
Penile and testicular prosthesis following gender-affirming phalloplasty and scrotoplasty: a narrative review and technical insights
Introduction
Transgender and gender diverse (TGD) individuals may seek gender-affirming phalloplasty with specific functional goals, including the ability to stand to void, and for some, to have erectile function sufficient for penetrative sexual intercourse. Phalloplasty technique, individual anatomic variation, and patient preferences will determine surgical options for erectile function, however, most will require a penile implant adapted for neophallic anatomy (1,2).
Radial forearm free flap and anterolateral thigh flaps, the most common phalloplasty approaches, and less common abdominal and groin flap phalloplasties, typically require an erectile device for penetrative sex. In addition to risks of injury to reconstructed phallic structures, absence of a defined crural space lined with tunica albuginea present challenges for implantation and prosthesis stabilization.
We review current practices at our center and literature discussing penile prosthesis and testicular prosthesis placement after phalloplasty and scrotoplasty, surgical outcomes, and quality of life outcomes where available. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-122/rc).
Methods
A scientific literature search utilizing PubMed was performed to comprehensively review advancements and surgical outcomes for gender-affirming phalloplasty patients from all years of publication (Table 1). Keywords used to identify articles included: “transgender”, “trans/transgender men/man/male”, “phalloplasty” and “erectile device”, “penile prosthesis”, “penile implant”, “testicular prosthesis”, or “neophallus” returned 134 publications. Peer reviewed articles published in English were included. Articles that included cisgender males or patients under 18 were excluded. Articles including results from retrospective, prospective, case and systematic reviews were selected for review if they evaluated comparative metrics (e.g., complications and patient reported outcomes) across penile and scrotal prosthesis types and reported surgical methods.
Table 1
| Items | Specification |
|---|---|
| Date of search | November 2022, repeated February 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “transgender”, “trans/transgender men/man/male”, “phalloplasty” and “erectile device”, “penile prosthesis”, “penile implant”, “testicular prosthesis”, or “neophallus” |
| Timeframe | 1975 to present |
| Inclusion and exclusion criteria | Inclusion: English language, any study type (retrospective, prospective, case and systematic reviews). Exclusion: articles that only included cisgender males or patients under 18 |
| Selection process | Reviewed by all authors independently |
Penile prostheses
Options for erectile function have evolved with phalloplasty technique over the last 100 years. The first penile reconstruction with tubularized skin flap adapted for erection was described by Bogoras in 1936, using rib cartilage as the erectile support (3). The rib cartilage maintained rigidity but did not allow for a flaccid state and added curvature, distorting the penis. Attempts to use bone with periosteum, including free radial osteocutaneous flaps and fibula flaps, are associated with resorption of bone over time (4). Latissimus dorsi myocutaneous free flaps may stiffen and shorten when contracted (5), however these may still require erectile device placement for adequate rigidity. Selvaggi et al. described a two-stage titanium bone anchoring to the pubis (6). Following preoperative computed tomography (CT) imaging to determine adequate pubic bone size, titanium fixtures were implanted onto the pubic bone and reassessed four weeks later for insertion of penile epithesis; interval imaging demonstrated successful titanium osseointegration in their five-patient series (6).
The first use of a hydraulic prosthesis was in the late 1970s by Puckett and Montie and was associated with high failure rates, cosmetic deformity of the phallus, and implant infection (7). Later series demonstrated more success, including successful intromission (8), erogenous sensation for orgasm (9), and device longevity (8,9). While the complication rate remains high, inflatable devices remain most frequently used (10-12). Although none of the commercially available penile prosthesis devices in the United States are designed for phalloplasty, modern inflatable and malleable prostheses are adapted for use in the post-phalloplasty setting.
Criteria and considerations prior to penile prosthesis placement
Individuals seeking penile prosthesis placement must accept the potential risks to their phallic anatomy. Early discussion of a staged approach to phallic construction with a last step of implant placement is important during initial phalloplasty counseling. To decrease risk of complications after prosthesis placement, we require that any major urethral complications (e.g., stricture, large fistulae) and/or cosmetic concerns (e.g., scrotal revision) must be addressed with appropriate interval of healing prior to proceeding with prosthesis (13). This aims to decrease risk of infection and need for additional surgeries risking device injury or exposure following prosthesis placement. The authors typically request 6 months of healing after urethroplasty or other genital surgeries prior to implant placement. In the absence of urethral or cosmetic issues, post-phalloplasty nerve coaptation and return of sensation may decrease risk of pressure necrosis (8-12,14-17).
Pre-operative preparation and counseling at our multi-disciplinary center includes: discussion of surgical history, complications, goals and priorities; physical exam to evaluate phallic size and position, scrotal size, and other anatomic findings that may influence prosthesis selection; urinary evaluation, including uroflowmetry with post-void residual, and a cystoscopy with retrograde urethrogram if indicated based on symptoms or urinary studies; and discussion of surgical risks, benefits, and alternatives. We utilize anonymous patient photos to demonstrate variations in patient anatomy and potential aesthetic and functional outcomes. Patients who choose an inflatable penile prosthesis are advised that the scrotal pump and reservoir will be placed contralateral to the vascular supply of the phallus. If desired, testicular implant size and style are selected.
Surgical technique and post-operative care
Many surgical approaches and perioperative strategies have been described for erectile device placement after phalloplasty. Here, we describe the current approach at our center, recognizing the evolving nature of surgical care in this nascent field.
Preoperative antibiotic prophylaxis is administered at the discretion of the surgeon (13), and is often institution dependent and may include cephalosporins and aminoglycosides prior to incision (18). Urinary catheterization is performed to limit the risk of urethral injury during dissection, and full bladder drainage is important to reduce risk of bladder injury if pre-vesical reservoir placement is anticipated. The patient may be asked to void prior to surgery and this may be all that is necessary in phalloplasty without urethral lengthening. We prefer urethral catheter placement in all patients with urethral lengthening, which may require cystoscopic guidance.
At our center, patients are positioned in a modified frog-leg position with padding supporting the knees (Figure 1). The patient is prepped and sterilized. Surgical draping ensures access to the perineum. A catheter is placed prior to prep and draping in patients without urethral lengthening. Otherwise, we attempt catheter placement with a 16 French coude catheter once; if unsuccessful, we attempt catheterization over a hydrophilic guide wire. If still unable to catheterize, we utilize a flexible cystoscope to navigate the phallic urethra and place a council tip catheter over a wire. We then use adhesive antimicrobial drapes (IobanTM, product of 3M, St. Paul, MN, USA) to wrap the penis and catheter, reducing prosthesis skin contact time.

We make note of the vascular pedicle location based on operative notes and surgical scars to ensure familiarity with the neurovascular supply of the flap (Figure 2). Intraoperative Doppler ultrasound is also used to localize and avoid the vasculature (19). This information may alter our surgical incision planning, skewing our incision away from the pedicle (Figure 3).


Several approaches have been described for placement of penile prostheses in phalloplasty: infrapubic or penopubic, penoscrotal, parascrotal, and perineal. The authors prefer a midline penopubic incision that may be shifted slightly askew of midline, favoring the direction opposite of the neurovascular pedicle. Dissection is carried through the incision and subcutaneous tissues, with care to avoid the vascular pedicle injury and urethra; while it is ideal to avoid the neural anastomoses, these are unlikely to be visualized. We perform dissection along the anterior aspect of the pubic symphysis and clear a space approximately two centimeters wide, down to the pubic arch or to the superior aspect of the inferior pubic ramus for prosthesis anchoring.
Distally, subcutaneous space for the erectile cylinder is created with a combination of sharp and blunt dissection. We begin with Metzenbaum scissors to create a space superior to the urethra, along the midline of the phallus. Hegar or Brooks dilators can then be used for dilation, with care distally to maintain some degree of “padding” between the device and glans tip. We aim to seat the prosthesis tip at the level of the mid glans. Following the dissection, intraoperative measurements (proximal, down to inferior pubic ramus, and distal, to mid-glans) are taken to determine optimal implant size.
We often use one cylinder, though two may be required based on the size of the phallus (11,15,19). A single cylinder may be aesthetically superior to dual cylinder devices given potential for asymmetry at the distal glans and insufficient cushioning with two cylinders (13,19). In a single-cylinder placement, both cylinders are filled, one cylinder is cut away, and the tubing end is then capped.
Following implant preparation, proximal anchoring of the erectile cylinder and neotunical reconstruction must be considered. Many institutions have adapted the use of vascular grafts or mesh, which are fashioned into a sheath and sutured over the proximal length of the implant cylinder, leaving an opening in the sheath for the tubing exit. The sheath and sometimes the proximal aspect of the cylinder are secured to the inferior aspect of the pubic symphysis for proximal fixation. A variety of proximal bone anchoring techniques have been described (9,10,12,15,17,19-21). Typically, nonabsorbable suture are used to attach the implant to the periosteum of the pubic symphysis. In our institution, we use a Hemagard knitted vascular graft and preplace three to four FiberWire suture through the pubic periosteum prior to seating the cylinder (Figures 4,5). After the Hemagard graft is trimmed and sutured to itself around the base of the prosthesis, the FiberWire sutures are placed through the Hemagard alone (in the setting of prosthesis cylinder reservoirs) or through the Hemagard and the prosthesis, ensuring adequate spacing to distribute the bone anchoring and fixation across the implant.

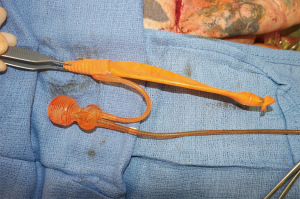
Cylinders are seated in the phallus using the Furlow tool if an inflatable implant is used; otherwise, the malleable device is seated in the pre-dilated phallic space. Bony fixation sutures are passed through cylinder or sheath and secured. If a multi-component prosthesis is used, then the reservoir is placed into the pre-peritoneal or subrectus space, through the infrapubic incision or a counter incision if unable to safely enter the inguinal ring for placement. The pump is placed into the hemiscrotum and a testicular prosthesis may be placed in the contralateral hemiscrotum (see later section on testicular prosthesis) (Figure 6).

Wound closure is performed in multiple layers at the midline incision, typically with delayed absorbable suture [Polydiaxonone Suture (PDS), Ethicon/Johnson and Johnson Medical, Bridgewater, NJ, USA; Maxon, Covidien/Medtronic, Minneapolis, MN, USA] and we prefer to close the skin with horizontal mattress nonabsorbable suture (Prolene, Ethicon/Johnson and Johnson Medical; Surgipro, Covidien/Medtronic), though absorbable suture for skin closure is also common. Use of nonabsorbable suture, removed at 2-week follow-up, is performed to reduce infrapubic scar widening and optimize aesthetics.
Post-operatively, patients may be discharged home or admitted for monitoring overnight. Foley catheters are removed on the day of discharge. We leave the inflatable devices partially inflated for 7–10 days with initial cycling at 4–6 weeks and clearance for penetrative sexual activity at 3 months (Figure 7).

Prosthesis types
Patient goals and anatomy may determine which implantable erectile prosthesis will work best. Currently, the main categories of implantable erectile devices are semirigid (malleable) and inflatable prostheses. AMS 700 (Boston Scientific, Marlborough, MA, USA), Ambicor (Boston Scientific), Titan (Coloplast, Minneapolis, MN, USA) are commercially available inflatable penile prostheses in the United States. Common malleable prostheses include Spectra, Tactra (both Boston Scientific) and Genesis (Coloplast). ZSI (Zephyr Surgical Implants, Geneva, Switzerland, e.g., malleable ZSI 100 FTM, hydraulic ZSI 475 FTM) are commonly reported in the literature from European centers. These erectile prostheses have several modifications including a rear tip amenable to bony fixation, a wider distal tip to decrease distal pressure and therein decrease erosion risk, and a single wider cylinder for rigidity and girth (22-24). These are not currently commercially approved by the Food and Drug Administration (FDA) for use in the U.S.
Semirigid or malleable prosthesis, while less common, carry the advantage of fewer components, with the absence of a scrotal pump or reservoir (25). This makes them more appropriate in patients with limited scrotal space, no scrotoplasty or significant prior pelvic surgery. To date, studies comparing malleable and inflatable penile prosthesis in phalloplasty are limited. One 31-patient cohort reported an increased overall complication rate in the malleable device group (28% vs. 10% inflatable) and was not powered to reach statistical significance (17). A 2019 cohort of 32 patients demonstrated high complication rates between malleable and inflatable devices (75% vs. 62.5%) (26).
Inflatable prostheses are widely used. Early series described the use of a single cylinder hydraulic prosthesis known as the Dynaflex (AMS, Boston Scientific) (6,16) which was subsequently withdrawn from the market in 1997 following increased adaptation of multi-component inflatable prosthesis (27). Inflatable prostheses allow for depressurized states improving the concealability (28,29) and may decrease the risk of glans discomfort and extrusion from chronic pressure (10,15). The multi-component nature is subject to mechanical failure. There is limited data regarding prosthesis longevity in the phalloplasty population; median device expectancy ranged from 4–5 years (12,21). Given that phalloplasty patients tend to undergo prosthesis placement at a younger age, patients are counseled that they will likely require multiple prosthesis revisions over their lifetime (6,11,12,18,21).
Complications
Complications after phalloplasty prosthesis placement are common. Infection requiring explantation, device extrusion, erosion, migration or malposition, inadequate rigidity, poor aesthetic result, pain, decrease or loss of erogenous and/or tactile sensation, device failure, injury to the urethra, and injury to the neurovascular supply of the penis with resultant partial or complete flap loss should all be discussed during preoperative counseling. Scientific literature does not qualify these complications well, reporting ranges of complications from 20% to 80% (12,14,17,26,30,31).
Based on our described technique, our complication rate is reported in Table 2.
Table 2
| Variables | Values |
|---|---|
| Patients, n | 45 |
| Devices, n | |
| Malleable | 11 |
| Two-piece | 7 |
| Three-piece | 25 |
| Testicular implants | 41† |
| Primary vs. revision, n (%) | 36 (80.0) vs. 9 (20.0) |
| Complications, n (%) | |
| Pubic or pelvic pain | 3 (6.7) |
| Change in sensation | 1 (2.2) |
| Cellulitis | 5 (11.1)‡ |
| Migration | 2 (4.4) |
| Mechanical failure | 3 (6.7) |
| Extrusion of testicular implant | 2 (4.4) |
| Penile prosthesis erosion | 2 (4.4) |
Notably, both penile prosthesis erosions occurred in revision patients. Seven patients in total required device explantation and revision. †, three patients underwent testicular implant placement only; ‡, cellulitis managed non-operatively with oral or parenteral antibiotics.
Infection
Prosthesis infection is higher in phalloplasty compared with natal penises (10% vs. 1.1%, respectively) (13,32,33). These infections are typically managed with device removal and wound washout followed by delayed device replacement. In our experience, superficial soft tissue infections may be managed with oral antibiotics and close observation; however, progression to deeper infections with device implications require explantation. The largest cohort study of 247 transgender men after phalloplasty and penile prosthesis demonstrated an infection rate of 8.5% without any predictive factors (12). The group assessed number of cylinders and protective measures such as antibiotic device coating and neotunical constructed grafts (12). Another large cohort of 129 patients demonstrated a 11.9% infection rate (11). Briles et al. reported an infection rate of 20% in a cohort of 80 patients and found that prior urethral revision and concurrent procedures were not associated with increased infection rate (18). The same group also reported lower rate of infection among semirigid devices compared to inflatable (1/13 semirigid vs. 15/67 inflatable) and a declining rate of infections noted as surgeon case number increased (18).
Vascular pedicle injury
Patients are counseled pre-operatively on the risk of full or partial flap loss. Injury to the vascular supply of the penis is rare and reported in a single patient from a single study (17). This required prompt recognition and immediate microvascular repair, followed by delayed implant placement. Intraoperative measures to reduce risk of injury to the vascular pedicle include use of intra-operative doppler, which is particularly important if the surgeon placing the implant was not present for or is unfamiliar with the initial phalloplasty approach.
Migration or loss of anchoring
Given the lack of native corpora cavernosa in phalloplasty and need for surgical fixation, device migration or inadequate fixation are potential risks. Because improperly anchored devices are typically insufficient for penetrative sex, these may require revision of the anchoring mechanism or complete device replacement. Device migration occurred in 3.2–10% among case series (17,18,30,31). Using an implant graft as an attempt to mitigate surgical fixation has been associated with device dysfunction by several groups (11,12,21); they posit that this may be a result of increased friction on the implant (21), though the grafts remain in use for proximal fixation (12,21). One study compared outcomes with and without vascular grafts and was unable to find any significant difference between malposition or device malfunction (21).
Cylindrical malposition within glans
Estimating the location of the distal cylindrical tip within the glans is challenging in phalloplasty due to the variable nature of phalloplasty anatomy and lack of natural glans cushion. Phallic size, fat composition, and position, as well as the degree of suprapubic or mons fat all influence the ultimate “settling” of the cylindrical tip within the glans, and can also change with patient weight fluctuation and greater time after initial phalloplasty. For some, the cylindrical tips may not extend far enough into the glans when fully erect due to undersized cylinders, shifts in positioning, changes in body weight and fat distribution. This may lead to supersonic transporter deformity or an unsupported glans (34), leading to aesthetic dissatisfaction and difficulty inserting the phallus for penetrative intercourse (35). On the other hand, patients who have minimal fat within the phallus may have visible and palpable cylindrical tips under the glans, which are not necessarily a cause for surgical revision if the skin remains mobile over the tip and the patient is not experiencing pain or discomfort related to this (36).
Device erosion and extrusion
Due to the lack of protective corporal tissue, erosion and extrusion are thought to be more prevalent in phalloplasty than in natal penises. Rates of extrusion and erosion range from 2–33% (11,18,19,21,31) and are higher among individuals with malleable prostheses. Excess pressure of the distal cylinders under the glans will present as persistent discoloration and skin changes over the cylindrical tips; these findings signal impending erosion and should prompt surgical revision with shorter cylinders or proximal repositioning (36). Complete erosion and extrusion of an implant through the urethra or glans tip are indications for device explantation and require period of complete wound healing (at least 6 months) before reimplantation. Subsequent prosthesis placement is typically more challenging due to phallic contraction and scar tissue development.
Mechanical device failure
Mechanical device failure in phalloplasty is reported at high rates, ranging from 7.0% to 15.4% (12,14,19,21). The Falcone et al. series of 247 patients showed that cylinder rupture (69%), cylinder aneurysm (19%), and rupture of connective tubing (12%) were the most common mechanical dysfunctions (12). Hoebeke et al. reported in their experience that dysfunction and leakage were higher in transgender men compared to cisgender men: a dysfunction rate of 14.5% to 4.3% in implants in natal penises and a 17.4% rate of device leakage compared to 10.8% (using three-piece prostheses) (11). Other studies reviewed did not distinguish from mechanical device failure, leak, or dysfunction (18,30,31).
Need for surgical revision
Any of the above complications may be an indication of explantation or device replacement, contributing to the lower average lifespan of erectile devices after phalloplasty. Other indications for revision may include scrotal pump migration (Figure 8), post-operative pain from pubic fixation (19), aesthetic dissatisfaction or change in patients’ device preference (26), or inadequate rigidity (19,26). The most robust retrospective studies of prosthesis in patients after phalloplasty have demonstrated a lifespan ranging from 4 to 5 years for 60–78% of patients (11,12,21).
Patient reported outcomes
Patient reported outcomes of erectile devices after phalloplasty remain limited by lack of standardized tools to assess patient satisfaction and other subjective outcomes. Sensation outcomes, particularly tactile and erogenous sensation, are not reported consistently from before and after implantation (30). Falcone et al. reported an 83% satisfaction rate with phallic sensation via non-validated questionnaire (12).
Several cohort studies demonstrate a high patient satisfaction after phalloplasty penile prosthesis. Young et al. surveyed twelve patients with 66% of the prosthesis cohort currently sexually active; compared to the non-prosthesis cohort, both groups demonstrated similar scores of penile perception and sexual quality of life (37). Assessments of larger cohorts suggest on average high rates of successful penetrative intercourse, near 84% (30). Falcone et al. reported 88% overall satisfaction and 60% partner satisfaction in their large series (12).
As part of a pilot study for an external erectile device, Boskey et al. asked 15 transgender men about concerns regarding erectile prostheses with 100% of respondents reporting concerns due to pain and damage to the penis as well as risk of device failure (38). These authors felt a strong need to advocate for a lower risk, non-surgical external device option (the Elator) (39).
Erectile devices designed for phalloplasty
Although not approved by the FDA for use in the U.S., Zephyr Surgical Implants (Geneva, Switzerland) has designed both inflatable and malleable prostheses for phalloplasty anatomy. These devices are approved for use in Europe, Cuba, and South America.
The three-piece ZSI 475 FtM was specifically designed for a neophallus and consists of a single cylinder. It has several advantages over prior devices designed for a natal phallus including: a large implantation base for pubic bone fixation to assist with anchoring, a realistically shaped hard glans to protect against distal tip erosion in the less vascularized neophallus as well as rigidity for penetration, and a pump shaped like a testicle (24). Results from recent studies on the ZSI 475 show an acceptable safety profile and high patient satisfaction with estimated explant-free survival rate of 80% at one year. The overall complication rate was 32% similar to other devices, however there was a high infection rate compared to devices typically implanted in cisgender men possibly due to the lack of vascularization and scarring of the neophallus compared with a natal phallus (23).
The single-component ZSI 100 FtM malleable implant is also designed for phalloplasty anatomy. The explanation rate was 16% with a common cause being protrusion and limitations to social activities (e.g., fitness). A learning curve exists for surgeons in cutting the prosthesis to adequate size and correct placement onto the pubic bone; a device left too long could lead to distal erosion, however a device cut too short could lead to insufficient glans support and drooping, causing difficulty with penetration.
Longer term follow-up is required to better understand surgical complications, implant survival, and patient satisfaction with these devices.
Prosthesis in metoidioplasty
Maintenance of native erectile function is a benefit of metoidioplasty. However, erectile devices have also been designed for this patient population. Neuville et al. described a surgical technique for implantation of a semirigid Zephyr device (ZSI 100 D4) in metoidioplasty, wherein the clitoral corpora are entered prior to urethral reconstruction (40). In a total of 15 patients, they reported a median 8.5 cm of prosthesis length, a minor complication rate of 46.7%, and one severe complication requiring surgical intervention (evacuation of hematoma). 85% of survey respondents in this series reported being “very satisfied” or “satisfied” with the appearance of their genitalia (40). No similar studies have been reported in the U.S.
Testicular implants
Testicular prosthesis implantation is often performed as a secondary procedure at least 6 months after scrotoplasty to reduce the risk of wound complications (41,42). We recommend all genital surgeries—including any scrotoplasty or urethral revisions—be healed completely prior to prosthesis placement. The length of time between initial metoidioplasty or phalloplasty to when the testicular prostheses are inserted ranges from immediately to 1 year (41-43), with immediate testicular implant placement typically performed during Belgrade scrotoplasty (44). Strict aseptic technique is critical during placement of testicular prostheses to prevent infection. Widely used routines include pre-scrub, perioperative antibiotics, surgeon glove change prior to prosthesis handling and irrigation within the wound with an antimicrobial solution (45).
Testicular prostheses range in size from <15 to 30 mL, with selection based on patient anatomy and preference. Over the past years, there has been a trend to use smaller and lighter testicular prosthesis (46). In the United States the Torosa (saline-filled, Coloplast) prosthesis made by Coloplast is the only FDA approved prosthesis available. Other testicular prosthesis options include solid silicone, saline-filled, or silicone gel filled implants (e.g., Implantech, Ventura, CA, USA and Alpha Aesthetics, Carson City, NV, USA).
Surgical technique and post-operative care
Patients are typically in supine frog-leg position. We utilize horizontal incisions overlying the hemiscrotum. A subcutaneous pocket is bluntly created and the size of the implant is determined by the pocket size and skin laxity. The subcutaneous tissue is closed in multiple layers of delayed absorbable sutures. The skin is closed with either absorbable or nonabsorbable (Prolene, Surgipro) which are removed at 10–14 days post-operatively. Patients are advised to refrain from prolonged pressure on the scrotum for at least 4 weeks.
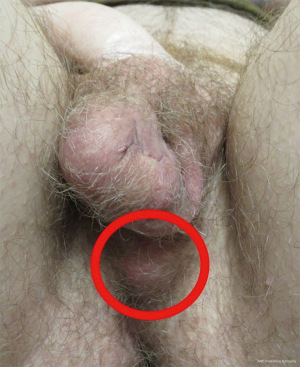
Complications
Though fewer studies have described outcomes following testicular prosthesis placement after gender-affirming scrotoplasty, the most common complications include infection (3–11%) and extrusion (7–14%) (Figure 9) (41,46). Other issues that may arise include hematoma, prosthesis migration (approximately 15%), and genital pain (1%) (46). Surgical revision rates are not well reported, however explant of the prosthesis rates range from 0.2% to 32% (41,44,46-49). In limited cohorts of TGD patients with testicular implants after scrotoplasty, the most common reported cause for explantation was infection (41). Despite complications, nearly 70% of patient opted for replacement prosthesis (41). Explantation rates were significantly lower in patients with smaller or lighter prostheses (41) and patients who had a history of smoking were at increased risk for explantation (46). The authors also speculated this may be a selected patient population prone to complicated healing due to prior surgery and soft tissue damage (46).

Patient reported outcomes
Satisfaction rates with testicular implants are high in cisgender men who have undergone radical orchiectomy, with regards to size, weight, texture, shape, position, and comfort level (50). There is a paucity of literature studying the clinical outcomes of testicular prosthesis in TGD individuals (41). The impact of impact testicular prostheses on gender and genital dysphoria has yet to be described from the patient perspective and warrants future research.
Conclusions
Erectile function is an important aspect of surgical transition for many TGD individuals who undergo phalloplasty. Due to the lack of native corpora cavernosa, highly variable phallic anatomy, and the need to adapt implants designed for natal penile anatomy, complication rates of prosthesis placement after phalloplasty remain high. As our understanding of clinical and patient-reported outcomes improves, it is critical for surgeons to elicit each patient’s goals and priorities to navigate individualized decision-making and improve patient experiences.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Gross, Jay Simhan, and David Barham) for the series “Complex Penile Prosthesis Surgery” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-122/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-122/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-122/coif). The series “Complex Penile Prosthesis Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Robinson IS, Blasdel G, Cohen O, et al. Surgical Outcomes Following Gender Affirming Penile Reconstruction: Patient-Reported Outcomes From a Multi-Center, International Survey of 129 Transmasculine Patients. J Sex Med 2021;18:800-11. [Crossref] [PubMed]
- Parker A, Blasdel G, Kloer C, et al. Postulating Penis: What Influences the Interest of Transmasculine Patients in Gender Affirming Penile Reconstruction Techniques? J Sex Med 2022;19:385-93. [Crossref] [PubMed]
- Bogoras N. Uber die volle plastische Wiederherstellung eines zum Koitus fahigen Penis (Peniplastica totalis). Zentralbl Chir 1936;63:1271-6.
- Ali M. Surgical treatment of the male genitalia; with special reference to the use of periosteal bone graft in constructing the penis. J Int Coll Surg 1957;27:352-8. [PubMed]
- Vesely J, Hyza P, Ranno R, et al. New technique of total phalloplasty with reinnervated latissimus dorsi myocutaneous free flap in female-to-male transsexuals. Ann Plast Surg 2007;58:544-50. [Crossref] [PubMed]
- Selvaggi G, Branemark R, Elander A, et al. Titanium-bone-anchored penile epithesis: preoperative planning and immediate postoperative results. J Plast Surg Hand Surg 2015;49:40-4. [Crossref] [PubMed]
- Puckett CL, Montie JE. Construction of male genitalia in the transsexual, using a tubed groin flap for the penis and a hydraulic inflation device. Plast Reconstr Surg 1978;61:523-30. [Crossref] [PubMed]
- Levine LA, Zachary LS, Gottlieb LJ. Prosthesis placement after total phallic reconstruction. J Urol 1993;149:593-8. [Crossref] [PubMed]
- Jordan GH, Alter GJ, Gilbert DA, et al. Penile prosthesis implantation in total phalloplasty. J Urol 1994;152:410-4. [Crossref] [PubMed]
- Hoebeke P, de Cuypere G, Ceulemans P, et al. Obtaining rigidity in total phalloplasty: experience with 35 patients. J Urol 2003;169:221-3. [Crossref] [PubMed]
- Hoebeke PB, Decaestecker K, Beysens M, et al. Erectile implants in female-to-male transsexuals: our experience in 129 patients. Eur Urol 2010;57:334-40. [Crossref] [PubMed]
- Falcone M, Garaffa G, Gillo A, et al. Outcomes of inflatable penile prosthesis insertion in 247 patients completing female to male gender reassignment surgery. BJU Int 2018;121:139-44. [Crossref] [PubMed]
- Levine LA, Becher EF, Bella AJ, et al. Penile Prosthesis Surgery: Current Recommendations From the International Consultation on Sexual Medicine. J Sex Med 2016;13:489-518. [Crossref] [PubMed]
- Leriche A, Timsit MO, Morel-Journel N, et al. Long-term outcome of forearm flee-flap phalloplasty in the treatment of transsexualism. BJU Int 2008;101:1297-300. [Crossref] [PubMed]
- Cohen AJ, Bhanvadia RR, Pariser JJ, et al. Novel Technique for Proximal Bone Anchoring of Penile Prosthesis After Radial Forearm Free Flap Neophallus. Urology 2017;105:2-5. [Crossref] [PubMed]
- Hage JJ. Dynaflex prosthesis in total phalloplasty. Plast Reconstr Surg 1997;99:479-85. [Crossref] [PubMed]
- Zuckerman JM, Smentkowski K, Gilbert D, et al. Penile Prosthesis Implantation in Patients with a History of Total Phallic Construction. J Sex Med 2015;12:2485-91. [Crossref] [PubMed]
- Briles BL, Middleton RY, Celtik KE, et al. Penile Prosthesis Placement by a Dedicated Transgender Surgery Unit: A Retrospective Analysis of Complications. J Sex Med 2022;19:641-9. [Crossref] [PubMed]
- Segal RL, Massanyi EZ, Gupta AD, et al. Inflatable penile prosthesis technique and outcomes after radial forearm free flap neophalloplasty. Int J Impot Res 2015;27:49-53. [Crossref] [PubMed]
- Large MC, Gottlieb LJ, Wille MA, et al. Novel technique for proximal anchoring of penile prostheses in female-to-male transsexual. Urology 2009;74:419-21. [Crossref] [PubMed]
- Neuville P, Morel-Journel N, Maucourt-Boulch D, et al. Surgical Outcomes of Erectile Implants After Phalloplasty: Retrospective Analysis of 95 Procedures. J Sex Med 2016;13:1758-64. [Crossref] [PubMed]
- Pigot GLS, Sigurjónsson H, Ronkes B, et al. Surgical Experience and Outcomes of Implantation of the ZSI 100 FtM Malleable Penile Implant in Transgender Men After Phalloplasty. J Sex Med 2020;17:152-8. [Crossref] [PubMed]
- Verla W, Goedertier W, Lumen N, et al. Implantation of the ZSI 475 FTM Erectile Device After Phalloplasty: A Prospective Analysis of Surgical Outcomes. J Sex Med 2021;18:615-22. [Crossref] [PubMed]
- Neuville P, Morel-Journel N, Cabelguenne D, et al. First Outcomes of the ZSI 475 FtM, a Specific Prosthesis Designed for Phalloplasty. J Sex Med 2019;16:316-22. [Crossref] [PubMed]
- Sun HH, Isali I, Mishra K, et al. Surgical Outcomes at a Single Institution of Infrapubic Insertion of Malleable Penile Prosthesis in Transmen. Urology 2023;173:209-14. [Crossref] [PubMed]
- van der Sluis WB, Pigot GLS, Al-Tamimi M, et al. A Retrospective Cohort Study on Surgical Outcomes of Penile Prosthesis Implantation Surgery in Transgender Men After Phalloplasty. Urology 2019;132:195-201. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology 1973;2:80-2. [Crossref] [PubMed]
- Kim SK, Kim TH, Yang JI, et al. The etiology and treatment of the softened phallus after the radial forearm osteocutaneous free flap phalloplasty. Arch Plast Surg 2012;39:390-6. [Crossref] [PubMed]
- Papadopulos NA, Schaff J, Biemer E. The use of free prelaminated and sensate osteofasciocutaneous fibular flap in phalloplasty. Injury 2008;39:S62-7. [Crossref] [PubMed]
- Rooker SA, Vyas KS, DiFilippo EC, et al. The Rise of the Neophallus: A Systematic Review of Penile Prosthetic Outcomes and Complications in Gender-Affirming Surgery. J Sex Med 2019;16:661-72. [Crossref] [PubMed]
- Chen ML, Patel DP, Moses RA, et al. Infrapubic Insertion of Penile Implants in Transmen After Phalloplasty. Urology 2021;152:79-83. [Crossref] [PubMed]
- Gross MS, Phillips EA, Carrasquillo RJ, et al. Multicenter Investigation of the Micro-Organisms Involved in Penile Prosthesis Infection: An Analysis of the Efficacy of the AUA and EAU Guidelines for Penile Prosthesis Prophylaxis. J Sex Med 2017;14:455-63. [Crossref] [PubMed]
- Carson CC 3rd, Mulcahy JJ, Harsch MR. Long-term infection outcomes after original antibiotic impregnated inflatable penile prosthesis implants: up to 7.7 years of followup. J Urol 2011;185:614-8. [Crossref] [PubMed]
- Mulhall JP, Kim FJ. Reconstructing penile supersonic transporter (SST) deformity using glanulopexy (glans fixation). Urology 2001;57:1160-2. [Crossref] [PubMed]
- Salgado CJ, Fein LA, Williams EA, et al. Supersonic Transporter Deformity in Transgender Men following Phalloplasty. Plast Reconstr Surg 2019;144:225-7. [Crossref] [PubMed]
- Trost L, Wanzek P, Bailey G. A practical overview of considerations for penile prosthesis placement. Nat Rev Urol 2016;13:33-46. [Crossref] [PubMed]
- Young EE, Friedlander D, Lue K, et al. Sexual Function and Quality of Life Before and After Penile Prosthesis Implantation Following Radial Forearm Flap Phalloplasty. Urology 2017;104:204-8. [Crossref] [PubMed]
- Boskey ER, Mehra G, Jolly D, et al. Concerns About Internal Erectile Prostheses Among Transgender Men Who Have Undergone Phalloplasty. J Sex Med 2022;19:1055-9. [Crossref] [PubMed]
- Boskey ER, Jolly D, Mehra G, et al. Feasibility of an External Erectile Prosthesis for Transgender Men Who have Undergone Phalloplasty. Sex Med 2022;10:100560. [Crossref] [PubMed]
- Neuville P, Carnicelli D, Paparel P, et al. Metoidioplasty With Implantation of a Specific Semirigid Prosthesis. J Sex Med 2021;18:830-6. [Crossref] [PubMed]
- Legemate CM, de Rooij FPW, Bouman MB, et al. Surgical outcomes of testicular prostheses implantation in transgender men with a history of prosthesis extrusion or infection. Int J Transgend Health 2020;22:330-6. [Crossref] [PubMed]
- Monstrey SJ, Ceulemans P, Hoebeke P. Sex Reassignment Surgery in the Female-to-Male Transsexual. Semin Plast Surg 2011;25:229-44. [Crossref] [PubMed]
- Djordjevic ML, Stanojevic D, Bizic M, et al. Metoidioplasty as a single stage sex reassignment surgery in female transsexuals: Belgrade experience. J Sex Med 2009;6:1306-13. [Crossref] [PubMed]
- Djordjevic ML, Bizic MR. Comparison of two different methods for urethral lengthening in female to male (metoidioplasty) surgery. J Sex Med 2013;10:1431-8. [Crossref] [PubMed]
- Lucas JW, Lester KM, Chen A, et al. Scrotal reconstruction and testicular prosthetics. Transl Androl Urol 2017;6:710-21. [Crossref] [PubMed]
- Pigot GLS, Al-Tamimi M, Ronkes B, et al. Surgical Outcomes of Neoscrotal Augmentation with Testicular Prostheses in Transgender Men. J Sex Med 2019;16:1664-71. [Crossref] [PubMed]
- Hage JJ, van Turnhout AA. Long-term outcome of metaidoioplasty in 70 female-to-male transsexuals. Ann Plast Surg 2006;57:312-6. [Crossref] [PubMed]
- Noe JM, Sato R, Coleman C, et al. Construction of male genitalia: the Stanford experience. Arch Sex Behav 1978;7:297-303. [Crossref] [PubMed]
- Schaff J, Papadopulos NA. A new protocol for complete phalloplasty with free sensate and prelaminated osteofasciocutaneous flaps: experience in 37 patients. Microsurgery 2009;29:413-9. [Crossref] [PubMed]
- Clifford TG, Burg ML, Hu B, et al. Satisfaction With Testicular Prosthesis After Radical Orchiectomy. Urology 2018;114:128-32. [Crossref] [PubMed]


