
Efficacy of keishibukuryogan for hot flashes in prostate cancer patients receiving androgen deprivation therapy: a sub-analysis focusing on hormonal and cytokine levels
Highlight box
Key findings
• Keishibukuryogan was more effective in patients with higher levels of TNF-α (P=0.0372) and IL-8 (P=0.0312) during androgen deprivation therapy for prostate cancer, with no significant change quantities of these blood levels by the medication.
What is known and what is new?
• Keishibukuryogan is an efficient and safe treatment for hot flashes in patients receiving androgen deprivation therapy.
• The present study elucidated hormonal and cytokine conditions in the patients with hot flashes, and showed that some effects of keishibukuryogan was potentially correlated with serum IL-8 and TNF-α.
What is the implication, and what should change now?
• Keishibukuryogan may prevent some of the actions of IL-8 and TNF-α, rather than suppressing their production, which is one possible mechanism for its effects.
Introduction
Treatment options for prostate cancer (PC), one of the most common cancers in man globally, are generally divided into hormonal therapy, prostatectomy, and radiotherapy, and these options are often chosen for each patient in consideration of the quality of life (QOL) and some complications associated with treatments, such as erectile dysfunction and urinary incontinence (1). Androgen deprivation therapy (ADT), which is medical castration by luteinizing hormone-releasing hormone (LH-RH) agonists or LH-RH antagonists combined with androgen blockade (CAB) therapy, is the most commonly used hormonal therapy for PC. In Japan, ADT is most commonly used, particularly in the elderly or patients with multiple complications (2,3).
In recent years, not only the efficiency of hormone therapy but also its adverse effects have attracted attention. Hot flashes were observed in approximately 80% of hormone therapy patients, which can frequently result in a decrease in QOL (4). Although the effectiveness of some agents for hot flashes caused by ADT has been reported (5,6), none have been presently established.
Keishibukuryogan, a Japanese traditional herbal medicine, is used to treat menopausal symptoms (headache, dizziness, hot flashes, stiff shoulders, etc.), and, has been used to treat hot flashes in hypogonadal men (7,8). Keishibukuryogan (TJ-25, TSUMURA & CO, Tokyo, Japan) was found to be an efficient and safe treatment for hot flashes in PC patients receiving ADT in a previous prospective study (9). However, the mechanisms of the efficacy of TJ-25 for hot flashes have been not well understood. We attempted to elucidate the mechanisms of TJ-25 efficacy by focusing on hormonal and cytokine levels in the current study. We present this article following the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-121/rc).
Methods
Study subjects
The current study is a sub-analysis of serum hormonal and cytokine levels extracted from the single-arm prospective study (UMIN 000031606) that evaluated the efficacy and safety of TJ-25 for hot flashes in PC patients receiving ADT (9). According to the study protocol, all participants were given TJ-25 at a dose of 2.5 g three times per day for 12 weeks (9). During the trial, all patients competed for a diary of their hot flashes conditions. The diary contained information on the strength and frequency. The intensity of hot flashes was measured using a visual analog scale (VAS) and quantified using a 100 mm VAS ruler Frequency was counted by the number of hot flashes per day. The strength and frequency of hot flashes were evaluated as the mean value recorded in a diary for 1 week before baseline and a 12-week visit.
Participants provided clinical information at the outset, including body mass index, duration of PC, clinical stage of PC, use of LH-RH agonist or antagonist, and presence of radical prostatectomy, radiation, and complications. At baseline and 12-week visits, various hormonal [total testosterone (TT), dehydroepiandrosterone (DHEA), dihydrotestosterone (DHT), all patients had [interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), macrophage chemotactic protein-1 (MCP-1, also known as CCL2), macrophage inflammatory protein-1β (MIP-1β, also known as CCL-4), and vascular endothelial growth factor (VEGF)] levels measured.
All participants provided written informed consent following a protocol approved by the Ethics Committee of the Kanazawa University Graduate School of Medical Science (No. 2017-214), and the study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Laboratory assays
All blood samples were collected between 09:00 and 11:00 in the morning at each visit. Blood samples were immediately centrifuged at 4 ℃, and serum was kept at −80 ℃ until assays were performed. Each laboratory received aliquots of each serum sample. Each hormone value was measured by Liquid Chromatograph - Mass Spectrometry (LC-MS/MS) (ASKA Pharma Medical Co., Ltd., Kanagawa, Japan). Each cytokine level was assessed in a TSUMURA & CO laboratory using a Bio-Plex Suspension Array System (Bio-Rad Laboratories, Hercules, CA, USA) and Luminex xMAP technology.
Sample size
This was a sub-analysis of a prospective observational pilot study (9). In the original study, the sample size had been calculated based on the number of patients undergoing hormone therapy per year at research facilities and incidence rate of hot flashes.
Statistical analysis
Initially, changes in hormone and cytokine levels from baseline to 12-week visit were analyzed by paired t-test. A correlation between the strength and consistency of hot flashes was examined by Spearman’s test. To elucidate the link between hormones or cytokines and hot flashes, the correlation of hot flashes with hormonal and cytokine levels at baseline was also examined employing Spearman’s test.
Next, we tried to examine the predictive factors for response to TJ-25 for hot flashes. According to the baseline median values of every hormone and cytokine, all subjects were split into two groups. The changes from baseline to 12-week visit in strength and frequency of hot flashes in both groups were compared by unpaired t-test. Furthermore, the relationship in change amounts (Δ values) by TJ-25 administration between hot flashes and each parameter was also conducted using Spearman’s test.
Two-tailed analysis was carried out in all analyses, and then a P value of <0.05 shows statistical significance.
Results
Patients characteristics
Twenty-five patients who completed 12-week treatment were included in the present sub-analysis. The age [mean ± standard deviation (SD)] of a total of 25 participants was 69.5±6.0 years (Table 1). As shown in our previous study (9), mean values of VAS and frequency of hot flashes were 51.8±21.3 and 4.2±2.2 at baseline, and 37.6±15.8 and 3.7±2.0 at the 12-week visit, and TJ-25 treatment for 12 weeks had significantly improved strength and frequency of hot flashes. Hormone and cytokine levels at baseline and 12-week visit are shown in Table 2. No significant changes in all of hormone and cytokine levels were observed by TJ-25 treatment.
Table 1
| Variables | Mean ± SD or number |
|---|---|
| Age (years) | 69.5±6.0 |
| BMI (kg/m2) | 24.0±3.7 |
| PSA levels (ng/mL) | 0.1±0.3 |
| Gleason score | 9.2±0.9 |
| TNM stage | |
| T1 | 1 |
| T2 | 9 |
| T3 | 12 |
| T4 | 3 |
| N | |
| N0 | 20 |
| N1 | 5 |
| M | |
| M0 | 21 |
| M1 | 4 |
| Duration of prostate cancer (years) | 2.8±3.2 |
| Hormonal therapy | |
| LH-RH agonist | 19 |
| LH-RH antagonist | 6 |
| Anti-androgenic therapy* | 20 |
| Radical radiation therapy | 9 |
| Prostatectomy | 7 |
| Complications | 6 |
| Strength of hot flashes (VAS) (mm) | 51.8±21.3 |
| Frequency of hot flashes (times/day) | 4.2±2.2 |
*, all cases used bicaltamide. BMI, body mass index; PSA, prostate specific antigen; LH-RH, luteinizing hormone-releasing hormone; VAS, visual analogue scale.
Table 2
| Variables | Baseline, mean ± SD (median) | 12-week visit, mean ± SD | P value |
|---|---|---|---|
| Hormone | |||
| Testosterone (ng/dL) | 74.2±40.9 (61.6) | 79.1±63.3 | 0.737 |
| DHT (ng/dL) | 10.5±9.0 (7.1) | 11.6±8.7 | 0.514 |
| Estradiol (pg/mL) | 1.7±0.8 (1.7) | 1.5±0.5 | 0.318 |
| DHEA (ng/mL) | 1.2±0.7 (1.1) | 1.2±0.7 | 1.000 |
| Progesterone (ng/mL) | 14.3±7.7 (13.2) | 16.2±8.0 | 0.261 |
| Cytokine | |||
| IFN-γ (IU/mL) | 29.7±33.9 (16.1) | 31.4±30.9 | 0.766 |
| IL-1β (pg/mL) | 10.2±21.3 (3.0) | 11.3±24.8 | 0.513 |
| IL-2 (pg/mL) | 4.8±7.0 (2.4) | 5.4±8.0 | 0.378 |
| IL-4 (pg/mL) | 62.2±83.1 (30.5) | 70.0±87.8 | 0.599 |
| IL-6 (pg/mL) | 3.4±3.5 (1.6) | 3.7±4.0 | 0.563 |
| IL-8 (pg/mL) | 4.1±2.5 (2.8) | 4.1±2.6 | 0.939 |
| IL-10 (pg/mL) | 29.0±51.7 (10.6) | 30.3±39.8 | 0.809 |
| MCP-1 (pg/mL) | 229.4±71.9 (209.1) | 242.9±66.1 | 0.256 |
| MIP-1β (pg/mL) | 43.3±30.1 (30.0) | 46.8±36.5 | 0.490 |
| TNF-α (pg/mL) | 19.4±14.3 (13.3) | 20.5±12.2 | 0.702 |
| VEGF (pg/mL) | 161.4±103.9 (129.8) | 176.4±100.3 | 0.446 |
SD, standard deviation; DHT, dihydrotestosterone; DHEA, dehydroepiandrosterone; IFN, interferon; IL, interleukin; MCP, macrophage chemotactic protein; MIP, macrophage inflammatory, protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
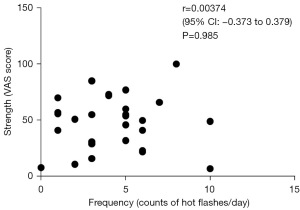
Initially, we investigated whether or not the intensity and frequency of hot flashes should be considered separately. Prior to treatment, there was no discernible relationship between VAS and hot flash frequency (r=0.00374, 95% CI: −0.373 to 0.379, P=0.985) (Figure 1). As a result, hot flashes were examined separately for strength and frequency in the present study.
The relationship between the intensity and frequency of hot flashes and hormone/cytokine levels
Strength was inversely correlated with estradiol levels (r=−0.433, P=0.019), and frequency revealed an inverse correlation with progesterone levels (r=−0.415, P=0.025) (Table 3). However, there were no significant correlations between hot flashes symptoms and all of the cytokine levels (Table 4).
Table 3
| Testosterone | DHEA | DHT | Estradiol | Progesterone | |
|---|---|---|---|---|---|
| Strength (VAS) | |||||
| r | −0.139 | 0.051 | 0.187 | −0.433 | −0.041 |
| P value | 0.471 | 0.795 | 0.332 | 0.019* | 0.833 |
| Frequency | |||||
| r | −0.315 | −0.105 | 0.041 | −0.044 | −0.415 |
| P value | 0.096 | 0.588 | 0.835 | 0.821 | 0.025* |
*, significant difference (two-tailed test). VAS, visual analog scale; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone.
Table 4
| IL-1β | IL-2 | IL-4 | IL-6 | IL-8 | IL-10 | TNF-α | IFN-γ | MCP-1 | MIP-1β | VEGF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength (VAS) | |||||||||||
| r | 0.259 | 0.065 | 0.206 | 0.238 | 0.066 | 0.119 | 0.146 | 0.109 | 0.161 | 0.088 | −0.006 |
| P value | 0.175 | 0.736 | 0.284 | 0.214 | 0.734 | 0.538 | 0.45 | 0.572 | 0.405 | 0.649 | 0.974 |
| Frequency | |||||||||||
| r | −0.075 | 0.11 | 0.061 | 0.182 | 0.33 | 0.071 | 0.194 | 0.153 | 0.237 | 0.239 | −0.012 |
| P value | 0.7 | 0.57 | 0.755 | 0.346 | 0.08 | 0.715 | 0.313 | 0.429 | 0.216 | 0.212 | 0.951 |
VAS, visual analog scale; IL, interleukin; TNF, tumor necrosis factor; MCP, macrophage chemotactic protein; MIP, macrophage inflammatory protein; VEGF, vascular endothelial growth factor.
Responder analysis based on hormone/cytokine levels
All subjects were divided into two groups according to the baseline median values (Table 2) of every hormone and cytokine, and the responder analysis was performed. The efficacy of TJ-25 for the strength of hot flashes was significantly correlated with higher levels of TNF-α at baseline (P=0.0372) (Figure 2). Moreover, TJ-25 was more effective in frequency among the patients with higher levels of IL-8 (P=0.0312). A slight improvement in frequency was discovered in patients with higher baseline MCP-1 levels, but it was not significant (P=0.0652). Improvement in hot flashes with TJ-25 treatment was not linked with any other cytokine or hormone levels. However, ΔTNF-α and ΔIL-8 were not significantly correlated with Δstrength and Δfrequency of hot flashes (Figure 3). In addition, the Δstrength or Δfrequency of hot flashes by TJ-25 administration were not significantly associated with Δ values of the other hormonal and cytokine (Tables 5,6).


Table 5
| ΔTestosterone | ΔDHEA | ΔDHT | ΔEstradiol | ΔProgesterone | |
|---|---|---|---|---|---|
| ΔStrength (VAS) | |||||
| r | 0.071 | 0.126 | 0.136 | −0.091 | 0.125 |
| P | 0.735 | 0.548 | 0.517 | 0.666 | 0.553 |
| ΔFrequency | |||||
| r | 0.253 | 0.244 | 0.172 | 0.152 | 0.167 |
| P | 0.223 | 0.240 | 0.412 | 0.467 | 0.425 |
TJ-25, keishibukuryogan; VAS, visual analog scale; DHEA, dehydroepiandrosterone, DHT, dihydrotestosterone.
Table 6
| ΔIL-1β | ΔIL-2 | ΔIL-4 | ΔIL-6 | ΔIL-8 | ΔIL-10 | ΔTNF-α | ΔIFN-γ | ΔMCP-1 | ΔMIP-1β | ΔVEGF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔStrength (VAS) | |||||||||||
| r | 0.053 | −0.180 | −0.138 | −0.088 | −0.272 | −0.138 | −0.224 | −0.156 | −0.213 | −0.195 | −0.186 |
| p | 0.801 | 0.389 | 0.512 | 0.677 | 0.188 | 0.509 | 0.282 | 0.456 | 0.307 | 0.351 | 0.373 |
| ΔFrequency | |||||||||||
| r | 0.093 | 0.015 | 0.077 | 0.077 | 0.015 | 0.031 | 0.009 | 0.026 | −0.244 | 0.038 | −0.027 |
| p | 0.658 | 0.945 | 0.714 | 0.713 | 0.942 | 0.884 | 0.967 | 0.903 | 0.241 | 0.856 | 0.900 |
TJ-25, keishibukuryogan; VAS, visual analogue scale; IFN, interferon; IL, interleukin; MCP, macrophage chemotactic protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Discussion
In the previous prospective study, we showed that TJ-25 was an efficient and safe treatment for hot flashes in PC patients. The strength of hot flashes significantly improved from 4 weeks after TJ-25 administration, and frequency was significantly decreased at the 8-week visit (9). The goal of the current investigation was to clarify the processes underlying the effectiveness of TJ-25 with a particular emphasis on serum cytokine and hormone levels. To our knowledge, this is the first study to examine the relationships of hot flashes with serum cytokine and hormonal levels among PC patients with ADT.
Initially, no significant correlation between VAS and the frequency of hot flashes was not observed, and consequently, the strength and frequency of hot flashes were examined separately in the present analysis, which is likely to be a unique study. We discovered that the strength of hot flashes was inversely associated with estradiol levels, and frequency was inversely correlated with progesterone levels. Numerous earlier studies have shown that the development of hot flashes in hypogonadal men or male castrated rats is significantly influenced by calcitonin gene-related peptide (CGRP), a powerful vasodilator and a factor in increased regional blood flow (10-15). The decrease in estrogen and progesterone is reported to be associated with the CGRP-induced elevation of skin temperature (13-15). The passed reports explained the upregulation of vessel CGRP receptors in the rats with estrogen and testosterone-deficient rats, and consequently, the skin temperature elevation induced by CGRP injection was significantly greater (15,16). Certain pressures or stimulations may cause the release of CGRP when there is a lack of sex hormones, which could increase the frequency of hot flashes (17). These opinions are likely to support our results that estradiol was importantly correlated with the strength of hot flashes. Furthermore, progesterone inhibited CGRP-induced skin temperature elevation in a dose-dependent manner in the castrated rats (15). Indeed, some placebo-regulated researches have illustrated that medroxyprogesterone alleviated hot flashes in the mail with ADT (5,18). However, mechanisms of how progesterone can affect hot flashes have remained unclear.
On the other hand, no important correlations between hot flashes and any cytokine levels at baseline were observed in the current research, although consistent hot flashes revealed a weak positive correlation with IL-8 levels without a statistical difference. various passed research involving menopausal and postmenopausal women demonstrated that multiple inflammatory cytokines and chemokines, such as IL-6, IL-8, TNF-α, and MIP-1β, also played some significant roles in the improvement of hot flashes, and indicated that hot flashes might be associated with low-grade systemic inflammation (19-21). Particularly, a potential association of IL-8 with hot flashes in women was supported by various passed research (19-21). As with the roles of estrogen in women, it has been widely accepted that testosterone also has some significant roles in the creation and activity of many cytokines in men (22,23). However, our research showed that hot flashes were not importantly correlated with any cytokine levels. For this reason, our researched population was made up of PC patients with ADT, and consequently, the castrated surroundings are likely to be completely different from the menopausal and postmenopausal conditions. Furthermore, the number of subjects included in the current research was extremely limited. There have been currently no passed studies to investigate the link between hot flashes and serum cytokine levels among PC patients with ADT, and further studies involving a large number of subjects need to get to a more definite conclusion.
In the current research, TJ-25 was more efficient in the patients with greater levels of TNF-α for the strength of hot flashes, and IL-8 for consistency, which may also be an interesting finding. Some passed studies targeted to women contributed that the efficacy of TJ-25 was included in circulating cytokine and chemokine levels. TJ-25 has been reported to be reduced inflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α, MCP-1, and MIP-1β (21,24-26). One earlier study found that premenopausal, perimenopausal, and postmenopausal women who received TJ-25 for hot flashes experienced a substantial decrease in blood IL-8 and MCP-1 concentrations in the treatment-responder group (21). We observe significant correlations between baseline serum IL-8 and TNF-α levels, which is likely to be partially consistent with the previous findings. However, there was no correlation between the strength or frequency of hot flashes caused by TJ-25 administration and the levels of these cytokines from baseline to 12-month visits. In particular, ΔIL-8 and ΔTNF-α were not significantly correlated with Δstrength and Δfrequency of hot flashes. TJ-25’s effects, contrary to previous findings (21), were not due to a decrease in these cytokine levels. These findings suggest that TJ-25 does not directly suppress IL-8 and TNF-α production in PC patients with ADT, but it may inhibit some of these cytokines’ actions that are associated with the development of hot flashes. However, differences in the relevance of cytokines in the strength and frequency of hot flashes have remained unclear, and further research is expected.
As an alternative mechanism of the effects of TJ-25 on hot flashes, one previous report demonstrated that TJ-25 decreased plasma CGRP levels in postmenopausal women with hot flashes (17). According to one experimental study using human keratinocyte cell lines, CGRP could upregulate IL-8 receptor mRNA expression without increasing IL-8 production (27). This suggests that CGRP suppression may inhibit some IL-8 actions by downregulating its receptor, with no changes in IL-8 as our results indicate. However, there have been no vivo studies to examine the correlation between CGRP and IL-8/IL-8 receptor. Furthermore, because CGRP was not measured in the current study, more research is needed to support this hypothesis.
There are several limitations in the present study that are required to be addressed. To begin with, the number of study subjects is extremely limited. Next, this was a single-arm cross-sectional study with no controls. Furthermore, duration of ADT was not examined in the original study (9), and then a correlation between hot flashes and duration of ADT was not discussed. Duration of ADT may have some effects on cytokine levels. In addition, hot flashes are a subjective reporting of symptoms that can be influenced by many factors such as TJ-25 placebo effects, memory, and other psychological factors. However, this is the first study to examine the efficacy of TJ-25 in men with ADT, focusing on different hormonal and cytokine levels, and it is likely to be a valuable preliminary study. As a result, additional randomized controlled studies involving a sizable number of participants and controls are necessary to verify the findings of this investigation.
Conclusions
The strength of hot flashes was inversely correlated with estradiol levels, and frequency was inversely correlated with progesterone levels. TJ-25 was more effective in patients with higher levels of TNF-α for hot flash strength and IL-8 for frequency, with no significant change in serum levels caused by the treatment. TJ-25 may inhibit some cytokine actions but does not suppress their production.
Acknowledgments
The authors want to highlight the significant technical contributions of Toshitaka Kido and Keita Mizuno from Tsumura & Company, Tokyo, Japan. The study team deeply appreciates the technical support in data collection by some coordinators at Innovative Clinical Research Center, Kanazawa University. Furthermore, we would like to thank Enago (www.enago.jp) for the English language review.
Funding: This work was supported by specific funding from Tsumura & Co., Tokyo, Japan.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-121/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-121/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-121/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-121/coif). The authors report that this study was supported by specific funding from Tsumura & Co., Tokyo, Japan. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethical Committee of the Kanazawa University Graduate School of Medical Science (No. 2017-214) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants in the study provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int 2013;1:117-24. [Crossref] [PubMed]
- Fujimoto H, Nakanishi H, Miki T, et al. Oncological outcomes of the prostate cancer patients registered in 2004: report from the Cancer Registration Committee of the JUA. Int J Urol 2011;18:876-81. [Crossref] [PubMed]
- Akaza H. Future prospects for luteinizing hormone-releasing hormone analogues in prostate cancer treatment. Pharmacology 2010;85:110-20. [Crossref] [PubMed]
- Nishiyama T, Kanazawa S, Watanabe R, et al. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol 2004;11:735-41. [Crossref] [PubMed]
- Irani J, Salomon L, Oba R, et al. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial. Lancet Oncol 2010;11:147-54. [Crossref] [PubMed]
- Moraska AR, Atherton PJ, Szydlo DW, et al. Gabapentin for the management of hot flashes in prostate cancer survivors: a longitudinal continuation Study-NCCTG Trial N00CB. J Support Oncol 2010;8:128-32. [PubMed]
- Amano T, Imao T, Takemae K. Clinical efficacy of Japanese traditional herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male 2010;13:166-73. [Crossref] [PubMed]
- Tsujimura A, Nonomura N. Recent topics related to testosterone deficiency syndrome in Japan. Asian J Androl 2011;13:558-62. [Crossref] [PubMed]
- Shigehara K, Izumi K, Nakashima K, et al. Efficacy and safety of keishibukuryogan, a traditional Japanese Kampo medicine, for hot flashes in prostate cancer patients receiving androgen deprivation therapy. Transl Androl Urol 2020;9:2533-40. [Crossref] [PubMed]
- Spetz AC, Pettersson B, Varenhorst E, et al. Momentary increase in plasma calcitonin gene-related peptide is involved in hot flashes in men treated with castration for carcinoma of the prostate. J Urol 2001;166:1720-3. [Crossref] [PubMed]
- Wyon Y, Spetz AC, Hammar M, et al. Urinary excretion of calcitonin gene-related peptide in males with hot flushes after castration for carcinoma of the prostate. Scand J Urol Nephrol 2001;35:92-6. [Crossref] [PubMed]
- Yuzurihara M, Ikarashi Y, Noguchi M, et al. Involvement of calcitonin gene-related peptide in elevation of skin temperature in castrated male rats. Urology 2003;62:947-51. [Crossref] [PubMed]
- Keast JR, Gleeson RJ. Androgen receptor immunoreactivity is present in primary sensory neurons of male rats. Neuroreport 1998;9:4137-40. [Crossref] [PubMed]
- Gangula PR, Lanlua P, Wimalawansa S, et al. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod 2000;62:1033-9. [Crossref] [PubMed]
- Yuzurihara M, Ikarashi Y, Noguchi M, et al. Prevention by 17beta-estradiol and progesterone of calcitonin gene-related peptide-induced elevation of skin temperature in castrated male rats. Urology 2004;64:1042-7. [Crossref] [PubMed]
- Noguchi M, Ikarashi Y, Yuzurihara M, et al. Effects of the Japanese herbal medicine Keishi-bukuryo-gan and 17beta-estradiol on calcitonin gene-related peptide-induced elevation of skin temperature in ovariectomized rats. J Endocrinol 2003;176:359-66. [Crossref] [PubMed]
- Chen JT, Shiraki M. Menopausal hot flash and calciotonin gene-related peptide; effect of Keishi-bukuryo-gan, a kampo medicine, related to plasma calciotonin gene-related peptide level. Maturitas 2003;45:199-204. [Crossref] [PubMed]
- Langenstroer P, Kramer B, Cutting B, et al. Parenteral medroxyprogesterone for the management of luteinizing hormone releasing hormone induced hot flashes in men with advanced prostate cancer. J Urol 2005;174:642-5. [Crossref] [PubMed]
- Huang WY, Hsin IL, Chen DR, et al. Circulating interleukin-8 and tumor necrosis factor-α are associated with hot flashes in healthy postmenopausal women. PLoS One 2017;12:e0184011. [Crossref] [PubMed]
- Malutan A, Costin N, Duncea I, et al. Interleukin-8 and vasomotor symptoms in natural and surgically induced menopause. Acta Endo (Buc) 2013;9:133-44. [Crossref]
- Yasui T, Matsui S, Yamamoto S, et al. Effects of Japanese traditional medicines on circulating cytokine levels in women with hot flashes. Menopause 2011;18:85-92. [Crossref] [PubMed]
- Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019;22:129-40. [Crossref] [PubMed]
- Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 2006;91:345-7. [Crossref] [PubMed]
- Tanaka K, Chiba K, Nara K. A Review on the Mechanism and Application of Keishibukuryogan. Front Nutr 2021;8:760918. [Crossref] [PubMed]
- Yoshihisa Y, Furuichi M, Ur Rehman M, et al. The traditional Japanese formula keishibukuryogan inhibits the production of inflammatory cytokines by dermal endothelial cells. Mediators Inflamm 2010;2010:804298. [Crossref] [PubMed]
- Nakagawa T, Tashiro I, Fujimoto M, et al. Keishibukuryogan reduces renal injury in the early stage of renal failure in the remnant kidney model. Evid Based Complement Alternat Med 2011;2011:914249. [Crossref] [PubMed]
- Kiss M, Kemény L, Gyulai R, et al. Effects of the neuropeptides substance P, calcitonin gene-related peptide and alpha-melanocyte-stimulating hormone on the IL-8/IL-8 receptor system in a cultured human keratinocyte cell line and dermal fibroblasts. Inflammation 1999;23:557-67. [Crossref] [PubMed]


