but moderate to severe with Gore-Tex | No difference of nerve induced erection among groups | NA |
| Leungwattanakij (6), 2000 | Cadaveric pericardium | Graft vs. control | Rat | 4 months | Fibrosis under graft, no graft replacement with normal TA | Comparable to controls | NA |
| Stefanovic (7), 1994 | Fascia | Temporoparietal fascia; vascularized vs. non-vascularized graft | Rat | 3 months | Secondary degeneration in all non-vascularized and one-third of vascularized graft | Strait erections | NA |
| Hafez (8), 2004 | Fibrin glue | Cover a 5 mm × 15 mm tunica defect | Rabbit | 12 weeks | Complete regeneration of TA | Straight pharmacologic-induced erection | No narrowing or venous leakage |
| Eberli (9), 2007 | Matrix | Acellular bladder matrix grafts | Rabbit | 3 months | Biomechanically compatible tunica substitution | maintenance of normal intracavernous pressure | normal anatomy |
| Joo (10), 2006 | Matrix | Acellular porcine bladder matrix vs. normal controls | Rabbit | 6 months | No significant histological differences between the implanted tunica and the normal control tunica | NA | NA |
| Shokeir (11), 2004 | Matrix | TA acellular matrix; cover a 30 mm × 10 mm tunica defect | Dog | 6 months | Regeneration of tunica histologically similar to normal tunica | Straight, rigid pharmacologic-induced erection | Patent corpora cavernosa |
| Wefer (12), 2002 | Matrix | Acellular matrix; penile reconstruction | Rabbit | 6 months | No difference between natural and regenerated tunica | Papaverine erection in 15/18 animals, 11/18 rigid and straight | Four penile deviations |
| Ferretti (13), 2012 | Polyglycolic acid | Scaffold vs. scaffold seeded with autologous fibroblast vs. autograft vs. sham | Rat | 4 months | Seeded TA displayed higher collagen density and preserved but small sinusoidal spaces; outer layer longitudinal reorganization and external collagen bundles have the same spatial orientation as the original | Significantly less than controls | NA |
| Bazeed (14), 1983 | Polyglycolic acid | Mesh | Dog | 6 months | Replacement by fibrous connective tissue | NA | No curvature of the penis, bulging, or obstruction |
| Blumenfrucht (15), 1983 | Polytetrafluoroethylene | Replacement of segmentally excised TA | Dog | – | None had an inflammatory reaction | – | – |
| Gundogdu (16), 2021 | Silk fibroin scaffold | Silk fibroin scaffold, SIS, TV vs. nonsurgical controls | Rabbit | 3 months | Fibroin scaffold, SIS: neo tissue similar to control TA; SIS and TV: the corpus cavernosum showed significant fibrosis and loss of smooth muscle cells | TV and fibroin scaffold: 80% of animals had a full pharmacologic erection; SIS: only 40% | No leak in all grafted. |
| Ma (17), 2012 | SIS | SIS vs. SIS seeded with stem cells | Rat | 8 weeks | Mild fibrosis around the graft and the elastic fibers of the graft were orientated in two layers similar to the adjacent TA | Seeded grafts were comparable to controls | NA |
| Leungwattanakij (18), 2006 | SIS | Penile enhancement | Rat | 2 months | Replaced with circularly oriented elastic fibers with minimal fibrosis under the graft | NA | NA |
| El-Assmy (19), 2004 | SIS | SIS vs. TV flap | Rabbit | 12 weeks | Replaced by normal TA-like tissue | Straight, rigid pharmacologic-induced erection | Intact veno-occlusive mechanism |
| Hafez (20), 2004 | SIS | SIS 1 layer vs. 4-layer graft | Rabbit | 12 weeks | One layer of SIS graft was entirely replaced by tissue similar to normal TA with no inflammatory infiltrate; 4-layer graft showed dense fibrosis, chronic inflammation, and focal calcification | NA | NA |
| Kropp (21), 2002 | SIS | SIS graft vs. autologous tunica graft | Rat | 6 months | Complete integration and histological similarity to autologous tunica graft | No graft ballooning or expansion was noted with pharmacologically induced erection | NA |
| Monga (22), 2002 | SIS | SIS vs. sham | Rabbit | 45 days | Minimal inflammation, and no cavernous smooth muscle loss or fibrosis underneath the graft | Preservation of pharmacologic erection | NA |
| El-Assmy (23), 2007 | Surgicel | Cover a 15 mm × 5 mm tunica defect | Rabbit | 3 months | Complete replacement of the defect by regenerated tunica, absorption of the Surgicel | Straight and rigid erection | straight erection with patent corpora and no evidence of narrowing or a venous leak |
| Sansalone (24), 2017 | TA | Allotransplantation | Rat | 6 months | Biointegration with surrounding TA | NA | NA |
| Ferretti (25), 2014 | TA | Autograft vs. allograft | Rat | 12 weeks | Penile curvature 25 degrees, decreased elastin fiber length, intense scarring and cartilage formation, chronic inflammation, and localized osteogenesis | Erection maintained below 12 weeks | NA |
| Seyam (26), 2007 | TA | Autologous crura TA | Baboon | 12 months | Graft was indistinguishable from the adjacent tunica with no fibrosis | No change in pressure | Venous leak in 2/8, angulation of penis in 4/8 animals |
| Bagbanci (27), 2015 | TV | TV or rectus muscle fascia; penile enhancement | Rat | 2 months | Complete resorption due to lack of vascularization | NA | NA |
| Hafez (28), 2001 | TV | Compare TV graft vs. TV flap | Rabbit | 12 weeks | Contracted by a mean of 42%, fibrosis | NA | NA |
| Brannigan (29), 1998 | Vein | Dorsal vein vs. silicon vs. skin | Dog | 3 months | Reformation of tunica, moderate fibrosis, loss of typical venous architecture | Comparable to preoperative pressures | NA |
| Brock (30), 1993 | Vein | Femoral vein vs. control | Rat | 6 months | A return of near normal tunica structure with areas of mild fibrosis | Comparable to controls | NA |
TA, tunica albuginea; NA, not available; SIS, small intestinal submucosa; TV, tunica vaginalis.
TachoSil is a fibrin sealant patch indicated as an adjunct to hemostasis during bleeding control by standard surgical technique (40). It consists of human fibrinogen and human thrombin coated onto an equine patch.
The histopathological consequences of the TachoSil patch to cover corpus cavernous tunica defects is unknown. The characteristics of resultant scar, risk of colonization, neovascularization, promotion of tunica regeneration, or changes in the corpus cavernosum tissue are unknown. However, all these factors may explain the repair outcome regarding corporal function, risk of erectile dysfunction, infection rate, and protection of the underneath penile prosthesis if present. This information may help to understand the difference in outcome between graft materials for PD in the clinical setting.
In this experiment, we are looking for a histological evaluation of the fate of TachoSil graft application to the TA and its effect on the underlying corpora cavernosa. We present this article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-229/rc).
Methods
Experimental protocol
Six Sprague Dawley adult male rats were used. The experiments and animal care were conducted in the Comparative Medicine Department of our institution. The house veterinarian and veterinarian assistants carried out anesthesia and perioperative care. Rats were allowed free access to water and food perioperatively. All procedures were carried out under general inhalation anesthesia with isoflurane. Cefazolin 100 mg/kg/i.m. was injected at induction. Under sterile conditions and operative microscope magnification up to ×20, the penis was degloved through a circumferential subcoronal incision. A 1 mm × 10 mm defect was created at the base of the lateral aspect of the penis. A TachoSil patch (Takeda, Japan) was fashioned 5 mm × 15 mm, applied dry to the defect, and trimmed. Pressure on the patch was applied for three minutes. The penile skin covering was restored and fixed with interrupted 5–6 zero absorbable sutures. Postoperatively, for analgesia, 2 mg/kg of Carprofen was administered subcutaneously every 24 hours for 5 days. The rats were inspected daily for general condition and wound appearance.
At 2 months under general inhalation anesthesia, the rat penis was excised. Euthanasia was carried out with an overdose of inhalation anesthesia and chest opening to induce pneumothorax. The excised penis was fixed in 10% formalin and sent to the anatomical pathology lab. Tissue cross sections of the penis were stained with hematoxylin, eosin, and trichrome. The pathologist evaluated the tissue sections for fibrosis, inflammation, new vascularity, graft resorption, and new tissue regeneration.
Statistical analysis
The mean and standard deviation of the body weight of rats was reported. The endpoint was preset at 2 months. No other analysis was performed on the histopathology images.
Literature review
A literature review of PubMed citations was conducted using the search terms combining TA with either graft, rat, rabbit, or dog. The search was limited to the English language literature but not the publication date. The search syntax was as follows: Search: ((((((tunica albuginea) AND (("rat"[All Fields]) OR (rats))) OR ((tunica albuginea) AND (("rabbit"[All Fields]) OR ("rabbits"[All Fields])))) OR ((tunica albuginea) AND (("dog"[All Fields]) OR ("dogs"[All Fields])))) OR ((("graft"[All Fields]) AND (TUNICA ALBUGINEA)) AND (("dog"[All Fields]) OR ("dogs"[All Fields])))) OR ((("graft"[All Fields]) AND (TUNICA ALBUGINEA)) AND (("rat"[All Fields]) OR (rats)))) OR ((("graft"[All Fields]) AND (TUNICA ALBUGINEA)) AND (("rabbit"[All Fields]) OR ("rabbits"[All Fields]))).
Ethical statement
A protocol was prepared before the study without registration. Experiments were performed under a project license (No. 2210008) granted by the Animal Use and Care Committee of King Faisal Specialist Hospital and Research Center Office of Research Affairs, in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition.
Results
Experimental animal study
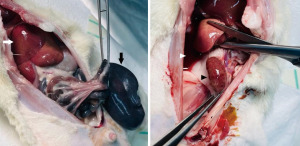
At the time of surgery, the rats’ weight was 369.2 gm (standard deviation: 31.5). Five rats showed at gross examination a normal-looking penis at 2 months with complete healing, no scaring, tethering, or gross inflammatory features (Figure 1). No induration could be felt upon palpation of the graft site of the rat penis. However, upon excision, two rats had subcutaneous pockets of pus. Histopathologic examination of the site of the TachoSil patches showed fibrosis, chronic inflammation, and foreign body giant cell reaction in all rats (Figure 2). There was no generation of a new TA, or new vascularity. No inflammatory or pathological reaction affected the underlying corpus cavernous tissue.

One rat died on the 6th postoperative day. Postmortem showed massive gastrointestinal bleeding, intraperitoneal hemorrhage, and punctate hemorrhages of the parenchyma of both suprarenal glands, both kidneys, and the liver (Figure 3). A clinical diagnosis of disseminated intravascular coagulopathy (DIC) was made. Histopathology of the penis, however, did not show micro thrombosis.
Literature review
The literature review search retrieved 259 articles. A review of the abstracts and selected full-text articles resulted in eliminating irrelevant articles or articles with no clear methodology or conclusion on TA grafting or shorter than the one-month follow-up, leaving 27 with adequate reporting on histopathology fate of the grafts (Table 1). Seventeen articles reported complete or similar tissue regeneration of TA. Six of these grafts were SIS, four acellular matrices, two veins, two auto or allografts, one fibrin glue, one Surgicel, and one polyglycolic acid scaffold. The other four articles reported degeneration of the graft area associated with tunica vaginalis, cadaveric pericardium, temporalis fascia, and polyglycolic acid mesh. Three papers described no inflammation and no or minimal fibrosis of the cavernous tissue, while two articles reported moderate to severe fibrosis.
Discussion
Experimental animal and clinical studies indicated that various graft materials for penile surgery have different advantages. The ideal graft for PD surgery has yet to be discovered. Multiple literature reviews reported that these grafts are comparable regarding clinical outcomes; however, the heterogeneity of the series published makes it difficult to conclude (1-3,41-43). Nevertheless, TachoSil stands out as an operative time saver compared to others (3,36,37,44,45). Due to its ready availability, sutureless application, and hemostatic effect, TachoSil gained popularity in PD surgery. Furthermore, TachoSil was used to repair failed grafting of Peyronie’s disease (46).
Regeneration of TA
Unlike other grafts that were tested in the experimental animal, the fate of the TachoSil graft for the penile TA defect is not known. The literature review of other graft materials yields varied results extending from complete regeneration of the TA on one end of the spectrum to complete degeneration and fibrosis at the other (Table 1). SIS, vein, and TA acellular matrix grafts were the most commonly reported grafts replaced with identical or nearly identical TA with minimal impact on the underlying cavernous tissue (8-13,16-21,23,29,30). In another set of experiments, however, tunica vaginalis, cadaveric pericardium, temporalis fascia grafts, and polyglycolic acid mesh did not show regeneration. These studies, however, reported degeneration, complete absorption, lack of vascularization, replacement by fibrous connective tissue, and fibrosis of the underlying cavernous tissue (6,7,14,27). In our experiment, there is no evidence of regeneration of the TA or neovascularity commonly reported.
Tissue reaction
After 2 months, TachoSil grafts almost resorb, leaving granulation tissue and chronic inflammatory cells. No fibrous sheet bridging the TA defect was seen. There was no development of fibrosis. Clinically, when compared to SIS, TachoSil was associated with less recurrence of curvature and less shortening of the penis (44). These differences might be related to the tendency of SIS to contract even with techniques to mitigate penile shortening (1,47,48).
How TachoSil sustains penile rigidity?
Early experience with TachoSil patching and plaque incision or excision in patients with an erection hardness score of a mean of 3.4 showed that the score decreased significantly to 2.6 (31). In subsequent larger and multicenter clinical studies, the TachoSil patch, even with a relatively large size, was associated with nearly ~ 16% de novo erectile dysfunction (36,37). The reported mean longitudinal length of TA defect is 21.9 mm (range, 5–40) for plaque excision (38). After patching, the only support to the graft is from the reapproximated buck’s fascia. Early postoperative assessment of erection was not reported; however, longer follow-up results from the same author indicated that 84.3% of patients had unchanged or improved erection (36). This figure is comparable to or even better than all other graft applications for patients with PD and absent erectile dysfunction.
One factor which may have contributed to the support of the cavernous pressure is the tensile power of TachoSil demonstrated in other surgical applications (46,49,50). In the dog, a 1 cm longitudinal incision in the external iliac artery, non-sutured and patched for 5 minutes with TachoSil similar graft (Tachotop, Hormonchemie, Munich, Germany) could stand pressure up to 260 mmHg (49). At four weeks, resorption of the patch occurred and was replaced by mature granulation tissue and cell-depleted collagen tissue. Subsequently, TachoSil is used to bolster the high-pressure cardiovascular structures during surgery, such as the cardiac ventricles and aorta (50).
Another contributing factor to the preservation of erection is the intact underlying cavernous smooth muscle. Our experiment showed that TachoSil protects the underlying corpus cavernous from superficial inflammation by forming a barrier. There is no evidence of fibrosis or necrosis as well. Furthermore, the effect on erection compared to other graft materials may not be related to the tensile strength of the graft but rather the incision or excision of TA during surgery. Autologous TA grafts are associated with 11–18% ED, implicating that the damage of the underlying layer at the TA cavernous tissue junction is a significant contributing factor (26,51,52). Clinical reports of grafts that are replaced by a TA-like tissue still were associated with 4.5–56.5% de novo ED (44,47,48,53-57). The development of ED is directly proportional to the size of the graft (47).
The tensile strength of TachoSil is also important in patients undergoing penile prosthesis implantation. Grafting materials including TachoSil in association with penile prosthesis did not show significant differences in the outcomes (3). To our knowledge, there is no report of aneurysmal dilation at the site of TachoSil patch in those patients.
DIC
One of our rats developed DIC on the sixth postoperative day and expired. Reviewing the literature for the association of TachoSil and DIC or thrombosis did not provide similar reports in PubMed. TachoSil is impregnated with thrombin. However, a search of DIC and thrombin identified some studies and case reports (58-67). The thrombovascular event like DVT/PE could be related to two mechanisms. One is the direct activation of the hemostatic agent to the coagulation mechanism as a dose-dependent phenomenon. The other is an immune-mediated mechanism associated with bovine-driven thrombin use in humans. As TachoSil contains human-derived thrombin, an immune reaction of the rat may have mediated the DIC in our experiment.
In the prescription details of TachoSil approved by the FDA, there is a warning of thrombosis if the TachoSil gets into systemic circulation. FDA safety reports DIC in a six-month-old female patient in association with the TachoSil arm of hemostasis in a liver resection trial (68). On the 12th day, the patient developed DIC and mycobacterium septicemia, and on the 13th-day portal vein thrombosis. After protracted surgical morbidity, the patient died. The reviewer concluded that there is no evidence that these events are related to the TachoSil application. However, there is a concern, though theoretical, of perturbation of the coagulation system, and routine laboratory vigilance is needed.
Fortunately, there are no clinical reports of thrombosis associated with TachoSil applications in PD surgery. The surgeon, however, should be vigilant as the cavernous tissue exposed is vascular tissue with direct drainage into the systemic venous system. The problem might be more significant in patients with healthy cavernous tissue and a large TA defect created during surgery when venous drainage is unimpeded. A factor that may have lessened the risk of thrombin reaching the systemic circulation in patients is the avoidance of artificial erection by infusing saline in the corpora not to dislodge the un-sutured TachoSil patch. Although saline infusion at this test is usually applied with a tourniquet at the base of the penis, the release of the tourniquet will immediately lead to cavernous drainage into the systemic veins.
The strength of our study is that it is the first to demonstrate the histopathology of the TachoSil patch applied to the penile TA defect. In addition, the results help to understand the outcomes of TachoSil applications in PD surgery and how it compares to other grafting materials.
The weakness of this study is that it lacks control animals, a short follow-up time, and a lack of evaluation of erection.
Conclusions
TachoSil grafting of the TA protects the underlying cavernous tissue from inflammation, fibrosis, and necrosis. However, there is no regeneration of TA-like tissue bridging the created defect. TachoSil almost resorbs by 2 months of grafting, leaving a chronic inflammation with foreign body giant cells in place. Fatal DIC developed in one of our rats. Although clinically not proven, laboratory vigilance toward developing DIC is advised.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-229/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-229/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-229/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-229/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2210008) granted by the Animal Use and Care Committee of King Faisal Specialist Hospital and Research Center Office of Research Affairs, in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krishnappa P, Fernandez-Pascual E, Carballido J, et al. Surgical Management of Peyronie's Disease With Co-Existent Erectile Dysfunction. Sex Med 2019;7:361-70. [Crossref] [PubMed]
- Osmonov D, Ragheb A, Ward S, et al. ESSM Position Statement on Surgical Treatment of Peyronie's Disease. Sex Med 2022;10:100459. [Crossref] [PubMed]
- Sokolakis I, Pyrgidis N, Ziegelmann MJ, et al. Penile Prosthesis Implantation Combined With Grafting Techniques in Patients With Peyronie's Disease and Erectile Dysfunction: A Systematic Review. Sex Med Rev 2022;10:451-9. [Crossref] [PubMed]
- Leungwattanakij S, Tiewthanom V, Hellstrom WJ. Evaluation of corporal fibrosis in cadaveric pericardium and vein grafts for tunica albuginea substitution in rats. Asian J Androl 2003;5:295-9. [PubMed]
- Leungwattanakij S, Bivalacqua TJ, Yang DY, et al. Comparison of cadaveric pericardial, dermal, vein, and synthetic grafts for tunica albuginea substitution using a rat model. BJU Int 2003;92:119-24. [Crossref] [PubMed]
- Leungwattanakij S, Bivalacqua TJ, Caulfield JJ, et al. Evaluation of cadaveric pericardium in the rat for the surgical treatment of Peyronie's disease. Urology 2000;56:1075-80. [Crossref] [PubMed]
- Stefanovic KB, Clark SA, Buncke HJ. Microsurgical vascularized free temporoparietal fascia transfer for Peyronie's disease: an experimental study. J Reconstr Microsurg 1994;10:39-45; discussion 45-6. [Crossref] [PubMed]
- Hafez AT, El-Assmy A, El-Hamid MA. Fibrin glue for the suture-less correction of penile chordee: a pilot study in a rabbit model. BJU Int 2004;94:433-6. [Crossref] [PubMed]
- Eberli D, Susaeta R, Yoo JJ, et al. Tunica repair with acellular bladder matrix maintains corporal tissue function. Int J Impot Res 2007;19:602-9. [Crossref] [PubMed]
- Joo KJ, Kim BS, Han JH, et al. Porcine vesical acellular matrix graft of tunica albuginea for penile reconstruction. Asian J Androl 2006;8:543-8. [Crossref] [PubMed]
- Shokeir AA, Osman Y, El-Azab M, et al. Tunica albuginea acellular matrix graft for treatment of Peyronie's disease--an experimental study in dogs. Scand J Urol Nephrol 2004;38:499-503. [Crossref] [PubMed]
- Wefer J, Schlote N, Sekido N, et al. Tunica albuginea acellular matrix graft for penile reconstruction in the rabbit: a model for treating Peyronie's disease. BJU Int 2002;90:326-31. [Crossref] [PubMed]
- Ferretti L, Giuliani M, Bessède T, et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J Sex Med 2012;9:625-31. [Crossref] [PubMed]
- Bazeed MA, Thüroff JW, Schmidt RA, et al. New surgical procedure for management of Peyronie disease. Urology 1983;21:501-4. [Crossref] [PubMed]
- Blumenfrucht M, Bhattacharya S, Brunner R, et al. Penile corporoplasty with polytetrafluoroethylene (PTFE) grafts. Urology 1983;22:46-8. [Crossref] [PubMed]
- Gundogdu G, Okhunov Z, Starek S, et al. Evaluation of Bi-Layer Silk Fibroin Grafts for Penile Tunica Albuginea Repair in a Rabbit Corporoplasty Model. Front Bioeng Biotechnol 2021;9:791119. [Crossref] [PubMed]
- Ma L, Yang Y, Sikka SC, et al. Adipose tissue-derived stem cell-seeded small intestinal submucosa for tunica albuginea grafting and reconstruction. Proc Natl Acad Sci U S A 2012;109:2090-5. [Crossref] [PubMed]
- Leungwattanakij S, Pummangura N, Ratana-Olarn K. Penile enhancement using a porcine small intestinal submucosa graft in a rat model. Int J Impot Res 2006;18:39-43. [Crossref] [PubMed]
- El-Assmy A, El-Hamid MA, Abo-Elghar ME, et al. Single-layer small intestinal submucosa or tunica vaginalis flap for correcting penile chordee. BJU Int 2004;94:1097-101. [Crossref] [PubMed]
- Hafez AT, El-Assmy A, El-Hamid MA. 4 layer versus 1 layer small intestinal submucosa for correction of penile chordee: experimental study in a rabbit model. J Urol 2004;171:2489-91. [Crossref] [PubMed]
- Kropp BP, Cheng EY, Pope JC 4th, et al. Use of small intestinal submucosa for corporal body grafting in cases of severe penile curvature. J Urol 2002;168:1742-5; discussion 1745. [Crossref] [PubMed]
- Monga M, Cosgrove D, Zupkas P, et al. Small intestinal submucosa as a tunica albuginea graft material. J Urol 2002;168:1215-21. [Crossref] [PubMed]
- El-Assmy A, Eassa W, El-Hamid MA, et al. Use of oxidized cellulose for corporal body grafting and suture-less correction of severe penile chordee: an experimental study in rabbits. BJU Int 2007;99:1098-102. [Crossref] [PubMed]
- Sansalone S, Loreto C, Leonardi R, et al. Microsurgical tunica albuginea transplantation in an animal model. Asian J Androl 2017;19:694-9. [Crossref] [PubMed]
- Ferretti L, Fandel TM, Qiu X, et al. Tunica albuginea allograft: a new model of LaPeyronie's disease with penile curvature and subtunical ossification. Asian J Androl 2014;16:592-6. [Crossref] [PubMed]
- Seyam RM, Mokhtar AA, Chishti MA, et al. Crural tunica albuginea autograft for corporoplasty: an experimental animal study of hemodynamic, histopathological, and molecular effects in the long term. J Sex Med 2007;4:1277-90. [Crossref] [PubMed]
- Bagbanci S, Dadali M, Emir L, et al. Penile enhancement with rectus muscle fascia and testicular tunica vaginalis grafts: an experimental animal study. Int Urol Nephrol 2015;47:915-20. [Crossref] [PubMed]
- Hafez AT, Smith CR, McLorie GA, et al. Tunica vaginalis for correcting penile chordee in a rabbit model: is there a difference in flap versus graft? J Urol 2001;166:1429-32. [Crossref] [PubMed]
- Brannigan RE, Kim ED, Oyasu R, et al. Comparison of tunica albuginea substitutes for the treatment of Peyronie's disease. J Urol 1998;159:1064-8. [Crossref] [PubMed]
- Brock G, Nunes L, von Heyden B, et al. Can a venous patch graft be a substitute for the tunica albuginea of the penis? J Urol 1993;150:1306-9. [Crossref] [PubMed]
- Horstmann M, Kwol M, Amend B, et al. A self-reported long-term follow-up of patients operated with either shortening techniques or a TachoSil grafting procedure. Asian J Androl 2011;13:326-31. [Crossref] [PubMed]
- Lahme S, Götz T, Bichler KH. Collagen fleece for defect coverage following plaque excision in patients with Peyronie's disease. Eur Urol 2002;41:401-5. [Crossref] [PubMed]
- Hatzichristodoulou G, Yang DY, Ring JD, et al. Multicenter Experience Using Collagen Fleece for Plaque Incision With Grafting to Correct Residual Curvature at the Time of Inflatable Penile Prosthesis Placement in Patients With Peyronie's Disease. J Sex Med 2020;17:1168-74. [Crossref] [PubMed]
- Hatzichristodoulou G. The PICS Technique: A Novel Approach for Residual Curvature Correction During Penile Prosthesis Implantation in Patients With Severe Peyronie's Disease Using the Collagen Fleece TachoSil. J Sex Med 2018;15:416-21. [Crossref] [PubMed]
- Fernández-Pascual E, Gonzalez-García FJ, Rodríguez-Monsalve M, et al. Surgical Technique for Complex Cases of Peyronie's Disease With Implantation of Penile Prosthesis, Multiple Corporeal Incisions, and Grafting With Collagen Fleece. J Sex Med 2019;16:323-32. [Crossref] [PubMed]
- Hatzichristodoulou G, Fiechtner S, Pyrgidis N, et al. Suture-Free Sealing of Tunical Defect with Collagen Fleece after Partial Plaque Excision in 319 Consecutive Patients with Peyronie's Disease: The Sealing Technique. J Urol 2021;206:1276-82. [Crossref] [PubMed]
- Fernández-Pascual E, Manfredi C, Torremadé J, et al. Multicenter Prospective Study of Grafting With Collagen Fleece TachoSil in Patients With Peyronie's Disease. J Sex Med 2020;17:2279-86. [Crossref] [PubMed]
- Hatzichristodoulou G, Gschwend JE, Lahme S. Surgical therapy of Peyronie's disease by partial plaque excision and grafting with collagen fleece: feasibility study of a new technique. Int J Impot Res 2013;25:183-7. [Crossref] [PubMed]
- Sokolakis I, Pyrgidis N, Hatzichristodoulou G. The use of collagen fleece (TachoSil) as grafting material in the surgical treatment of Peyronie's disease. A comprehensive narrative review. Int J Impot Res 2022;34:260-8. [Crossref] [PubMed]
- TACHOSIL Fibrin Sealant Patch, highlights of prescribing information. FDA 2015. Available online: https://www.fda.gov/media/78698/download (accessed Feb 24, 2023).
- Rice PG, Somani BK, Rees RW. Twenty Years of Plaque Incision and Grafting for Peyronie's Disease: A Review of Literature. Sex Med 2019;7:115-28. [Crossref] [PubMed]
- Verze P, Sokolakis I, Manfredi C, et al. Penile prosthesis implant in the management of Peyronies' disease. Minerva Urol Nephrol 2021;73:196-214. [Crossref] [PubMed]
- Garcia-Gomez B, Ralph D, Levine L, et al. Grafts for Peyronie's disease: a comprehensive review. Andrology 2018;6:117-26. [Crossref] [PubMed]
- Rosenhammer B, Sayedahmed K, Fritsche HM, et al. Long-term outcome after grafting with small intestinal submucosa and collagen fleece in patients with Peyronie's disease: a matched pair analysis. Int J Impot Res 2019;31:256-62. [Crossref] [PubMed]
- Falcone M, Preto M, Ceruti C, et al. A Comparative Study Between 2 Different Grafts Used as Patches After Plaque Incision and Inflatable Penile Prosthesis Implantation for End-Stage Peyronie's Disease. J Sex Med 2018;15:848-52. [Crossref] [PubMed]
- Fabiani A, Fioretti F, Filosa A, et al. Patch bulging after plaque incision and grafting procedure for Peyronie's disease. Surgical repair with a collagen fleece. Arch Ital Urol Androl 2015;87:173-4. [Crossref] [PubMed]
- Morgado A, Morgado MR, Tomada N. Penile lengthening with porcine small intestinal submucosa grafting in Peyronie's disease treatment: long-term surgical outcomes, patients' satisfaction and dissatisfaction predictors. Andrology 2018;6:909-15. [Crossref] [PubMed]
- Sayedahmed K, Rosenhammer B, Spachmann PJ, et al. Bicentric prospective evaluation of corporoplasty with porcine small intestinal submucosa (SIS) in patients with severe Peyronie's disease. World J Urol 2017;35:1119-24. [Crossref] [PubMed]
- Schelling G, Block T, Blanke E, et al. The effectiveness of a fibrinogen-thrombin-collagen-based hemostatic agent in an experimental arterial bleeding model. Ann Surg 1987;205:432-5. [Crossref] [PubMed]
- Maisano F, Kjaergård HK, Bauernschmitt R, et al. TachoSil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: a randomised controlled trial. Eur J Cardiothorac Surg 2009;36:708-14. [Crossref] [PubMed]
- Schwarzer JU, Mühlen B, Schukai O. Penile corporoplasty using tunica albuginea free graft from proximal corpus cavernosum: a new technique for treatment of penile curvature in Peyronie's disease. Eur Urol 2003;44:720-3. [Crossref] [PubMed]
- Da Ros CT, Graziottin TM, Ribeiro E, et al. Long-term follow-up of penile curvature correction utilizing autologous albugineal crural graft. Int Braz J Urol 2012;38:242-7; discussion 248-9. [Crossref] [PubMed]
- Wimpissinger F, Parnham A, Gutjahr G, et al. 10 Years' Plaque Incision and Vein Grafting for Peyronie's Disease: Does Time Matter? J Sex Med 2016;13:120-8. [Crossref] [PubMed]
- Staerman F, Pierrevelcin J, Ripert T, et al. Medium-term follow-up of plaque incision and porcine small intestinal submucosal grafting for Peyronie's disease. Int J Impot Res 2010;22:343-8. [Crossref] [PubMed]
- Cosentino M, Kanashiro A, Vives A, et al. Surgical treatment of Peyronie's disease with small intestinal submucosa graft patch. Int J Impot Res 2016;28:106-9. [Crossref] [PubMed]
- Valente P, Gomes C, Tomada N. Small Intestinal Submucosa Grafting for Peyronie Disease: Outcomes and Patient Satisfaction. Urology 2017;100:117-24. [Crossref] [PubMed]
- Kadıoğlu A, Salabaş E, Özmez A, et al. Peyronie's disease surgery: Surgical outcomes of 268 cases. Turk J Urol 2018;44:10-5. [Crossref] [PubMed]
- Pavlovich CP, Battiwalla M, Rick ME, et al. Antibody induced coagulopathy from bovine thrombin use during partial nephrectomy. J Urol 2001;165:1617. [Crossref] [PubMed]
- Sarfati MR, Dilorenzo DJ, Kraiss LW, et al. Severe coagulopathy following intraoperative use of topical thrombin. Ann Vasc Surg 2004;18:349-51. [Crossref] [PubMed]
- Diesen DL, Lawson JH. Bovine thrombin: history, use, and risk in the surgical patient. Vascular 2008;16:S29-36. [PubMed]
- Ferschl MB, Rollins MD. Thromboemboli, acute right heart failure and disseminated intravascular coagulation after intraoperative application of a topical hemostatic matrix. Anesth Analg 2009;108:434-6. [Crossref] [PubMed]
- Ness P, Creer M, Rodgers GM, et al. Building an immune-mediated coagulopathy consensus: early recognition and evaluation to enhance post-surgical patient safety. Patient Saf Surg 2009;3:8. [Crossref] [PubMed]
- Naoum JJ. Immune-mediated coagulopathy: a case report. Pharmacotherapy 2009;29:13S-7S. [Crossref] [PubMed]
- Rodgers GM. Immune-mediated coagulopathy associated with topical bovine thrombin: review of the pediatric literature. J Pediatr Hematol Oncol 2011;33:86-8. [Crossref] [PubMed]
- Safaee M, Sun MZ, Oh T, et al. Use of thrombin-based hemostatic matrix during meningioma resection: a potential risk factor for perioperative thromboembolic events. Clin Neurol Neurosurg 2014;119:116-20. [Crossref] [PubMed]
- Sagar AS, Vakil E, Jimenez CA, et al. Pulmonary embolism caused by thrombin-based haemostatic matrix. BMJ Case Rep 2017;2017:bcr2017223503. [Crossref] [PubMed]
- Gazzeri R, Galarza M, Conti C, et al. Incidence of thromboembolic events after use of gelatin-thrombin-based hemostatic matrix during intracranial tumor surgery. Neurosurg Rev 2018;41:303-10. [Crossref] [PubMed]
- Maplethorpe CM. TachoSil (Fibrin Sealant Patch) (Takeda Pharma A/S) as an adjunct to hemostasis for adult and pediatric hepatic resection surgery Clinical Review Memo. FDA 2015. Available online: https://www.fda.gov/media/93265/download (accessed Feb 24, 2023).



