Predictive models and prognostic factors for upper tract urothelial carcinoma: a comprehensive review of the literature
Introduction
Until recently, management and surveillance of upper tract urothelial carcinoma (UTUC) was patterned after that of bladder cancer (BC). But reports have demonstrated that, despite their pathological similarities, BC and UTUC had distinct biological behaviors, and therefore, required individualized recommendations (1,2).
However, due to the low incidence of UTUC [it accounts for only 5–10% of all urothelial carcinomas (3)], the majority of studies is mainly made of single-institution small cohorts. The resulting low-level of evidence did unfortunately not allow high-grade recommendations for UTUC management (2).
In a context where personalized patient care is necessary with kidney-sparing surgery (KSS) for localized tumors, neoadjuvant chemotherapy before radical nephro-ureterectomy (RNU), and lymph node (LN) dissection for high-risk tumors (2,4), accurate assessment of tumor aggressiveness is necessary for clinical decision-making.
Beyond the established prognostic factors such as tumor stage, grade and LN metastasis, numerous patient-, surgery- and pathology-related factors have been recently identified thanks to intense research based on collaborative studies. The integration of these factors in predictive tools has permitted to guide decision-making for customized/personalized care delivery.
The aim of this review was to provide a critical overview of existing predictive models and to review emerging promising prognostic factors for UTUC. We have previously reported on International Consultation on Urologic Diseases—Société Internationale d’Urologie (ICUD-SIU) guidelines (5). In this review, we updated the data and added non-consensus-based opinions of authors.
Evidence acquisition
A non-systematic literature search was conducted using PubMed/Medline database. Articles published in English between January 2000 and June 2016 were collected by using a combination of the following terms: “prognostic factor”, “predictive tool”, “nomograms”, “risk stratification”, “survival”, “biomarker” together with “upper tract urothelial carcinoma” or “upper tract transitional cell carcinoma”. All published studies on predictive tools or predictive/prognostic biomarkers were retained for the purpose of this review. In order to explore other emerging prognostic factors and biomarkers, retrospective studies and meta-analyses involving more than 300 patients were also retained.
Evidence synthesis
Preoperative prediction of disease invasiveness and oncological outcomes after surgery
RNU with bladder-cuff excision remains the gold standard for high-risk UTUC (2). However, indication of KSS has slowly shifted from absolute indication in patients with solitary kidney, bilateral disease or patient-related comorbidities towards elective indication for a broader spectrum of patients with low-risk UTUC (2,4). Therefore, before considering KSS, preoperative assessment of tumor invasiveness but also after risk of extra-luminal recurrence, metastasis and cancer-specific mortality are essential to support an evidence-based assessment of the risks, benefits and alternatives in a shared decision-making process.
Imaging and ureteroscopy findings: cornerstones of preoperative prediction in UTUC
Several predictive models based on preoperative imaging and diagnostic ureteroscopy findings have been designed to assess muscle-invasive and/or non-organ-confined (NOC) UTUC (Table 1).
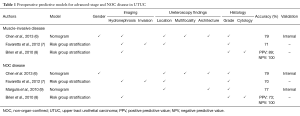
Full table
Hydronephrosis (6,10-12) and local invasion (7) are both features associated with advanced disease that can be detected on high definition computed tomography (CT) urography. Hydronephrosis is also associated with an increased risk of tumor metastasis (6).
The increased use of high-definition flexile digital ureteroscopes has facilitated the preoperative identification of features associated with high-risk UTUC such as sessile architecture (13-17) and tumor multifocality (18-20). When combined with biopsies, ureteroscopy also permit to identify high-grade tumors with high accuracy and reproductibility (14,16,21,22).
Predictive tools for advanced-stage and NOC UTUC assessment
Brien et al. showed that the knowledge of hydronephrosis, ureteroscopic grade and urinary cytology can predict muscle-invasive and NOC with a positive predictive value (PPV) of 89% and 73%, respectively (8). More importantly, if all three are negative, the negative predictive value (NPV) was 100%.
Chen et al. constituted a nomogram based on gender, tumor architecture, multifocality, tumor location, grade and hydronephrosis that reached an accuracy of 79% for both NOC and muscle-invasive disease assessment (6). Even if gender appeared as a predictor of advanced-stage disease in this dataset, its influence on tumor aggressiveness and oncological outcomes in UTUC is controversial with most studies showing no effect (23-27). Therefore, international guidelines on UTUC do not consider gender as a predictor of oncological outcomes in UTUC (2).
By combining tumor grade, architecture and tumor location in a nomogram, Margulis et al. reached an accuracy of 77% for NOC-disease assessment (9). However, the impact of tumor location on UTUC prognosis is still debated. Contradictory findings have been reported concerning its correlation with advanced UTUC (6,22,28,29), disease recurrence (19,22,26,29,30) and cancer-specific survival (CSS) (18-20,22,28,30,31). Even if meta-analyses found no correlation between NOC disease and tumor location (20), ureteral tumors seem associated with shorter recurrence-free survival (RFS) in various studies (20,24,25). However, the current meta-analyses suffer from poor quality as they are based on methodologically weak studies.
From a dataset of 274 UTUC patients treated with RNU, Favaretto et al. constituted a risk group stratification model for muscle-invasive UTUC with an accuracy of 71% (7). From the same dataset, the association of tumor grade, location, invasion and hydronephrosis on imaging predicted NOC-UTUC with an accuracy of 70%. Unfortunately, these findings are still waiting for external validation.
Emerging demographic and preoperative prognostic factors
As in most diseases, patient’s physical condition influences immediate postoperative outcomes such as time of recovery, duration of hospitalization and surgery-related complications (32). Few patient-related factors are associated with UTUC aggressiveness and oncologic outcomes (Table 2).
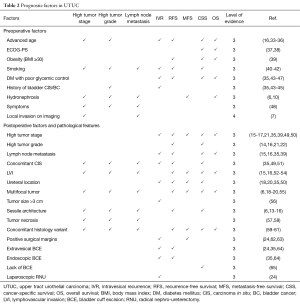
Full table
Advanced-age & ECOG-PS
For a long-time, advanced chronical age was thought to be an independent factors associated with invasive tumor patterns (33), tumor recurrence (34,35) and shorter CSS (16,33,34,36) based on nationwide epidemiologic studies. However, large multi-institutional studies have shown that advanced-age was not a predictor of survival anymore when it was adjusted for the effect of performance status (34,36-38). Therefore, international guidelines do not recommend age as reason to not offer RNU with potential curable intent (2). However, assessment of performance status helps identify patients who are likely to have serious morbidity and therefore not benefit from RNU.
Symptoms
At the time of diagnosis, patient’s physical condition can also be altered by systemic symptoms related to advanced-stage disease such as night sweat, anorexia and weight loss (48). Flank pain, when related to hydronephrosis, is also a marker of NOC disease (12). Similarly to all cancers, symptoms of systemic disease portend metastatic cancer with poor outcomes.
Ethnicity
Data on the influence of ethnicity on UTUC-related oncologic outcomes are very sparse. While a population-based US study found that African-American patients with UTUC had a shorter survival than other ethnic groups (66), an international study comparing Japanese with European and US Caucasian patients did not find any difference in survival between these two groups (67). Further investigations on both biological and sociological factors underlying these results must be performed. Access to care could also influence the worse outcomes of African-American patients.
Smoking status
Similarly to BC, cumulative smoking exposure is a well-established predictor of poor outcomes in UTUC. Heavy long-term smokers (more than 20 cigarettes per day for more than 20 years) were more likely to have advanced-stage disease, and experience disease recurrence and cancer-specific mortality after RNU (40,41). Interestingly, after 10 years of smoking cessation, former smokers had similar outcomes to non-smokers (40,42). Therefore, counseling smoking cessation should be strongly encouraged.
History of BC
Despite being recognized as separate entities, the upper urinary tract and bladder share the same fertile soil for development of urothelial carcinoma. Therefore, it is not surprising that history of BC is associated with higher tumor grade and increase risk of intravesical recurrence after treatment of UTUC (35,43-45). In general, BC recurrence after UTUC treatment is as high as 30–40% (35).
Metabolic disorders
Obese patients [body mass index (BMI) >30] (39) or diabetes mellitus (DM) with poor glycemic control (46,68,69) are more likely to develop tumors with aggressive behavior and suffer, therefore, from worse survival. On the other hand, underweight, defined as BMI in the lowest quartile of a cohort, is also associated with worse survival (70). These findings need to be confirmed in all ethnic groups and in large controlled studies.
Tumor necrosis
Tumor necrosis is a pathological feature that is associated with muscle-invasive UTUC. However, after adjustment for the effects of established pathologic features, its association with oncological outcomes either weakened or totally disappeared (57,58,67).
Preoperative assessment of tumor aggressiveness remains challenging despite the identification of solid new predictors/prognosticators. Clinical use of existing predictive models is mostly questioned due to the lack of external validation. However, the combination of emerging prognostic factors together with high definition imaging and ureteroscopically-obtained biopsies might help building more accurate predictive models for a more accurate customized care delivery.
Postoperative assessment of survival outcomes in UTUC
After surgery, accurate risk estimation would allow optimal decision-making regarding adjuvant chemotherapy and follow-up scheduling.
Postoperative predictive models for disease recurrence and distant metastasis
Several predictive tools have been designed to assess the risk of intravesical recurrence, local and distant recurrence after RNU (Table 3). These models share several factors that have been described as independent predictors for each outcome.

Full table
Concomitant carcinoma in situ (CIS) is a well-known predictor of worse survival in BC. In UTUC, concomitant CIS is associated with advanced-stage UTUC (49,51), intravesical and loco-regional recurrence (35,49,51) as well as CSS (49,51).
Lymphovascular invasion (LVI) is also an independent predictor of worse oncologic outcomes after RNU (52-54,73).
Positive surgical margins and lack of complete bladder cuff excision (BCE) are associated with higher risk of both intravesical recurrence and shorter survival (24,62,65,74).
Latest meta-analyses demonstrated that endoscopic and extravesical BCE resulted in higher recurrence rates compared to complete intravesical removal (22,35,64,74,75).
Xylinas et al. identified prognosticators of intravesical recurrence from a cohort study including more than 1,900 patients (35). Independent prognostic factors for nomogram building were patient age, gender, history of BC, tumor location, clinical stage, concomitant CIS, LN metastasis, BCE and surgical approach. The combination of these factors helped to reach an accuracy of 69% for prediction of intravesical recurrence risk at 2 years.
Ishioka et al. also proposed a risk group stratification model and a nomogram predicting intravesical recurrence after RNU (17). By combining, tumor architecture, tumor stage, LVI and gender, they obtained an accuracy of 62%.
For the prediction of 5-year RFS in patients with high grade UTUC after RNU, Youssef et al. developed a simplified risk stratification model called TALL score. Based on tumor stage, architecture, LVI and LN metastasis, this predictive scoring model reached an accuracy of 73% (71).
Colin et al. published a risk group stratification model that assessed 2- and 5-year metastasis-free survival (MFS) by combining tumor location, stage, LVI and surgical margin (50).
Postoperative predictive models for CSS
Existing postoperative models predicting CSS are mostly constructed from established prognosticators such as tumor stage, grade or LN metastases (Table 4). They reach an accuracy up to 82% for prediction of 5-year CSS. However, they almost all suffer from the same limitation: lack of external validation and lack of decision-analysis.
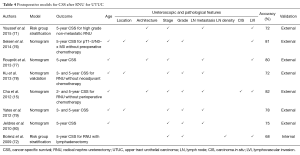
Full table
The exception is the study from Ku et al. (78) who performed an online external validation of Yates et al.’s (79) model in a dataset of patients from a Korean institution. This permitted to confirm that Yates et al.’s model based on age, tumor stage, grade, location and LN metastasis had an accuracy of more than 70% from 3- and 5-year CSS prediction.
Emerging prognostic factors of disease RFS or MFS
Some more prognostic factors of disease recurrence have been described and would benefit from more in depth investigations (Table 2).
Tumor size
Surgeons’ experimental knowledge has demonstrated that large tumors were not necessarily muscle-invasive tumors. However, no large multicenter study has investigated this question yet. A meta-analysis gathering seven studies showed that tumor larger than 3 cm were more likely to recur (56). However, these results are limited by the small number of patients included and the heterogeneity of studies.
Variant histology
Non-pure urothelial carcinoma with the presence of variant histology is another marker of aggressive disease that can sometimes be assessed on ureteroscopically-obtained biopsies (59,60,81). Variant histology has been associated with intravesical and loco-regional recurrence (60). A large retrospective study compared survival of patients presenting variant histology versus pure urothelial carcinoma. At 5-year, patients with variant histology had a 30% lower CSS compared to patients with pure urothelial carcinoma (60).
Before integration of the described predictive tools in clinical decision-making, external validations in independent cohorts such as Ku et al. (78) performed should be done. Variant urothelial carcinoma also appears to be a pathological feature associated with high risk UTUC and should therefore be emphasized on pathological reports and during multidisciplinary discussions for patient care management. Similarly to BC, it will/can change management significantly (82).
Biomarkers predicting oncologic outcomes after RNU
The increase in UTUC research has permitted to identify numerous tissue-, blood- and urine-based biomarkers associated with UTUC survival outcomes (Table 5). Through a better understanding of biological mechanisms associated with UTUC carcinogenesis, progression and metastasis, UTUC diagnosis, surveillance and treatment are likely to be improved.
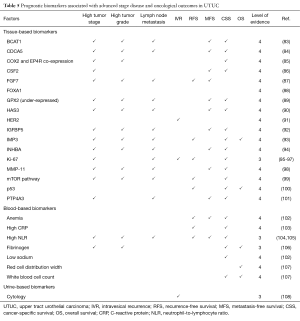
Full table
Blood-based predictive tools for survival outcomes
Inflammatory response and immune system reaction toward cancer are well-described phenomenon in various types of malignancies. Changes in level of biomarkers such as hemoglobin (102), CRP (103) or neutrophil-to-lymphocyte ratio (NLR) (104,105) have been correlated with muscle-invasiveness and/or NOC disease as well as worse oncologic outcomes after RNU (Table 5).
Kim et al. integrated NLR in a postoperative nomogram for RFS and CSS (109). When combined with tumor stage, LVI and BCE, the model predicted 2- and 5-year RFS with an accuracy of 78%, and CSS with an accuracy of 80%.
Fujita et al. (102) and Sakano et al. (110) both also integrated inflammatory biomarkers (hemoglobin level and white blood cell count) in the construction of a preoperative risk group stratification model predicting CSS.
Preoperative estimated glomerular filtration rate (eGFR) is also a predictor of disease recurrence and CSS (111,112). By adding eGFR to tumor stage, grade and LN metastasis, Ehdaie et al. constructed a nomogram predicting RFS and CSS with an accuracy of 82% and 83%, respectively (112).
Upcoming prognostic molecular biomarkers
Numerous prognostic molecular biomarkers in UTUC have been described (Table 5). These biomarkers are implicated in every steps of tumorigenesis and progression from cell-cycle regulation [mTOR pathway (99)] to cell-proliferation [HER2 (91), Ki-67 (95-97,113), BCAT1 (83), CDCA5 (84)] and apoptosis [p53 (100)]. Unfortunately, most of them have been described in single-institution cohorts and very few factors beneficiated from external validation.
Ki-67 seems to be, to date, the most promising biomarker. High proliferation based on Ki67 staining has been associated with disease invasiveness, disease recurrence and CSS in both retrospective and prospective studies (95-97,113,114).
Potentially, the combination of tissue-based biomarkers such as Ki-67 and inflammation-related blood-based preoperative markers could constitute the future of UTUC prognostication and prediction.
Conclusions
Current international guidelines encourage a risk-adapted approach to UTUC management. Whether it is for preoperative tumor invasiveness assessment when considering KSS or for postoperative determination of patients who could benefit from adjuvant intravesical instillations or chemotherapy, predictive models and prognostic factors have been described. However, due to their low level of evidence and lack of external validation, none of these predictive tools has been recommended in daily decision-making yet (2,4). Still, noteworthy developments have been achieved thanks to international collaborations, and more accurate predictors are highly likely to change current practice.
We expect the combination of patient-, pathology-, surgery- and biomarkers-related factors will eventually reach an accuracy high enough for a wide-spread use for customized decision-making in UTUC.
Acknowledgements
None.
Footnote
Conflicts of Interest: Shahrokh F. Shariat owns or co-owns the following patents: methods to determine prognosis after therapy for prostate cancer. Granted 2002-09-06; methods to determine prognosis after therapy for bladder cancer. Granted 2003-06-19; prognostic methods for patients with prostatic disease. Granted 2004-08-05; soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010-07-20. He is advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi, Wolff. He is speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, Wolff. Romain Mathieu is consultant for Astellas, Ipsen, Janssen; and he is speaker of Janssen, Sanofi, Novartis, Takeda. The other authors have no conflicts of interest to declare.
References
- Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013;189:1214-21. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol 2015;68:868-79. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Gakis G, Schubert T, Alemozaffar M, et al. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of localized high-risk disease. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Mbeutcha A, Rouprêt M, Kamat AM, et al. Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Chen XP, Xiong GY, Li XS, et al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China. BJU Int 2013;112:917-24. [PubMed]
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int 2012;109:77-82. [Crossref] [PubMed]
- Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184:69-73. [Crossref] [PubMed]
- Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 2010;184:453-8. [Crossref] [PubMed]
- Messer JC, Terrell JD, Herman MP, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol 2013;31:904-8. [Crossref] [PubMed]
- Bozzini G, Nison L, Colin P, et al. Influence of preoperative hydronephrosis on the outcome of urothelial carcinoma of the upper urinary tract after nephroureterectomy: the results from a multi-institutional French cohort. World J Urol 2013;31:83-91. [Crossref] [PubMed]
- Yeh HC, Jan HC, Wu WJ, et al. Concurrent Preoperative Presence of Hydronephrosis and Flank Pain Independently Predicts Worse Outcome of Upper Tract Urothelial Carcinoma. PLoS One 2015;10:e0139624. [Crossref] [PubMed]
- Fritsche HM, Novara G, Burger M, et al. Macroscopic sessile tumor architecture is a pathologic feature of biologically aggressive upper tract urothelial carcinoma. Urol Oncol 2012;30:666-72. [Crossref] [PubMed]
- Remzi M, Haitel A, Margulis V, et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int 2009;103:307-11. [Crossref] [PubMed]
- Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 2012;61:818-25. [Crossref] [PubMed]
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33. [Crossref] [PubMed]
- Ishioka J, Saito K, Kijima T, et al. Risk stratification for bladder recurrence of upper urinary tract urothelial carcinoma after radical nephroureterectomy. BJU Int 2015;115:705-12. [Crossref] [PubMed]
- Ouzzane A, Colin P, Xylinas E, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol 2011;60:1258-65. [Crossref] [PubMed]
- Williams AK, Kassouf W, Chin J, et al. Multifocality rather than tumor location is a prognostic factor in upper tract urothelial carcinoma. Urol Oncol 2013;31:1161-5. [Crossref] [PubMed]
- Wu Y, Dong Q, Liu L, et al. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: a meta-analysis. Sci Rep 2014;4:6361. [Crossref] [PubMed]
- Shariat SF, Zigeuner R, Rink M, et al. Subclassification of pT3 urothelial carcinoma of the renal pelvicalyceal system is associated with recurrence-free and cancer-specific survival: proposal for a revision of the current TNM classification. Eur Urol 2012;62:224-31. [Crossref] [PubMed]
- Raman JD, Ng CK, Scherr DS, et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol 2010;57:1072-9. [Crossref] [PubMed]
- Lughezzani G, Sun M, Perrotte P, et al. Gender-related differences in patients with stage I to III upper tract urothelial carcinoma: results from the Surveillance, Epidemiology, and End Results database. Urology 2010;75:321-7. [Crossref] [PubMed]
- Seisen T, Granger B, Colin P, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol 2015;67:1122-33. [Crossref] [PubMed]
- Yuan H, Chen X, Liu L, et al. Risk factors for intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinoma: a meta-analysis. Urol Oncol 2014;32:989-1002. [Crossref] [PubMed]
- Kusuda Y, Miyake H, Terakawa T, et al. Gender as a significant predictor of intravesical recurrence in patients with urothelial carcinoma of the upper urinary tract following nephroureterectomy. Urol Oncol 2013;31:899-903. [Crossref] [PubMed]
- Shariat SF, Favaretto RL, Gupta A, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2011;29:481-6. [Crossref] [PubMed]
- Isbarn H, Jeldres C, Shariat SF, et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J Urol 2009;182:2177-81. [Crossref] [PubMed]
- Favaretto RL, Shariat SF, Chade DC, et al. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol 2010;58:574-80. [Crossref] [PubMed]
- Catto JW, Yates DR, Rehman I, et al. Behavior of urothelial carcinoma with respect to anatomical location. J Urol 2007;177:1715-20. [Crossref] [PubMed]
- Yafi FA, Novara G, Shariat SF, et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: a homogeneous series without perioperative chemotherapy. BJU Int 2012;110:E7-13. [Crossref] [PubMed]
- Raman JD, Jafri SM. Complications Following Radical Nephroureterectomy. Curr Urol Rep 2016;17:36. [Crossref] [PubMed]
- Yap SA, Schupp CW, Chamie K, et al. Effect of age on transitional cell carcinoma of the upper urinary tract: presentation, treatment, and outcomes. Urology 2011;78:87-92. [Crossref] [PubMed]
- Chromecki TF, Ehdaie B, Novara G, et al. Chronological age is not an independent predictor of clinical outcomes after radical nephroureterectomy. World J Urol 2011;29:473-80. [Crossref] [PubMed]
- Xylinas E, Kluth L, Passoni N, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol 2014;65:650-8. [Crossref] [PubMed]
- Shariat SF, Godoy G, Lotan Y, et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int 2010;105:1672-7. [Crossref] [PubMed]
- Bagrodia A, Kuehhas FE, Gayed BA, et al. Comparative analysis of oncologic outcomes of partial ureterectomy vs radical nephroureterectomy in upper tract urothelial carcinoma. Urology 2013;81:972-7. [Crossref] [PubMed]
- Martinez-Salamanca JI, Shariat SF, Rodriguez JC, et al. Prognostic role of ECOG performance status in patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int 2012;109:1155-61. [Crossref] [PubMed]
- Ehdaie B, Chromecki TF, Lee RK, et al. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol 2011;186:66-72. [Crossref] [PubMed]
- Rink M, Xylinas E, Margulis V, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol 2013;63:1082-90. [Crossref] [PubMed]
- Xylinas E, Kluth LA, Rieken M, et al. Impact of smoking status and cumulative exposure on intravesical recurrence of upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int 2014;114:56-61. [Crossref] [PubMed]
- Rink M, Xylinas E, Trinh QD, et al. Gender-specific effect of smoking on upper tract urothelial carcinoma outcomes. BJU Int 2013;112:623-37. [Crossref] [PubMed]
- Youssef RF, Shariat SF, Lotan Y, et al. Prognostic effect of urinary bladder carcinoma in situ on clinical outcome of subsequent upper tract urothelial carcinoma. Urology 2011;77:861-6. [Crossref] [PubMed]
- Pignot G, Colin P, Zerbib M, et al. Influence of previous or synchronous bladder cancer on oncologic outcomes after radical nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol 2014;32:23.e1-8. [Crossref] [PubMed]
- Fang D, Zhang L, Li X, et al. Presence of Concomitant Non-muscle-invasive Bladder Cancer in Chinese Patients with Upper Tract Urothelial Carcinoma: Risk Factors, Characteristics, and Predictive Value. Ann Surg Oncol 2015;22:2789-98. [Crossref] [PubMed]
- Rieken M, Xylinas E, Kluth L, et al. Diabetes mellitus without metformin intake is associated with worse oncologic outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol 2014;40:113-20. [Crossref] [PubMed]
- Kang SG, Hwang EC, Jung SI, et al. Poor preoperative Glycemic Control is Associated with Dismal Prognosis After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Korean Multicenter Study. Cancer Res Treat 2016. [Epub ahead of print]. [PubMed]
- Raman JD, Shariat SF, Karakiewicz PI, et al. Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol Oncol 2011;29:716-23. [Crossref] [PubMed]
- Wheat JC, Weizer AZ, Wolf JS Jr, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ confined urothelial carcinoma following radical nephroureterectomy. Urol Oncol 2012;30:252-8. [Crossref] [PubMed]
- Colin P, Ghoneim TP, Nison L, et al. Risk stratification of metastatic recurrence in invasive upper urinary tract carcinoma after radical nephroureterectomy without lymphadenectomy. World J Urol 2014;32:507-12. [Crossref] [PubMed]
- Otto W, Shariat SF, Fritsche HM, et al. Concomitant carcinoma in situ as an independent prognostic parameter for recurrence and survival in upper tract urothelial carcinoma: a multicenter analysis of 772 patients. World J Urol 2011;29:487-94. [Crossref] [PubMed]
- Hurel S, Rouprêt M, Ouzzane A, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int 2013;111:1199-207. [Crossref] [PubMed]
- Novara G, Matsumoto K, Kassouf W, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 2010;57:1064-71. [Crossref] [PubMed]
- Ku JH, Byun SS, Jeong H, et al. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer 2013;49:2665-80. [Crossref] [PubMed]
- Chromecki TF, Cha EK, Fajkovic H, et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol 2012;61:245-53. [Crossref] [PubMed]
- Kates M, Badalato GM, Gupta M, et al. Secondary bladder cancer after upper tract urothelial carcinoma in the US population. BJU Int 2012;110:1325-9. [Crossref] [PubMed]
- Zigeuner R, Shariat SF, Margulis V, et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 2010;57:575-81. [Crossref] [PubMed]
- Seitz C, Gupta A, Shariat SF, et al. Association of tumor necrosis with pathological features and clinical outcome in 754 patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma: an international validation study. J Urol 2010;184:1895-900. [Crossref] [PubMed]
- Masson-Lecomte A, Colin P, Bozzini G, et al. Impact of micropapillary histological variant on survival after radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2014;32:531-7. [Crossref] [PubMed]
- Shibing Y, Turun S, Qiang W, et al. Effect of concomitant variant histology on the prognosis of patients with upper urinary tract urothelial carcinoma after radical nephroureterectomy. Urol Oncol 2015;33:204.e9-16. [Crossref] [PubMed]
- Tang Q, Xiong G, Li X, et al. The prognostic impact of squamous and glandular differentiation for upper tract urothelial carcinoma patients after radical nephroureterectomy. World J Urol 2016;34:871-7. [Crossref] [PubMed]
- Colin P, Ouzzane A, Yates DR, et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann Surg Oncol 2012;19:3613-20. [Crossref] [PubMed]
- Ouzzane A, Colin P, Ghoneim TP, et al. The impact of lymph node status and features on oncological outcomes in urothelial carcinoma of the upper urinary tract (UTUC) treated by nephroureterectomy. World J Urol 2013;31:189-97. [Crossref] [PubMed]
- Kapoor A, Dason S, Allard CB, et al. The impact of method of distal ureter management during radical nephroureterectomy on tumour recurrence. Can Urol Assoc J 2014;8:E845-52. [Crossref] [PubMed]
- Lughezzani G, Sun M, Perrotte P, et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol 2010;57:956-62. [Crossref] [PubMed]
- Raman JD, Messer J, Sielatycki JA, et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int 2011;107:1059-64. [Crossref] [PubMed]
- Matsumoto K, Novara G, Gupta A, et al. Racial differences in the outcome of patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int 2011;108:E304-9. [Crossref] [PubMed]
- Hu CY, Tsai YC, Wang SM, et al. Ureteral involvement and diabetes increase the risk of subsequent bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Biomed Res Int 2015;2015:527976.
- Tai YS, Chen CH, Huang CY, et al. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev 2015;31:307-14. [Crossref] [PubMed]
- Kang HW, Jung HD, Ha YS, et al. Preoperative Underweight Patients with Upper Tract Urothelial Carcinoma Survive Less after Radical Nephroureterectomy. J Korean Med Sci 2015;30:1483-9. [Crossref] [PubMed]
- Youssef RF, Krabbe LM, Shariat SF, et al. TALL score for prediction of oncological outcomes after radical nephroureterectomy for high-grade upper tract urothelial carcinoma. World J Urol 2015;33:1965-72. [Crossref] [PubMed]
- Bolenz C, Shariat SF, Fernández MI, et al. Risk stratification of patients with nodal involvement in upper tract urothelial carcinoma: value of lymph-node density. BJU Int 2009;103:302-6. [Crossref] [PubMed]
- Kikuchi E, Margulis V, Karakiewicz PI, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 2009;27:612-8. [Crossref] [PubMed]
- Xylinas E, Rink M, Cha EK, et al. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 2014;65:210-7. [Crossref] [PubMed]
- Seisen T, Nison L, Remzi M, et al. Oncologic Outcomes of Kidney Sparing Surgery versus Radical Nephroureterectomy for the Elective Treatment of Clinically Organ Confined Upper Tract Urothelial Carcinoma of the Distal Ureter. J Urol 2016;195:1354-61. [Crossref] [PubMed]
- Seisen T, Colin P, Hupertan V, et al. Postoperative nomogram to predict cancer-specific survival after radical nephroureterectomy in patients with localised and/or locally advanced upper tract urothelial carcinoma without metastasis. BJU Int 2014;114:733-40. [Crossref] [PubMed]
- Rouprêt M, Hupertan V, Seisen T, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol 2013;189:1662-9. [Crossref] [PubMed]
- Ku JH, Moon KC, Jung JH, et al. External validation of an online nomogram in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. Br J Cancer 2013;109:1130-6. [Crossref] [PubMed]
- Yates DR, Hupertan V, Colin P, et al. Cancer-specific survival after radical nephroureterectomy for upper urinary tract urothelial carcinoma: proposal and multi-institutional validation of a post-operative nomogram. Br J Cancer 2012;106:1083-8. [Crossref] [PubMed]
- Jeldres C, Sun M, Lughezzani G, et al. Highly predictive survival nomogram after upper urinary tract urothelial carcinoma. Cancer 2010;116:3774-84. [Crossref] [PubMed]
- Rink M, Robinson BD, Green DA, et al. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol 2012;188:398-404. [Crossref] [PubMed]
- Perez-Montiel D, Suster S. Upper urinary tract carcinomas: histological types and unusual morphological variants. Diagn Histopathol 2008;14:48-54. [Crossref]
- Chang IW, Wu WJ, Wang YH, et al. BCAT1 overexpression is an indicator of poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. Histopathology 2016;68:520-32. [Crossref] [PubMed]
- Chang IW, Lin VC, He HL, et al. CDCA5 overexpression is an indicator of poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. Am J Transl Res 2015;7:710-22. [PubMed]
- Miyata Y, Kanda S, Nomata K, et al. Expression of cyclooxygenase-2 and EP4 receptor in transitional cell carcinoma of the upper urinary tract. J Urol 2005;173:56-60. [Crossref] [PubMed]
- Lee YY, Wu WJ, Huang CN, et al. CSF2 Overexpression Is Associated with STAT5 Phosphorylation and Poor Prognosis in Patients with Urothelial Carcinoma. J Cancer 2016;7:711-21. [Crossref] [PubMed]
- Fan EW, Li CC, Wu WJ, et al. FGF7 Over Expression is an Independent Prognosticator in Patients with Urothelial Carcinoma of the Upper Urinary Tract and Bladder. J Urol 2015;194:223-9. [Crossref] [PubMed]
- Raman JD, Warrick JI, Caruso C, et al. Altered Expression of the Transcription Factor Forkhead Box A1 (FOXA1) Is Associated With Poor Prognosis in Urothelial Carcinoma of the Upper Urinary Tract. Urology 2016;94:314.e1-7. [Crossref] [PubMed]
- Chang IW, Lin VC, Hung CH, et al. GPX2 underexpression indicates poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. World J Urol 2015;33:1777-89. [Crossref] [PubMed]
- Chang IW, Liang PI, Li CC, et al. HAS3 underexpression as an indicator of poor prognosis in patients with urothelial carcinoma of the upper urinary tract and urinary bladder. Tumour Biol 2015;36:5441-50. [Crossref] [PubMed]
- Soria F, Moschini M, Haitel A, et al. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Liang PI, Wang YH, Wu TF, et al. IGFBP-5 overexpression as a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. J Clin Pathol 2013;66:573-82. [Crossref] [PubMed]
- Lee DJ, Xylinas E, Rieken M, et al. Insulin-like growth factor messenger RNA-binding protein 3 expression helps prognostication in patients with upper tract urothelial carcinoma. Eur Urol 2014;66:379-85. [Crossref] [PubMed]
- Lee HY, Li CC, Huang CN, et al. INHBA overexpression indicates poor prognosis in urothelial carcinoma of urinary bladder and upper tract. J Surg Oncol 2015;111:414-22. [Crossref] [PubMed]
- Krabbe LM, Bagrodia A, Haddad AQ, et al. Multi-institutional validation of the predictive value of Ki-67 in patients with high grade urothelial carcinoma of the upper urinary tract. J Urol 2015;193:1486-93. [Crossref] [PubMed]
- Krabbe LM, Bagrodia A, Lotan Y, et al. Prospective analysis of Ki-67 as an independent predictor of oncologic outcomes in patients with high grade upper tract urothelial carcinoma. J Urol 2014;191:28-34. [Crossref] [PubMed]
- Jeon HG, Jeong IG, Bae J, et al. Expression of Ki-67 and COX-2 in patients with upper urinary tract urothelial carcinoma. Urology 2010;76:513.e7-12. [Crossref] [PubMed]
- Li WM, Wei YC, Huang CN, et al. Matrix metalloproteinase-11 as a marker of metastasis and predictor of poor survival in urothelial carcinomas. J Surg Oncol 2016;113:700-7. [Crossref] [PubMed]
- Bagrodia A, Krabbe LM, Gayed BA, et al. Evaluation of the prognostic significance of altered mammalian target of rapamycin pathway biomarkers in upper tract urothelial carcinoma. Urology 2014;84:1134-40. [Crossref] [PubMed]
- Ku JH, Byun SS, Jeong H, et al. The role of p53 on survival of upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Clin Genitourin Cancer 2013;11:221-8. [Crossref] [PubMed]
- Yeh HC, Li CC, Huang CN, et al. PTP4A3 Independently Predicts Metastasis and Survival in Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. J Urol 2015;194:1449-55. [Crossref] [PubMed]
- Fujita K, Uemura M, Yamamoto Y, et al. Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Clin Oncol 2015;20:156-63. [Crossref] [PubMed]
- Luo Y, Fu SJ, She DL, et al. Preoperative C-reactive protein as a prognostic predictor for upper tract urothelial carcinoma: A systematic review and meta-analysis. Mol Clin Oncol 2015;3:924-928. [PubMed]
- Tanaka N, Kikuchi E, Kanao K, et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Ann Surg Oncol 2014;21:4041-8. [Crossref] [PubMed]
- Vartolomei MD, Mathieu R, Margulis V, et al. Promising role of preoperative neutrophil-to-lymphocyte ratio in patients treated with radical nephroureterectomy. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Pichler M, Dalpiaz O, Ehrlich GC, et al. Validation of the preoperative plasma fibrinogen level as a prognostic factor in a European cohort of patients with localized upper tract urothelial carcinoma. J Urol 2014;191:920-5. [Crossref] [PubMed]
- Cheng YC, Huang CN, Wu WJ, et al. The Prognostic Significance of Inflammation-Associated Blood Cell Markers in Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol 2016;23:343-51. [Crossref] [PubMed]
- Tanaka N, Kikuchi E, Kanao K, et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: a multi-institutional study. Urol Oncol 2014;32:48.e19-26. [Crossref] [PubMed]
- Kim M, Moon KC, Choi WS, et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World J Urol 2015;33:1439-57. [Crossref] [PubMed]
- Sakano S, Matsuyama H, Kamiryo Y, et al. Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol 2013;20:4389-96. [Crossref] [PubMed]
- Morizane S, Yumioka T, Yamaguchi N, et al. Risk stratification model, including preoperative serum C-reactive protein and estimated glomerular filtration rate levels, in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Int Urol Nephrol 2015;47:1335-41. [Crossref] [PubMed]
- Ehdaie B, Shariat SF, Savage C, et al. Postoperative nomogram for disease recurrence and cancer-specific death for upper tract urothelial carcinoma: comparison to American Joint Committee on Cancer staging classification. Urol J 2014;11:1435-41. [PubMed]
- Wu P, Liu S, Zhang W, et al. Low-level Ki-67 expression as an independent predictor of bladder tumour recurrence in patients with primary upper tract urothelial carcinoma after radical nephroureterectomy. Jpn J Clin Oncol 2015;45:1175-81. [PubMed]
- Lei Y, Li Z, Qi L, et al. The Prognostic Role of Ki-67/MIB-1 in Upper Urinary-Tract Urothelial Carcinomas: A Systematic Review and Meta-Analysis. J Endourol 2015;29:1302-8. [Crossref] [PubMed]


