
Hydronephrosis in pediatric horseshoe kidneys: a comparative analysis of open and laparoscopic pyeloplasty and the influence of obstruction causes
Highlight box
Key findings
• Missed crossing vessels are the primary cause of reobstruction in hydronephrosis in horseshoe kidney (HSK).
What is known and what is new?
• HSK represents a unique challenge for ureteropelvic junction (UPJ) reconstruction due to its anomalous anatomy.
• We found that intrinsic obstruction and crossing vessels were the main causes of hydronephrosis in HSK, and missed crossing vessels were the primary cause of reobstruction.
What is the implication, and what should change now?
• Both open pyeloplasty (OP) and laparoscopic pyeloplasty (LP) are safe and effective in treating hydronephrosis in HSK patients, with LP being the less invasive option. It is crucial to search for crossing vessels actively and systematically when treating hydronephrosis in HSK patients.
IntroductionOther Section
Horseshoe kidney (HSK) is the most common renal fusion anomaly, occurring in approximately 1 in 500 children (1). HSK usually develops between 4 and 6 weeks of fetal development and can be associated with other kidney abnormalities, including duplex systems, vesicoureteral reflux (VUR), and hydronephrosis (2). Hydronephrosis is a common structural abnormality in about one-third of HSK patients, resulting from intrinsic obstruction, high ureteral insertions, abnormal ureteral courses anterior to the isthmus, crossing vessels, kinking ureter and other abnormal structures (3-5). Surgical treatment of this complex condition is challenging, and various techniques have been suggested, including conventional dismembered pyeloplasty, symphysiotomy, vascular hitch, endopyelotomy, and ureterocalicostomy (4-9). Pyeloplasty, proposed by Anderson and Hynes, has remained the gold standard for surgical treatment of hydronephrosis in children for decades, with a high success rate of more than 90% regardless of the methods (10,11). However, pyeloplasty can be challenging in patients with HSK due to the abnormal structure of the kidney. While several small case series studies have reported the feasibility and effectiveness of pyeloplasty in children with HSK, limited research has compared different surgical procedures (5,7,12). In the present study, we aimed to report our results in treating children with hydronephrosis in HSK and to investigate the differences in prognosis based on the cause of obstruction and the surgical methods. We also aimed to share our experiences by characterizing the success rates and complications after surgery. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-406/rc).
MethodsOther Section
Patients
Patients diagnosed with hydronephrosis in HSK and underwent pyeloplasty at our institution between August 2009 and June 2022 were retrospectively reviewed in this cohort observational study. Surgical intervention was performed if either ultrasound showed progression of hydronephrosis, patients had clinical symptoms, renal function of the hydronephrotic kidney <40%, decrease in split renal function >10%, or severe upper urinary tract dilatation (Society of Fetal Urology grade IV) (13) (http://uroweb.org/guidelines/compilations-of-all-guidelines/). Patients with a renal dysplasia, a VUR, or incomplete data were excluded.
Data collection and definition
Preoperative data included patient age, weight, gender, symptom, laterality, and ultrasound parameters. Ultrasonography recorded the anteroposterior pelvic diameter (APD) and the thickness of the renal parenchyma (C). The APD and P/C ratio (ratio of APD to the thickness of renal parenchyma) were used to evaluate the extent of hydronephrosis before and after surgery. Intraoperative findings and postoperative pathology were used to identify the cause of obstruction. Intrinsic obstruction was defined as ureteral lumen stenosis, and pathological examination showed muscular hypertrophy or dysplasia, fibrous tissue hyperplasia.
Assessment methods for follow-up included clinical manifestations, ultrasound, and urine routine. We choose to examine urine routine when patients undergoing symptoms such as fever, lower urinary tract symptoms or pyuria that make us doubt the possibility of urinary tract infections (UTI). Postoperative complications were defined as patients requiring further management, such as UTI, and reobstruction. UTI was defined as a symptomatic urine infection associated with a temperature over 38 ℃, and a pure bacterial growth >105 organisms on culture of a clean-catch urine sample (14). Reobstruction was defined as the need to redo dismemberment pyeloplasty if symptoms such as abdominal pain and vomiting were present, or if the Society of Fetal Urology grade was increased or the APD increased by more than 1 cm on ultrasound. All postoperative complications were classified according to the Clavien-Dindo classification, with postoperative UTI defined as Clavien-Dindo classification II and reobstruction defined as Clavien-Dindo classification IIIb (15). Surgical success was defined as the absence of reoperation and UTIs associated with this surgery. Ultrasound examination was performed 6 and 12 months after surgery and annually thereafter. Follow-up time, surgery methods, operation time, and postoperative length of stay (LOS) was also collected.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (16). This study obtained approval from the Ethics Committee of Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health (No. [2023]-E-043-R). Individual consent for this retrospective analysis was waived.
Surgery
Surgery was performed with open pyeloplasty (OP) or laparoscopic pyeloplasty (LP). All procedures for hydronephrosis in HSK do not involve division of the isthmus of the HSK. All procedures were performed by four surgeons with same qualifications and experience of OP and LP surgery. The choice of surgery modalities was largely determined by the experience and preference of the surgeon. For children younger than 1 year of age and of a weight less than 10 kg, we tend to perform OP. In addition to age and weight, the preference of the surgeon is also an important factor in the choice of surgical approach. Based on our institution’s experience, the use of LP in low-body-weight, small-age children can lead to limited access to ureter and increased incidence of intraoperative complications and side injuries due to the small space for abdominal manipulation. In a slightly inclined position with the affected side elevated, LP was performed using a transperitoneal methods with three ports (5 mm). The colon was mobilized to expose the renal pelvis up to the ureteropelvic junction (UPJ). When crossing vessels were detected, the UPJ and pelvis were delivered anterior to the crossing vessels and anastomosis was performed. If high ureteral insertions were encountered, the previous UPJ was ligated or cut with the redundant renal pelvis, and a neo-UPJ was anastomosed with 5-0 absorbable monofilament running suture at the most dependent position of the renal pelvis. The normal ureter was then identified distally, and dissection was carried out proximally toward the renal pelvis. Ureteropelvic anastomosis began from the most dependent portion of the incised pelvis to the most inferior point of the ureter. During the procedure, the surgeons first tried a double-J (DJ) stent if the stenotic segment had resolved halfway through the anastomosis. If the DJ stent was well placed in an antegrade fashion, a perinephric drain and urethral catheter were placed simultaneously. The appropriate catheter size was selected based on the patient’s age: 6, 8, 8–10, and 10–12 F catheters were selected for patients aged 0–2, 2–5, 5–10, and 10–16 years, respectively. In our study, all LP patients were placed 4.7 F DJ stent except for one patient under 1 year old was placed 4 F DJ stent. None of the patients had difficulty placing the DJ stent. For OP, an average size of 5 cm subcostal flank incision was made. OP followed the same steps as LP, and a nephrostomy tube and external ureteral stent were placed. The nephrostomy tube serves to drain the urine, while the external stent serves to support the neo-UPJ. The distal end of the external ureteral stent didn’t arrive to the bladder. External ureteral stent would be removed 6–7 days after surgery. The nephrostomy tube was clamped 24 hours before removal and the methylene blue test was performed at the time the nephrostomy tube was clamped. If the methylene blue urine was drained and there were no complications within 24 hours, the nephrostomy tube would be removed. The DJ stent was removed under general anesthesia using a cystoscope 4–6 weeks after the operation.
Statistical analysis
All statistical analyses were performed using R software (version 2244.2.2, http://www.r-project.org). Preoperative and postoperative ultrasound parameters were performed with GraphPad Prism (GraphPad Prism 9.5.0.730 for Windows; GraphPad Software; GraphPad, Bethesda, MD, USA). Normality test was performed before sample comparison (Shapiro-Wilk normality test or Pearson normality test). Continuous data following normal distribution showed as mean ± standard deviation were analyzed by t-test. Continuous data not following normal distribution are presented as median [interquartile range (IQR)], analyzed by Mann-Whitney U-test, while variables between groups were compared using Chi-square test or Fisher exact test. All statistical results were reported as two-tailed P<0.05 is considered statistical significant.
ResultsOther Section
Patient characteristics
One patient with renal dysplasia was excluded and three patients were excluded due to missing data. A total of 31 patients were included and followed up for more than 6 months. The median age of the overall cohort at the time of surgery was 51.0 months (IQR, 26.0–83.0 months), while the median weight was 17.4 kg (IQR, 13.5–22.5 kg). All patients had grade 4 hydronephrosis before treatment. Among them, 58.1% of patients (18/31) were symptomatic, with abdominal pain in 14, UTI in 2, abdominal mass in 1, pyuria in 1, and hematuria in 1. OP was performed in 16 patients (51.6%) and LP in 15 patients (48.4%). The causes of obstruction include intrinsic obstruction, crossing vessels, high ureter insertion, and kinking ureter. In the overall cohort, intrinsic obstruction (19/31, 61.3%) was the most common cause of obstruction. Crossing vessels were found in 16 patients (51.6%), including four patients combined with intrinsic obstruction, three patients combined with high ureter insertion, one patient combined with intrinsic obstruction and high ureter insertion, and one patient combined with intrinsic obstruction and kinking ureter (Table 1). In this study, 6 patients (19.4%) developed complications. In general, surgical success was achieved in 25 patients (80.6%).
Table 1
| Variables | Value (n=31) |
|---|---|
| Age (months) | 51.0 (26.0–83.0) |
| Weight (kg) | 17.4 (13.5–22.5) |
| Gender | |
| Male | 20 (64.5) |
| Female | 11 (35.5) |
| Follow-up time (months) | 31.0 (10.5–75.5) |
| Symptom | |
| No | 13 (41.9) |
| Yes | 18 (58.1) |
| Laterality | |
| Left | 22 (71.0) |
| Right | 9 (29.0) |
| Surgery modality | |
| OP | 16 (51.6) |
| LP | 15 (48.4) |
| Operation time (min) | 94.0 (72.8–130.0) |
| Cause of obstruction | |
| Crossing vessels only | 7 (22.6) |
| Intrinsic obstruction only | 7 (22.6) |
| High ureteral insertions only | 2 (6.5) |
| Crossing vessels with intrinsic obstruction | 4 (12.9) |
| Crossing vessels with high ureteral insertions | 3 (9.7) |
| Crossing vessels with intrinsic obstruction and high ureteral insertions | 1 (3.2) |
| Crossing vessels with intrinsic obstruction and kinking ureter | 1 (3.2) |
| Intrinsic obstruction with high ureteral insertions | 4 (12.9) |
| Intrinsic obstruction with kinking ureter | 2 (6.5) |
| Postoperative LOS (days) | 7.0 (5.5–11.0) |
| Complications | |
| UTI | 4 (12.9) |
| Reobstruction | 2 (6.5) |
Data are shown as median (interquartile range) or number (percentage). OP, open pyeloplasty; LP, laparoscopic pyeloplasty; LOS, length of stay; UTI, urinary tract infections.
Comparison of OP and LP
The comparison of OP group and LP group is shown in Table 2. There were no significant differences in age, weight, symptom, cause of obstruction, postoperative ultrasound parameters, and operation time between the two groups (P>0.05). The preoperative APD and P/C in the OP group were smaller than those in the LP group (P=0.010), while the preoperative C was thicker in the OP group (0.5 vs. 0.3, P=0.032). The results showed that there was no significant difference in complications between the two groups [12.5% (2/16) vs. 26.7% (4/15), P=0.374]. A total of 4 patients presented with UTI. Among them, one patient in the OP group had UTI with symptoms of abdominal pain and vomiting with nephrostomy tube placed. One patient in the LP group had a UTI with symptoms of abdominal pain and vomiting with DJ stent placement. After DJ stents being removed, two patients in the LP group developed UTI. All patients’ symptoms improved after antibiotic treatment. Reobstruction occurred in one patient each in the OP and LP groups, and crossing vessels were found during the reoperation, which were not found in the primary operation. LP performed longer operation time than OP with no significant statistical difference (P=0.550). Postoperative LOS was significantly longer in the OP group compared with the LP group (11.0 vs. 6.0, P<0.001).
Table 2
| Variables | OP (n=16) | LP (n=15) | P value |
|---|---|---|---|
| Age (months) | 40.0 (26.5–78.2) | 55.0 (29.0–88.0) | 0.418 |
| Weight (kg) | 15.2 (12.8–20.2) | 19.0 (14.5–23.3) | 0.373 |
| Symptom | 0.565 | ||
| No | 8 (50.0) | 5 (33.3) | |
| Yes | 8 (50.0) | 10 (66.7) | |
| Cause of obstruction | >0.99 | ||
| Crossing vessels | 8 (50.0) | 7 (46.7) | |
| No crossing vessels | 8 (50.0) | 8 (53.3) | |
| Preoperative | |||
| APD (cm) | 2.1±0.6 | 2.8±0.8 | 0.010 |
| C (cm) | 0.5±0.2 | 0.3±0.2 | 0.032 |
| P/C radio | 4.3 (2.7–8.9) | 11.7 (6.6–21.0) | 0.010 |
| 6 months postoperatively | |||
| APD (cm) | 1.5 (1.1–2.0) | 1.0 (0.8–1.6) | 0.068 |
| C (cm) | 0.4 (0.3–0.6) | 0.3 (0.3–0.5) | 0.367 |
| P/C radio | 4.3 (2.2–7.1) | 3.3 (2.0–4.5) | 0.259 |
| 12 months postoperatively | |||
| APD (cm) | 1.2 (0.9–1.5) | 0.7 (0.6–1.4) | 0.153 |
| C (cm) | 0.5±0.2 | 0.5±0.2 | 0.879 |
| P/C radio | 2.1 (1.5–4.0) | 1.5 (1.2–2.9) | 0.374 |
| Operation time (min) | 75.0 (71.0–130.0) | 100.0 (77.5–130.0) | 0.550 |
| Postoperative LOS (days) | 11.0 (9.8–12.0) | 6.0 (5.0–6.0) | <0.001 |
| Complications | 0.374 | ||
| UTI | 1 (6.3) | 3 (20.0) | |
| Reobstruction | 1 (6.3) | 1 (6.7) |
Data are shown as median (interquartile range), median ± standard deviation or number (percentage). OP, open pyeloplasty; LP, laparoscopic pyeloplasty; APD, anteroposterior pelvic diameter; P/C ratio, ratio of APD to the thickness of renal parenchyma; C, the thickness of the renal parenchyma; LOS, length of stay; UTI, urinary tract infections.
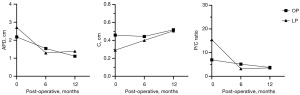
The trend of changes in preoperative and postoperative ultrasound parameters are shown in Figure 1. The decrease of APD and P/C ratio in the OP group was relatively gentle, and the decrease of APD and P/C ratio in the LP group was rapid at 6 months postoperatively, and the changes were not significant from 6 to 12 months postoperatively. The C thickness in the LP group increased more significantly than that in the OP group at 6 months postoperatively, and it was similar to rise between the two groups from 6 to 12 months postoperatively.
Comparison of with and without crossing vessels
As shown in Table 3, there were no significant differences in age, weight, symptom, surgery methods, preoperative and postoperative ultrasound parameters, operation time, and postoperative LOS between the with and without crossing vessels groups (P>0.05). In the crossing vessels group, 4 patients (12.9%) had UTI and 2 patients (6.5%) were found reobstruction and cured after reoperation, while there was no patient with complication in the no crossing vessels group (P=0.018). The success rate in crossing vessels was significantly lower than that in the no crossing vessels group (62.5% vs. 100%, P=0.018).
Table 3
| Variables | Crossing vessels (n=16) | No crossing vessels (n=15) | P value |
|---|---|---|---|
| Age (months) | 59.0 (36.0–88.5) | 41.0 (17.0–70.5) | 0.082 |
| Weight (kg) | 20.0 (15.0–23.1) | 15.0 (10.5–19.5) | 0.085 |
| Symptom | 0.378 | ||
| No | 5 (31.2) | 8 (53.3) | |
| Yes | 11 (68.8) | 7 (46.7) | |
| Surgery methods | >0.99 | ||
| OP | 8 (50.0) | 8 (53.3) | |
| LP | 8 (50.0) | 7 (46.7) | |
| Preoperative | |||
| APD (cm) | 2.3±0.9 | 2.6±0.7 | 0.409 |
| C (cm) | 0.4±0.2 | 0.3±0.2 | 0.337 |
| P/C radio | 5.6 (3.5–11.7) | 8.7 (5.3–18.5) | 0.268 |
| 6 months postoperatively | |||
| APD (cm) | 1.3 (1.0–2.1) | 1.0 (0.7–1.9) | 0.212 |
| C (cm) | 0.4 (0.3–0.5) | 0.3 (0.3–0.5) | 0.297 |
| P/C radio | 3.6 (2.2–5.2) | 3.3 (1.8–5.0) | 0.593 |
| 12 months postoperatively | |||
| APD (cm) | 1.1 (0.7–1.6) | 0.9 (0.6–1.4) | 0.405 |
| C (cm) | 0.5±0.2 | 0.5±0.2 | 0.752 |
| P/C radio | 1.6 (1.4–3.8) | 2.0 (1.2–3.4) | 0.797 |
| Operation time (min) | 94.0 (73.8–128.0) | 94.0 (72.8–130.0) | 0.979 |
| Postoperative LOS (days) | 8.0 (6.0–12.0) | 6.0 (5.0–10.5) | 0.390 |
| Complications | 0.018 | ||
| UTI | 4 (12.9) | 0 (0.0) | |
| Reobstruction | 2 (6.5) | 0 (0.0) |
Data are shown as median (interquartile range), median ± standard deviation or number (percentage). OP, open pyeloplasty; LP, laparoscopic pyeloplasty; APD, anteroposterior pelvic diameter; P/C ratio, ratio of APD to the thickness of renal parenchyma; C, the thickness of the renal parenchyma; LOS, length of stay; UTI, urinary tract infections.
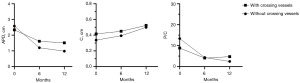
The trend of changes in preoperative and postoperative ultrasound parameters were shown in Figure 2. APD and P/C ratio decreased similarly between the two groups at 6 and 12 months postoperatively. The C thickness was similar to rise between the two groups at 6 and 12 months postoperatively.
DiscussionOther Section
HSK is the most common renal fusion anomaly. Hydronephrosis in HSK is a rare malformation with various anatomical variations (8). For treating hydronephrosis in a normal kidney, pyeloplasty is considered the gold standard treatment, with a success rate exceeding 90% regardless of the approach used (11,17). Nevertheless, a minimally invasive technique known as LP has emerged as a new gold standard for treating hydronephrosis in children, and has been increasingly adopted since its introduction in the 1990s (18). In recent years, robotic technology has been developing rapidly and becoming more widely used in pyeloplasty (19). The advent of robotic technology has mitigated some of the technical challenges associated with laparoscopic surgery, providing surgeons with advanced EndoWrist instrumentation and three-dimensional visualization (20).
In our study, we analyzed data from 31 patients of hydronephrosis in HSK, including 15 patients that underwent OP and 16 patients that underwent LP. We investigated the outcomes between OP and LP, with and without crossing vessels. Our findings aim to contribute to the management of hydronephrosis in HSK. In our study, structural abnormalities causing hydronephrosis included intrinsic obstruction, crossing vessels, high ureteral insertions, and kinking ureter. In the overall cohort, intrinsic obstruction was the most common cause of obstruction, which was consistent with previous report (21). Previous literature has reported that crossing vessels occurrence in 40–80% of patients, and high ureteral insertions in 20–40% (5,6,22). In our study, we observed crossing vessels in 51.6% of patients, and high ureteral insertions in 32.2% of patients. Crossing vessels that cause obstruction in isolation can theoretically be treated with a vascular hitch (23-25). However, sometimes crossing vessels are not the only cause, and intrinsic obstruction or high ureteral insertions may also be present. In these patients, dismembered pyeloplasty is the most effective way to address both extrinsic and intrinsic obstructions simultaneously. In our study, all patients with and without crossing vessels underwent pyeloplasty.
Our study demonstrated a surgical success rate of 80.6% (25/31) in treating HSK. Previous studies have reported success rates of pyeloplasty for HSK to be lower compared to that of normal kidneys, ranging from 55% to 80% and exceeding 90%, respectively (7,11,21,26). Moreover, previous study has shown no significant difference in success rates among OP, LP, and robot-assisted LP for treating hydronephrosis in HSK (12). Similarly, our present study showed no significant difference in success rates between OP and LP. For normal kidneys, the incidence of complications after pyeloplasty ranges from 0% to 29.3%, including urinary leakages, UTI, wound infections, ileus, restenosis, and hemorrhage (27). In our previous LP series in normal kidney, the success rate reached 93.8%, as 6.2% (33/535) of patients developed negative outcome including ten patients who was found of reobstruction and underwent secondary surgery (28).
In the present study, 19.4% of patients developed grade II or IIIb Clavien-Dindo postoperative complications. There was no significant difference in the incidence of UTI between the LP group, which left catheter in place longer, and the OP group, which left catheter in place for a shorter period of time. Our study did not find a correlation between the duration of indwelling catheterization and the occurrence of UTI, and prospective studies with larger samples are needed to further explore this question in the future. We found no significant difference in the rates of reobstruction between the OP and LP groups. Reobstruction occurred in two patients because missing crossing vessels in the primary operation. This may be due to rotation of the renal pelvis, variations in vascular alignment and poor visual field exposure in HSK patients. In our experience, the laparoscopic methods provide a more flexible and extensive way to identify crossing vessels in HSK. Compared with open surgery, laparoscopic surgery allows for better visualization of the renal pelvis and UPJ, which is critical for identifying the presence of crossing vessels (5). Additionally, the clear intraoperative anatomy of the inferior pole of the kidney in laparoscopic surgery provides improved surgical exposure, allowing for a more thorough exploration of the medial inferior region of the kidney. Given the special anatomy of the isthmus of HSK, adequate exploration of this region is essential for successful surgery. Laparoscopic surgery provides a more thorough dissection of the UPJ and can confirm whether crossing vessels exist or not more clearly, which is critical for selecting the appropriate surgical technique. Therefore, we believe that LP is a valuable method for the treatment of hydronephrosis in patients with HSK.
The median duration of postoperative LOS was relatively long in our study, because our institution is a medical referral center for the treatment of pediatric UPJO in China and most patients come from other provinces far away from our hospital, it is necessary to ensure steady postoperative recovery and avoid multiple hospital returns after discharge. In OP group, the LOS was significantly longer than LP group. Slower recovery and the requirement for removal of the nephrostomy tube in some patients during hospital stay may be the reasons for prolonged LOS in OP group.
The main limitations of this study are the retrospective nature, the small sample size and lack of robotic approach. However, this limitation is due to the high rarity of this pathology in the pediatric population. In addition, due to the long-time span of the study and economic conditions, only a few patients underwent CT angiogram and magnetic resonance urography which can evaluate vasculature and collecting system accurately. Despite above-mentioned limitations, our study highlights the consistent reasons for obstruction and causes for redo surgery in HSK patients undergoing pyeloplasty. Our findings suggest that both OP and LP are safe and effective surgical options for treating hydronephrosis in HSK, with high success rates and low complication rates. Nonetheless, further studies with larger sample sizes and prospective designs are warranted to confirm our findings and evaluate the long-term outcomes of these procedures in HSK patients.
ConclusionsOther Section
HSK represents a unique challenge for UPJ reconstruction due to its anomalous anatomy. Our study found that intrinsic obstruction and crossing vessels were the main causes of hydronephrosis in HSK, and missed crossing vessels were the primary cause of reobstruction. Our results demonstrate that both OP and LP are safe and effective in treating hydronephrosis in HSK patients, with LP being the less invasive option.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-406/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-406/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-406/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-406/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained approval from Ethics Committee of Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health (No. [2023]-E-043-R). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Glodny B, Petersen J, Hofmann KJ, et al. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU Int 2009;103:224-35. [Crossref] [PubMed]
- Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. J Pediatr Urol 2016;12:275-80. [Crossref] [PubMed]
- Keeley FX Jr, Bagley DH, Kulp-Hugues D, et al. Laparoscopic division of crossing vessels at the ureteropelvic junction. J Endourol 1996;10:163-8. [Crossref] [PubMed]
- Yohannes P, Smith AD. The endourological management of complications associated with horseshoe kidney. J Urol 2002;168:5-8. [Crossref] [PubMed]
- Blanc T, Koulouris E, Botto N, et al. Laparoscopic pyeloplasty in children with horseshoe kidney. J Urol 2014;191:1097-103. [Crossref] [PubMed]
- Bove P, Ong AM, Rha KH, et al. Laparoscopic management of ureteropelvic junction obstruction in patients with upper urinary tract anomalies. J Urol 2004;171:77-9. [Crossref] [PubMed]
- Nishi M, Iwamura M, Kurosaka S, et al. Laparoscopic Anderson-Hynes pyeloplasty without symphysiotomy for hydronephrosis with horseshoe kidney. Asian J Endosc Surg 2013;6:192-6. [Crossref] [PubMed]
- Houat AP, Guimarães CTS, Takahashi MS, et al. Congenital Anomalies of the Upper Urinary Tract: A Comprehensive Review. Radiographics 2021;41:462-86. [Crossref] [PubMed]
- Kumar S, Panigrahy B. Laparoscopic management of complex ureteropelvic junction obstruction. J Laparoendosc Adv Surg Tech A 2009;19:521-8. [Crossref] [PubMed]
- Szavay P, Zundel S. Surgery of uretero-pelvic junction obstruction (UPJO). Semin Pediatr Surg 2021;30:151083. [Crossref] [PubMed]
- Abdel-Karim AM, Fahmy A, Moussa A, et al. Laparoscopic pyeloplasty versus open pyeloplasty for recurrent ureteropelvic junction obstruction in children. J Pediatr Urol 2016;12:401.e1-6. [Crossref] [PubMed]
- Elmaadawy MIA, Kim SW, Kang SK, et al. A retrospective analysis of ureteropelvic junction obstructions in patients with horseshoe kidney. Transl Androl Urol 2021;10:4173-80. [Crossref] [PubMed]
- Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol 1993;23:478-80. [Crossref] [PubMed]
- Akinci A, Kubilay E, Solak VT, et al. Effect of continuous antibiotic prophylaxis in children with postoperative JJ stents: A prospective randomized study. J Pediatr Urol 2021;17:89-94. [Crossref] [PubMed]
- Yoon PD, Chalasani V, Woo HH. Use of Clavien-Dindo classification in reporting and grading complications after urological surgical procedures: analysis of 2010 to 2012. J Urol 2013;190:1271-4. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Penn HA, Gatti JM, Hoestje SM, et al. Laparoscopic versus open pyeloplasty in children: preliminary report of a prospective randomized trial. J Urol 2010;184:690-5. [Crossref] [PubMed]
- Peters CA, Schlussel RN, Retik AB. Pediatric laparoscopic dismembered pyeloplasty. J Urol 1995;153:1962-5. [Crossref] [PubMed]
- Esposito C, Masieri L, Blanc T, et al. Robot-assisted laparoscopic pyeloplasty (RALP) in children with horseshoe kidneys: results of a multicentric study. World J Urol 2019;37:2257-63. [Crossref] [PubMed]
- Boysen WR, Gundeti MS. Robot-assisted laparoscopic pyeloplasty in the pediatric population: a review of technique, outcomes, complications, and special considerations in infants. Pediatr Surg Int 2017;33:925-35. [Crossref] [PubMed]
- Moscardi PR, Lopes RI, Mello MF, et al. Laparoscopic Pyeloplasty in children with Horseshoe Kidney. Int Braz J Urol 2017;43:375. [Crossref] [PubMed]
- Oderda M, Calleris G, Allasia M, et al. Robot-assisted laparoscopic pyeloplasty in a pediatric patient with horseshoe kidney: surgical technique and review of the literature. Urologia 2017;84:55-60. [Crossref] [PubMed]
- Kim JK, Keefe DT, Rickard M, et al. Vascular hitch for paediatric pelvi-ureteric junction obstruction with crossing vessels: institutional analysis and systematic review with meta-analysis. BJU Int 2022;129:679-87. [Crossref] [PubMed]
- Miscia ME, Lauriti G, Riccio A, et al. Minimally invasive vascular hitch to treat pediatric extrinsic ureteropelvic junction obstruction by crossing polar vessels: A systematic review and meta-analysis. J Pediatr Urol 2021;17:493-501. [Crossref] [PubMed]
- Gundeti MS, Reynolds WS, Duffy PG, et al. Further experience with the vascular hitch (laparoscopic transposition of lower pole crossing vessels): an alternate treatment for pediatric ureterovascular ureteropelvic junction obstruction. J Urol 2008;180:1832-6; discussion 1836. [Crossref] [PubMed]
- Lallas CD, Pak RW, Pagnani C, et al. The minimally invasive management of ureteropelvic junction obstruction in horseshoe kidneys. World J Urol 2011;29:91-5. [Crossref] [PubMed]
- Mei H, Pu J, Yang C, et al. Laparoscopic versus open pyeloplasty for ureteropelvic junction obstruction in children: a systematic review and meta-analysis. J Endourol 2011;25:727-36. [Crossref] [PubMed]
- Li J, Li Z, He Y, et al. Development of the prediction model for negative outcomes after primary laparoscopic pyeloplasty in children: a retrospective study of 535 patients. Transl Androl Urol 2022;11:1680-90. [Crossref] [PubMed]


