
Lobaplatin induces apoptosis in T24 and 5637 bladder cancer cells by regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt signaling pathway
Highlight box
Key findings
• LBP demonstrated an in vivo and in vitro antitumor effect on T24 and 5637 BC cells.
What is known and what is new?
• In China, LBP has been used as one of the common drugs in the clinical treatment of some cancers.
• The inhibitory effect of LBP on T24 and 5637 BC cells may be related to the regulation of Bcl-2 and Bax expression and the inhibition of the PI3K/Akt sig-nalling pathway.
What is the implication, and what should change now?
• This provides data to explore the application of LBP in the field of bladder cancer.
Introduction
Bladder cancer (BC) is one of the most common cancers in the world and has a higher morbidity and mortality rate (1). According to prognostic features, BC is divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) (1-3). Clinically, 70% of patients are first diagnosed with NMIBC. However, after receiving transurethral resection, high-risk patients will relapse and develop MIBC (4). Therefore, it is of great significance to develop or identify safe and effective new drugs for BC treatment.
Lobaplatin (LBP), a third-generation platinum-based drug only available in the Chinese market, has been approved for treating metastatic BC, chronic granulocytic leukemia, and small cell lung cancer (5), for which surgical removal is not an option. In addition, some studies have confirmed that LBP is effective in treating other cancer types, including cervical cancer, triple-negative breast cancer, primary hepatocellular carcinoma, and T4 gastric cancer (6-9). Compared to other platinum-based chemotherapy drugs on the market, such as cisplatin (DDP), LBP is less toxic to the kidney, nerves and ear and bypasses the DDP resistance of cancer cells (10). Considering the similar antitumor mechanisms of LBP and DDP—both can cause DNA damage by generating DNA adducts, thereby inhibiting cell proliferation and inducing apoptosis—in the present study, for the first time, we explored whether LBP has a similar effect to DDP in treating BC while taking into account multiple pathways in the tumor cells related to the occurrence of BC, such as the overactivation of the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathway in MIBC (11). In this study, we further investigated whether the potential efficacy of LBP in treating BC is related to its ability to regulate B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax) expression and to inhibit the PI3K/Akt pathway in the hope of laying an experimental foundation for LBP application in the clinical treatment of BC. We present this article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/rc).
Methods
Materials
T24 human BC cells (SCSP-536) and the human BC cell line 5637 (TCHu 1) were provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Male BALB/c nude mice (BALB/c nude mice, little individual variation, commonly used in xenograft tumor models) 35–48 days old (grade: specific pathogen free) were purchased from Charles River Laboratories [license key: SCXK (Jing) 2016-0006] (Beijing, China). LBP lyophilized powder for injection was provided by Hainan Changan International Pharmaceutical Co., Ltd. (Hainan, China). Fetal bovine serum (FBS) was provided by HyClone (Logan, Utah, United States). Roswell Park Memorial Institute-1640 medium was provided by GE Healthcare Life Sciences (Pittsburgh, Pennsylvania, United States). Cell counting kit-8 (CCK-8) reagents were purchased from Dojindo Molecular Technologies (Tokyo, Japan). The FITC annexin V cell apoptosis assay kit was provided by BD Biosciences (Bergen, New Jersey, United States), and trypsin was purchased from Beijing Solarbio Life Sciences (Beijing, China).
Antibodies
Bcl-2 (D17C4) rabbit monoclonal antibody (mAb), Bax (D2E11) rabbit mAb, caspase-3 rabbit mAb, cleaved caspase-3 (Asp175) antibody, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (D16H11) XP rabbit mAb, β-Tubulin (9F3) rabbit mAb, PI3K p110a (C73F8) rabbit mAb, Akt (pan) (11E7) rabbit mAb, phospho-Akt (Ser473) (D9e) XP rabbit mAb and anti-rabbit IgG, Horseradish Peroxidase (HRP)-linked antibody was purchased from Cell Signalling Technology (Boston, Massachusetts, USA) were obtained for these studies. Fluorescent secondary antibodies were purchased from LI-COR Biosciences (Lincoln, Nebraska, United States).
Cell culture
T24 and 5637 cells were cultured separately in RPMI-1640 medium containing 1% penicillin-streptomycin and 10% FBS (complete medium). The complete medium was used and replaced every 2 days until 75–90% of cells were attached in a petri dish at 37 ℃ in an incubator with 5% CO2. The cells that reached the required number were used in experimental studies. The cells were subcultured at a 1:3 ratio.
CCK-8 assay for assessing cell viability
T24 and 5637 cells in the logarithmic growth phase were selected under a microscope. The original medium was discarded, and the cells were washed with phosphate buffered saline (PBS). Complete medium was used to resuspend the cell pellets after trypsin digestion. A total of 1×104 cells were counted in 100 µL per well and transferred to a 96-well plate. After the addition of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium Sodium Salt, the absorbance at 450 nm was measured by an Enzyme-labeled instrument. The experiment was repeated at least 3 times, and 3 experimental wells were set in each group. The optical density (OD) was measured at a wavelength of 450 nm:
Wound healing assay
A wound healing assay (12) was employed to determine the inhibitory effect of LBP on the migration of the two BC cell lines, namely, the T24 and 5637 lines. T24 and 5637 cells were seeded in a six-well culture plate (5×105 cells per well) until the cell confluency reached 80%. Then, the medium was removed, and a “wound” was created. After washing with PBS twice, we added culture medium containing LBP at different concentrations (0, 1, 2, 4, and 8 µg/mL) and captured images of the wound at the time of wounding (T0). After 24 hours (T24), we took photos of the same area and measured the wound size at T0 and T24, based on which the rate of cell migration was calculated to estimate the migration capacity of cells, with the formula shown below. The experiment was repeated at least 3 times.
Apoptosis detection with annexin V-FITC/PI staining
T24 and 5637 cells were treated with 0 and 8 µg/mL LBP for 24 hours. The adherent cells on the bottom were made into a cell suspension after trypsin digestion. After the suspension was centrifuged for 7 min at 1.0×103 r·min−1, PBS was used to wash the cells three times. According to the instructions of the Annexin V-FITC cell apoptosis kit. At room temperature, the cells were cultured in the dark for 15 min and analyzed with a flow cytometer. The experiment was repeated at least 3 times.
Western blot analysis of Bcl-2, Bax, caspase-3, cleaved caspase-3, PI3K, Akt and p-Akt protein expression
Whole-cell proteins were extracted using RIPA lysate containing 1 mM PMSF, and the same amount of protein (50 µg) was separated via electrophoresis and subsequently transferred to a polyvinylidene fluoride (PVDF) membrane. Tris Buffered Saline with Tween (TBS-T) solution containing 5% skimmed milk was used to block the PVDF membrane for 90 min at room temperature. Afterward, the blocked PVDF membrane was incubated with primary antibodies overnight at 4 ℃. The excess primary antibody was washed off, and then, the membrane was incubated with goat anti-rabbit IgG (1:1,000) at room temperature for 1.5 hours. Eventually, the membrane was evaluated using an Odyssey dual-color infrared laser imaging system, which was developed by LI-COR, and Image J for greyscale analysis [The infrared laser produces two wavelengths of laser light (680 and 780 nm) which excite the infrared fluorescent dye to produce emitted light at 720 and 820 nm respectively, which is then simultaneously detected by two highly sensitive avalanche photodiodes to detect the signal intensity].
Nude mouse xenograft assay
Eight male BALB/c nude mice from each experimental group were selected randomly, with two groups containing a total of 16 mice. Serum-free medium was used to dilute T24 and 5637 cells to 2×107 cells/mL. Then, 200 µL of cell suspension was injected subcutaneously into the backs of the nude mice, generating a solid tumor. When the solid tumor grew to 200 mm3, the nude mice were randomly split into the control group and the treatment group, and the latter group received an intravenous injection of 25 mg·kg−1 LBP. Then, we measured the weight of the nude mice and the tumor size every 3 days and extracted the tumor tissue after 21 days for weighing and cryopreservation. Mice who died were excluded from the experiment. Tumor size was calculated as follows:
(L refers to the length of the tumor tissue and W the width).
Immunohistochemical test
The tumor tissues were made into paraffin sections and deparaffinized with ethanol and xylene at different concentrations. Then, the deparaffinized sections were boiled in 0.01 M citrate buffer, incubated in 3% H2O2 for 10 min to reduce the activity of endogenous peroxidase, blocked in 5% nonfat milk for 60 min, and incubated with antibodies against Bax, Bcl-2, and cleaved caspase-3 at 4 ℃ overnight. The next day, we incubated the sections with HRP-linked antibody for 60 min, followed by staining through incubation in 3,3-diaminobenzidine (DAB) substrate for 10 min. Then, we took photos of the obtained sections with an optical microscope and processed the photos. Semi-quantitative analysis of the staining results: the intensity of the staining is combined with the percentage of positive cytology to give a composite score, with positive cells marked by a yellowish to brownish stain in the cytoplasm of the tissue section. The intensity of staining is based on the staining characteristics of the majority of cells (shades of staining in contrast to background staining): 0 points for no staining, 1 point for pale yellow, 2 points for brown, and 3 points for tan. The percentage of positive cells is the average number of positive cells over 5 fields of view (100 such cells counted in a 400× high magnification field) (13).
Animal experimental ethical inspection
The in vivo experiment was performed according to the Guide for the Care and Use of Laboratory Animals of Jilin University and was approved by the ethics committee of Jilin University School of Pharmaceutical Science (No. 20190055). A protocol was prepared before the study without registration.
Statistical and analytical methods
SPSS 24.0 software, developed by International Business Machines Corporation (IBM), was selected to process the data, and ImageJ software was used to quantitatively analyze the grayscale values of the protein bands and the cell scratch area. According to the experimental groups, the data from two groups were compared with unpaired t-tests, and multiple groups were compared via analysis of variance (ANOVA). A value of P<0.05 was regarded as statistically significant.
Estimation was carried out by means of the degrees of freedom (E) of the analysis of variance. The range of E should be between 10 and 20. If the calculated E is less than 10, it means that the number of animals in each group should be increased as a way to increase the likelihood of producing significance-level results; when the calculated E is greater than 20, it means that the number of animals estimated is too high and the likelihood of producing significance level results does not continue to increase, resulting in a waste of experimental animals; thus, the number of animals in each group should be reduced. E is calculated by the following formula:
The RAND function in Excel was used to create random arrays to group mice. Experiments were conducted by random number assignment results to reduce potential confounding factors.
Results
Inhibitory effects of LBP on T24 and 5637 cell viability
LBP impacts on the growth of BC cells were observed through the detection of cell viability. As shown in Figure 1, after exposure to LBP for 24 hours (LBP concentration ≥1 µg/mL), the viability of T24 and 5637 cells was obviously inhibited compared to that in the control group, and after 48 and 72 hours (LBP concentration ≥0.5 µg/mL), the viability of the cells was significantly inhibited, which suggests that the inhibition of cell viability is dependent on the dose of LBP. Since the effects of the treatment over different lengths of time were all significant, we chose 24 hours for the subsequent experiment (P<0.05). Under this condition, the half-maximal inhibitory concentration (IC50) of LBP in T24 cells was 11.623 µg/mL, while the IC50 in 5637 cells was 9.611 µg/mL. Based on the IC50, 8 µg/mL was chosen for the subsequent experiment.

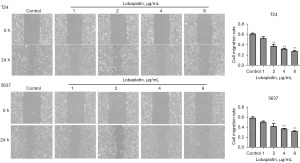
The effects of LBP on the migration rate of human BC cells
The duration of treatment was set as 24 hours according to the experimental results LBP at 0 µg/mL was set as the control group, and LBP at 1, 2, 4, and 8 µg/mL was set as the treatment group. As displayed in Figure 2, the migration rate of T24 cells in the control group was 60.49%±3.38%; the rate in the LBP 1 µg/mL group (51.67%±6.30%) was not significantly different (P>0.05); the rates at LBP 2 µg/mL (36.78%±6.67%), 4 µg/mL (31.43%±3.12%) and 8 µg/mL (26.85%±3.94%) were significantly different (P<0.01). The migration rate of 5637 cells in the control group was 58.37%±4.68%, the migration rate at LBP 1 µg/mL (50.45%±4.01%) was not significantly different (P>0.05); the rates obtained at LBP 2 µg/mL (41.77%±10.54%), 4 µg/mL (36.94%±1.78%) and 8 µg/mL (31.83%±2.51%) were significantly different from that in the control group (P<0.05).
The effects of LBP on BC cell apoptosis
According to the experimental results given above, we set the duration of treatment as 24 hours, with LBP at 0 µg/mL as the control group and LBP at 8 µg/mL as the treatment group. Annexin V-FITC/PI staining was employed to analyze the level of cell apoptosis. According to the results presented in Figure 3, the apoptosis of T24 cells in the treatment group (31.25%±1.20%) was greater than that in the control group (6.25%±0.64%); the apoptosis of 5637 cells in the treatment group (14.3%±2.24%) was greater than that in the control group (2.5%±0.78%). All the differences were significant (P<0.05).

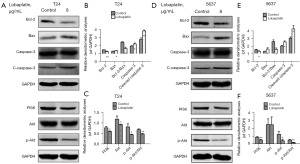
The effects of LBP on the expression of apoptosis-related proteins and PI3K/Akt pathway proteins
Western blotting was used to analyze the expression levels of apoptosis-related proteins (Bax, Bcl-2, caspase-3 and cleaved caspase-3) and proteins in the PI3K/Akt pathway in T24 and 5637 cells before and after LBP treatment. As displayed in Figure 4, compared with that in the control group, after 24 hours of exposure to LBP, the expression of Bcl-2 protein decreased in both cell lines and that of Bax protein increased; the Bcl-2/Bax ratio decreased; the activation of caspase-3 and cleaved caspase-3 was enhanced; the expression of PI3K, AKT and p-Akt in T24 cells decreased and that of PI3K and p-Akt in 5637 cells decreased (P<0.05); and no significant changes were observed in Akt (P>0.05).
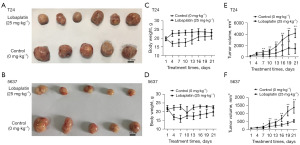
The effects of LBP on the growth of tumor xenografts
A nude mouse model with T24 and 5637 tumor xenografts was constructed to evaluate the effects of LBP in inhibiting BC. Compared with that in the control group, the tumor size in nude mice treated with LBP (25 mg·kg−1) was considerably smaller (Figure 5A,5B). There was no significant change in the body weight of nude mice in the treatment and model groups (Figure 5C,5D). The tumors in the treatment group grew slowly and tumor volumes were significantly less (Figure 5E,5F).
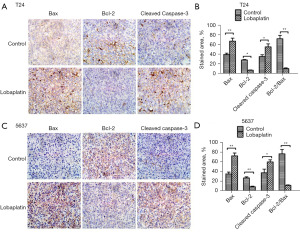
The effects of LBP on apoptosis-related proteins in tumor xenografts
Immunohistochemistry was used to detect the expression level of apoptosis-related proteins (Bcl-2, Bax, and cleaved caspase-3) in the tumor tissue. The staining results of tumor xenografts induced by T24 and 5637 BC cells are presented in Figure 6. Compared with that in the control group, the expression of Bax increased in mice treated with LBP (25 mg·kg−1), that of Bcl-2 protein declined, the Bcl-2/Bax ratio decreased significantly (P<0.05), and cleaved caspase-3 expression in the tissue was increased, indicating activation.
Discussion
The growth of bladder tumors is determined by the state of balance between the proliferation and apoptosis of tumor cells. Chemotherapy is a treatment that inhibits tumor growth by inducing cell cycle arrest and apoptosis. Shortly after its development, the third-generation platinum-based drug LBP [1,2-diamminomethyl-cyclobutane-platinum (II)-lactate] was found to be effective in inhibiting the growth of multiple types of tumors in mice (14-16). Later, further research confirmed its efficacy in inducing the apoptosis of many types of cancer cells, including BGC-823 gastric cancer cells (through the ROS-mitochondrial pathway) (17), nasopharyngeal cancer cells (by regulating cIAP1/2, ribosomes and ROS) (18), and non-small cell lung cancer cells (by acting on the p53/ROS/p38 MAPK pathway) (15).
In this study, for the first time, we explored whether LBP is effective in inhibiting the proliferation of BC cells. This study focused on T24 as the MIBC cell line and 5637 as the NMIBC cell line because of their different chemosensitivity levels and their wide application in BC research (19). The experimental results confirmed that LBP reduces the viability of T24 and 5637 cells and inhibits their migration in a dose-dependent manner. Moreover, when the concentration of LBP reached the IC50 value, LBP was able to induce apoptosis in the two types of BC cells. At the same time, necrotic and early apoptotic cells were further increased after drug administration. This dose-dependent characteristic of LBP was also confirmed in the study of Yu et al. in rectal cancer cells (20). In addition, cell migration ability is a key indicator to evaluate the ability of cancer cells to spread (21,22), and LBP was shown to have an inhibitory effect on the migration of the prostate cancer cell lines DU145 and PC3 (23). This inhibitory effect of LBP on cell migration was confirmed in T24 and 5637 cells in our study, providing some degree of data support for the ability of LBP to inhibit the migration of T24 and 5637 cells.
We employed Western blotting to detect the expression of Bcl-2, Bax, caspase-3 and cleaved caspase-3 in the two studied BC cell lines, which revealed that LBP can reduce the expression of Bcl-2, increase that of Bax, and activate caspase-3 and cleaved caspase-3 in both cell lines, thus confirming the efficacy of LBP in inhibiting proliferation and inducing apoptosis in the two BC cell lines. Bcl-2 is a protein in the Bcl-2 family that inhibits apoptosis. It is related to the survival, antiapoptotic activity and chemotherapy resistance of tumor cells and is also an important downstream effector in the PI3K/Akt pathway. It has been demonstrated that LBP inhibits the proliferation and migration of prostate cancer by regulating Bcl-2 and Bax (23). It also synergistically regulates cleaved caspase-3 to inhibit Ishikawa endometrial cancer cells (24). Anti-esophageal squamous cancer activity occurs through caspase-dependent apoptosis and enhanced Bax/Bcl-2 (10). This ability to adjust Bcl-2 and Bax expression also allows LBP to play a role in inhibiting the proliferation of human gastric cancer cells (25). The antiapoptotic PI3K/Akt pathway can cause an abnormal increase in cancer cells and accelerate the formation of tumors (26-30), and its PI3Kδ-specific inhibitor idelalisib (Zydelig®) received good feedback in phase III clinical trials and is already available in the United States as an oral agent for the treatment of B-cell hematological cancer (31-33). The proapoptotic protein Bax is in the Bcl-2 family, and Bcl-2 can combine to form heterodimers that induce the release of cytochrome C (Cyt-c) and inhibit the antiapoptotic function of Bcl-2, therefore promoting cell apoptosis. The regulation of the proliferation of different tumor cells by LBP through the PI3K/Akt pathway has been well studied. For example, Pan et al. found that LBP can regulate the proliferation of esophageal squamous cell carcinoma through this pathway (34). Furthermore, as the most critical apoptosis executor downstream of the caspase cascade reaction, Bcl-2 activation largely depends on the release of Cyt-c (35,36). By triggering Cyt-c release through the mitochondrial pathway, Bcl-2 and Bax can influence activation of the downstream caspase-3 protease and then regulate the caspase cascade reaction to affect the survival or apoptosis of cells.
We detected protein expression levels in the two types of bladder cells before and after LBP treatment and found that, while LBP can reduce the expression of PI3K, Akt and the phosphorylated protein p-Akt in T24 cells, it increases the expression of PI3K and p-Akt and does not significantly reduce the expression of Akt in 5637 cells. Considering the important role of p-Akt in the functions of AKT (37), more specific studies are needed to discover more facts about the pathway. The occurrence and development of BC is associated with the overactivation of protooncogenes and the loss of cancer suppressor genes, as well as with changes in multiple signaling pathways in tumor cells (38,39). As a main signaling pathway for protein synthesis, the PI3K/Akt pathway is involved in the growth, proliferation, division and migration of cells (40). Dysregulation of the PI3K/Akt pathway can cause various diseases, such as cancer, cardiovascular diseases, metabolic disorders, and immune dysfunction. In MIBC, the PI3K/Akt pathway is overactive, and in 72% of BC cases, changes in the PI3K/AKT pathway can be observed (41,42). PI3K is an esterase, and after being activated by cytokines and growth factors, it can phosphorylate phosphatidylinositol-3,4-bisphosphate and PIP2 in the intracellular membrane to form phosphatidylinositol-3,4,5-bisphosphate and PIP3 and act as the second messenger to activate Akt. Then, PI3K works with the downstream factor Akt to inhibit the apoptosis of tumor cells and promote their proliferation and migration (43). The function of the PI3K/Akt signaling pathway makes it an attractive target for BC treatment (40).
Apart from the aforementioned results, we wanted to understand whether LBP can effectively inhibit cell proliferation in vivo, so we implanted T24 and 5637 tumor xenografts in nude mice for further evaluation of the antitumor activity of LBP in vivo. The results showed that LBP treatment can significantly reduce the size of bladder tumor tissues. The immunohistochemical method verified the effectiveness of LBP in raising the expression level of the proapoptotic proteins Bax and cleaved caspase-3 and lowering the expression level of the antiapoptotic protein Bcl-2, indicating that LBP-induced cell apoptosis contributes to inhibiting the growth of bladder tumors.
Conclusions
The experiments in this study confirmed that LBP is effective in inhibiting the proliferation and suppressing the migration of T24 and 5637 BC cells both in vivo and in vitro and is potentially able to promote the apoptosis of BC cells through the PI3K/Akt pathway. However, the underlying molecular mechanism is still unknown. Further research must be conducted to evaluate the impacts of LBP on BC cells. In summary, this study lays a foundation for the application of LBP to treat BC.
Acknowledgments
We thank Dr. Giorgio Ivan Russo (University of Catania, Catania, Italy), and Dr. Hiroshi Fukushima (Tokyo Medical and Dental University, Tokyo, Japan) for the critical comments and valuable advice on this study.
Funding: This work was supported by the special fund for clinical research of Wu Jieping Medical Foundation (No. 320.6750.19089-41) and Jilin Provincial Technological Development Program (No. 20160101032JC).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang M, Chen X, Tan P, et al. Acquired semi-squamatization during chemotherapy suggests differentiation as a therapeutic strategy for bladder cancer. Cancer Cell 2022;40:1044-1059.e8. [Crossref] [PubMed]
- Teoh JY, Kamat AM, Black PC, et al. Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat Rev Urol 2022;19:280-94. [Crossref] [PubMed]
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016;388:2796-810. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Wheate NJ, Walker S, Craig GE, et al. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 2010;39:8113-27. [Crossref] [PubMed]
- Yan W, Wu X, Wang S, et al. Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial. Ther Adv Med Oncol 2022;14:17588359221107111. [Crossref] [PubMed]
- Chen J, Ge L, Shi X, et al. Lobaplatin Induces Pyroptosis in Cervical Cancer Cells via the Caspase-3/GSDME Pathway. Anticancer Agents Med Chem 2022;22:2091-7. [Crossref] [PubMed]
- Lu H, Zheng C, Liang B, et al. Efficacy and Safety of Lobaplatin-TACE in the Treatment of Primary Hepatocellular Carcinoma: A Retrospective Study. Anticancer Agents Med Chem 2023;23:461-9. [Crossref] [PubMed]
- Zhong Y, Kang W, Hu H, et al. Lobaplatin-based prophylactic hyperthermic intraperitoneal chemotherapy for T4 gastric cancer patients: A retrospective clinical study. Front Oncol 2023;13:995618. [Crossref] [PubMed]
- Du L, Fei Z, Song S, et al. Antitumor activity of Lobaplatin against esophageal squamous cell carcinoma through caspase-dependent apoptosis and increasing the Bax/Bcl-2 ratio. Biomed Pharmacother 2017;95:447-52. [Crossref] [PubMed]
- Costa C, Pereira S, Lima L, et al. Abnormal Protein Glycosylation and Activated PI3K/Akt/mTOR Pathway: Role in Bladder Cancer Prognosis and Targeted Therapeutics. PLoS One 2015;10:e0141253. [Crossref] [PubMed]
- Martinotti S, Ranzato E. Scratch Wound Healing Assay. Methods Mol Biol 2020;2109:225-9. [Crossref] [PubMed]
- Liu W, Chua CH, Wu XL, et al. Inhibiting scar formation in rat cutaneous wounds by blocking TGF-beta signaling. Zhonghua Yi Xue Za Zhi 2003;83:31-6. [PubMed]
- Lobaplatin: D 19466. Drugs R D 2003;4:369-72. [Crossref] [PubMed]
- Zhang H, Chen R, Wang X, et al. Lobaplatin-Induced Apoptosis Requires p53-Mediated p38MAPK Activation Through ROS Generation in Non-Small-Cell Lung Cancer. Front Oncol 2019;9:538. [Crossref] [PubMed]
- Gietema JA, de Vries EG, Sleijfer DT, et al. A phase I study of 1,2-diamminomethyl-cyclobutane-platinum (II)-lactate (D-19466; lobaplatin) administered daily for 5 days. Br J Cancer 1993;67:396-401. [Crossref] [PubMed]
- Li Y, Liu B, Yang F, et al. Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS- mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed Pharmacother 2016;83:1239-46. [Crossref] [PubMed]
- Chen Z, Xu G, Wu D, et al. Lobaplatin induces pyroptosis through regulating cIAP1/2, Ripoptosome and ROS in nasopharyngeal carcinoma. Biochem Pharmacol 2020;177:114023. [Crossref] [PubMed]
- Xu X, Wang X, Fu B, et al. Differentially expressed genes and microRNAs in bladder carcinoma cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp Pathol 2015;8:12678-87. [PubMed]
- Yu J, Li S, Qi J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis 2019;10:193. [Crossref] [PubMed]
- Bozzuto G, Ruggieri P, Molinari A. Molecular aspects of tumor cell migration and invasion. Ann Ist Super Sanita 2010;46:66-80. [Crossref] [PubMed]
- Lintz M, Muñoz A, Reinhart-King CA. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J Biomech Eng 2017;139:0210051-9. [Crossref] [PubMed]
- Cao H, Feng Y, Chen L, et al. Lobaplatin Inhibits Prostate Cancer Proliferation and Migration Through Regulation of BCL2 and BAX. Dose Response 2019;17:1559325819850981. [Crossref] [PubMed]
- He J, Zhang H. The antitumor effect of lobaplatin against Ishikawa endometrial cancer cells in vitro and in vivo. Biomed Pharmacother 2019;114:108762. [Crossref] [PubMed]
- Yin CY, Lin XL, Tian L, et al. Lobaplatin inhibits growth of gastric cancer cells by inducing apoptosis. World J Gastroenterol 2014;20:17426-33. [Crossref] [PubMed]
- Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 2019;18:26. [Crossref] [PubMed]
- Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res 2015;5:1602-9. [PubMed]
- Chen H, Zhou L, Wu X, et al. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 2016;21:1084-91. [Crossref] [PubMed]
- Ellis H, Ma CX. PI3K Inhibitors in Breast Cancer Therapy. Curr Oncol Rep 2019;21:110. [Crossref] [PubMed]
- Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019;698:120-8. [Crossref] [PubMed]
- Markham A. Idelalisib: first global approval. Drugs 2014;74:1701-7. [Crossref] [PubMed]
- Zirlik K, Veelken H. Idelalisib. Recent Results Cancer Res 2018;212:243-64. [Crossref] [PubMed]
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370:997-1007. [Crossref] [PubMed]
- Pan S, Sun Y, Sui D, et al. Lobaplatin promotes radiosensitivity, induces apoptosis, attenuates cancer stemness and inhibits proliferation through PI3K/AKT pathway in esophageal squamous cell carcinoma. Biomed Pharmacother 2018;102:567-74. [Crossref] [PubMed]
- Jiang W, Chen Y, Li B, et al. DBA-induced caspase-3-dependent apoptosis occurs through mitochondrial translocation of cyt-c in the rat hippocampus. Mol Biosyst 2017;13:1863-73. [Crossref] [PubMed]
- Ouyang Z, Yang B, Yi J, et al. Exposure to Fluoride induces apoptosis in liver of ducks by regulating Cyt-C/Caspase 3/9 signaling pathway. Ecotoxicol Environ Saf 2021; Epub ahead of print. [Crossref] [PubMed]
- Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res 2010;2:19-42. [PubMed]
- Zhan L, Zhang B, Tan Y, et al. Quantitative assessment of the relationship between RASSF1A gene promoter methylation and bladder cancer (PRISMA). Medicine (Baltimore) 2017;96:e6097. [Crossref] [PubMed]
- Siracusano S, Rizzetto R, Porcaro AB. Bladder cancer genomics. Urologia 2020;87:49-56. [Crossref] [PubMed]
- Yu X, Yang Y, Li Y, et al. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3K/Akt pathway. Int J Biochem Cell Biol 2018;94:107-18. [Crossref] [PubMed]
- Duan F, Mei C, Yang L, et al. Vitamin K2 promotes PI3K/AKT/HIF-1α-mediated glycolysis that leads to AMPK-dependent autophagic cell death in bladder cancer cells. Sci Rep 2020;10:7714. [Crossref] [PubMed]
- Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol Biol 2018;1655:335-50. [Crossref] [PubMed]
- Shu YJ, Weng H, Ye YY, et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer 2015;14:12. [Crossref] [PubMed]


