
Efficacy of medication for overactive bladder symptoms in 70 patients with Parkinson’s disease
Highlight box
Key findings
• The study presents an evidence that adopts tolterodine and/or tamsulosin as a pharmacologic treatment for overactive bladder symptoms in patients with Parkinson’s disease.
What is known and what is new?
• Managing overactive bladder symptoms in Parkinson’s disease is challenging, and tolterodine and/or tamsulosin are used empirically in clinic.
• We provide an evidence of treatment benefit from tolterodine and/or tamsulosin for overactive bladder symptoms in 70 patients with Parkinson’s disease. The work may aid future treatment.
What is the implication, and what should change now?
• The current work supports the usage of tolterodine and/or tamsulosin for overactive bladder symptoms in patients with Parkinson’s disease.
• Future research may include larger sample sizes and focus on the unsatisfactory symptoms after treatment.
Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disease characterized by motor and nonmotor symptoms (1). Urinary dysfunction is one of the most common nonmotor symptoms in PD patients, dominated by overactive bladder (OAB) symptoms (2). OAB is defined as a syndrome of urinary urgency, with or without urge incontinence, usually with urinary frequency and nocturia, and in the absence of infection or other obvious pathologic features (3). OAB seriously impacts the quality of life of PD patients.
Unlike motor disorders, urinary dysfunction is usually unresponsive to levodopa, and an add-on therapy for urinary dysfunction is necessary (2). Antimuscarinic drugs are recommended for OAB symptoms, and α-1 blockers are used in combination with antimuscarinic drugs or alone in male patients with voiding symptoms (2). However, few studies have comprehensively examined the features and management of OAB symptoms specifically in PD patients, such as urinary symptoms, bladder-urethral function, management schedule and remained symptoms after treatment. The aim of this study was to investigate the management of OAB symptoms in PD patients treated with tolterodine and/or tamsulosin in our daily work. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-378/rc).
Methods
Participants
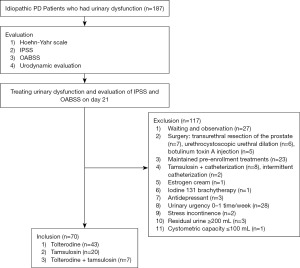
Idiopathic PD patients with OAB symptoms who were newly treated with tolterodine and/or tamsulosin were screened from a database of 187 PD patients (Figure 1). The database was prospectively collected from October 2013 to February 2019 in a single center for the assessment and management of urinary dysfunction in PD patients (4). The database included patients of 40–80 years of age with urinary dysfunction duration ≥1 month and sterile urine, and excluded patients with communication difficulty, acute urine retention, a history of prostate/urethra surgery, pelvic organ prolapse, cerebrovascular disease, coronary disease, and Hoehn-Yahr (H-Y) stage 5. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Xuanwu Hospital Capital Medical University (2013L013) and informed consent was taken from all the patients.
Questionnaire evaluation
Before treatment, the H-Y scale was used for neurological evaluation at “on” status, and the International Prostate Symptom Score (IPSS) and Overactive Bladder Symptom Score (OABSS) were used to evaluate OAB symptoms. The IPSS is composed of seven questions regarding storage symptoms (IPSS-s: questions 2, 4, and 7) and voiding symptoms (IPSS-v: questions 1, 3, 5, and 6), and it divides the severity of urination disorders into mild [0–7], moderate [8–19], and severe [20–35] symptoms (5). The OABSS is composed of four questions regarding storage symptoms, including daytime frequency, nocturia, urgency, and urge incontinence, and divides severity of urination disorders into mild [3–5], moderate [6–11], and severe [12–15] symptoms (5). In this study, daytime frequency ≥8 times, nocturia ≥2 times, urgency ≥1 time/day, and urge incontinence ≥1 time/week were deemed troublesome OAB symptoms and were further analyzed in OABSS.
Urodynamic evaluation
Before treatment, all the patients received urodynamic evaluation. We previously described the methods and definitions used for this evaluation (4). In men, a bladder outlet obstruction (BOO) index [detrusor pressure at Qmax (PDetQmax) – 2Qmax] value >40 was defined as BOO. A bladder contractility index (PDetQmax + 5Qmax) value <100 constituted detrusor underactivity (DUA) (6). In women, the following standards were used in the pressure-flow analysis: (I) BOO: PDetQmax >30 cmH2O together with Qmax <10 mL/s (7); (II) DUA: conforming to the International Continence Society definition (3) and PDetQmax ≤30 cmH2O together with Qmax <10 mL/s; (III) normal: PDetQmax >0 and Qmax >10 mL/s. Bladder hypersensitivity was defined as a decrease in the first sensation volume (100 mL < normal < 300 mL) without DO during the filling phase (8).
Treatment and follow-up
The therapeutic schedule was chosen by the urologists who performed the urodynamic evaluation based on the following principles: (I) tolterodine extended-release tablet (4 mg, orally, once a day) was prescribed when storage symptoms/DO were dominant in patients with a low risk of urinary retention; (II) tamsulosin extended-release capsule (0.2 mg, orally, once a night) was prescribed when obstructive symptoms/BOO/DUA were primarily found; and (III) combined therapy was used when both components were found. Follow-up was made by telephone on day 21 of treatment with IPSS and OABSS re-evaluation.
Statistical analyses
Data are expressed as absolute frequencies, percentages, mean ± standard deviation and median [interquartile range (IQR)]. A paired-samples t-test was used with P<0.05 considered statistically significant to evaluate the improvement of OAB symptoms.
Results
Demographic data
Seventy patients (28 males and 42 females) with a mean age of 62.2±7.9 years and a median H-Y stage of 2 (IQR 2–3) were enrolled (Figure 1; Table 1). The patients were from the hospital’s neurological outpatient clinic (n=8), urological outpatient clinic (n=21), and neurological ward (n=41). The median PD and OAB durations were 30 (IQR 12–60) months and 24 (IQR 10–36) months, respectively. Before urodynamic evaluation, 57 patients received drugs for motor disorder. A total of 33 patients had comorbidities, including 23 patients with benign prostatic hyperplasia.
Table 1
| Variables | Male (n=28) | Female (n=42) |
|---|---|---|
| Age (years) | 65.5±5.4 | 62±8.1 |
| BMI (kg/m2) | 25.1±3.4 | 24.3±3.3 |
| PD duration (months) | 36 [24, 60] | 24 [12, 72] |
| Urgency duration (months) | 24 [12, 60] | 21 [7.5, 36] |
| Hoehn-Yahr scale | ||
| I | 0 | 3 |
| II | 19 | 28 |
| III | 5 | 9 |
| IV | 4 | 2 |
| Anti-motor disorder drugs | ||
| No | 6 | 7 |
| Levodopa and benserazide hydrochloride tablet | 21 | 30 |
| Dopamine receptor agonists | 13 | 21 |
| Entacapone tablet | 7 | 10 |
| Amantadine hydrochloride tablet | 1 | 10 |
| Comorbidities | ||
| No | 3 | 34 |
| Hypertension | 4 | 2 |
| Diabetes | 1 | 5 |
| Nephrotic syndrome | 0 | 1 |
| Depression | 2 | 3 |
| Benign prostatic hyperplasia | 23 | – |
Data are presented as mean ± SD, median [IQR] or n. SD, standard deviation; BMI, body mass index; PD, Parkinson’s disease; IQR, interquartile range.
OAB symptoms at baseline
The IPSS (Figure 2A) and IPSS-v (10.9±5.3 vs. 4.8±4.6) in male patients were significantly higher than that in female patients (both P<0.01), whereas there was no significant difference in the OABSS (Figure 2B) and IPSS-s (9.1±3 vs. 9.6±3) between males and females (both P>0.05). According to the IPSS and OABSS, moderate and severe symptoms were predominant in male and female patients (Figure 2C,2D).

Urodynamic findings
Overall, 46 (65.7%) patients had DO and 22 (78.6%) male patients had BOO. There were also obvious gender differences in urodynamic findings. Among the 28 male patients, DO + DUA was the most common urodynamic finding and occurred in 10 (35.7%) patients, followed by DO + BOO in 8 (28.6%) patients, BOO in 4 (14.3%) patients, DO in 3 (10.7%) patients, DUA in 2 (7.1%) patients, and normal findings in 1 (3.6%) patient. Among the 42 female patients, DO was the most common urodynamic finding and occurred in 25 (59.5%) patients, followed by normal findings in 8 (19%) patients, bladder hypersensitivity in 7 (16.7%) patients, and BOO in 2 (4.8%) patients.
Treatment schedule
Among the 28 male patients, 18 (64.3%) received tamsulosin alone, including 4 patients with DO + BOO, 7 patients with DO + DUA, 4 patients with BOO, 2 patients with DUA and 1 patient with normal urodynamic findings. Seven (25%) male patients received tolterodine + tamsulosin, including 4 patients with DO + BOO and 3 patients with DO + DUA. Other 3 (10.7%) male patients who had DO received tolterodine alone. Among the 42 female patients, 40 (95.2%) received tolterodine alone, including 25 patients with DO, 8 patients with normal urodynamic findings and 7 patients with bladder hypersensitivity. Other 2 (4.8%) female patients who had BOO received tamsulosin alone.
Improvement of OAB symptoms
The IPSS-s (9.4±3 vs. 3.5±2.3) and OABSS (9±2.8 vs. 4.8±3.3) improved significantly after treatment (both P<0.01). After treatment, the IPSS (Figure 2A) and IPSS-v (5.2±3.7 vs. 2.6±3.4) in male patients were significantly higher compared to female patients (both P<0.01), whereas there was no significant difference in OABSS (Figure 2B) and IPSS-s (3.8±2 vs. 3.3±2.5) between males and females (both P>0.05). There were markedly fewer patients with moderate and severe symptoms according to IPSS or OABSS after treatment, although moderate symptoms remained relatively high (Figure 2C,2D). Similarly, troublesome OAB symptoms decreased but remained relatively high after treatment (Figure 3A-3D). The most common troublesome OAB symptom that remained was nocturia, followed by urgency, daytime frequency, and urge incontinence (Figure 3A-3D). Further analysis showed OAB in the male patients was improved with tamsulosin alone or tolterodine + tamsulosin (Figure 4A-4C).


Discussion
OAB symptoms deteriorate the quality of life in PD patients and the management is challenging. This study found that tolterodine and/or tamsulosin can significantly improve OAB symptoms in PD patients. The findings from the analysis of unsatisfactory OAB symptoms will shed light on further OAB management.
OAB symptoms in PD patients seem to be the result of an altered dopaminergic circuit in frontal-basal ganglion D1, which normally suppresses the micturition reflex (9). However, multiple causes can lead to urinary dysfunction, such as medications used to treat the motor disorders or benign prostatic hyperplasia (10). It is difficult to determine whether the patient’s OAB symptoms are caused by PD or have other etiologies, as well as to what extent PD is responsible for OAB symptoms in patients with multiple possible causes. Most OAB patients benefit from empiric therapy. In this study, urodynamics were performed in all the patients, and the reason was that PD patients were prospectively recruited from those who underwent urodynamic evaluation in our urodynamic center. However, urodynamics should be performed to explore the underlying bladder and voiding disorder for PD patients with refractory urinary dysfunction.
For OAB patients, the most distress symptom is urgency or urge incontinence (11,12), while voiding symptoms are also present in some patients. In this study, the IPSS, OABSS, and urodynamic evaluation were used to assess OAB symptoms. The IPSS includes 7 questions on voiding symptoms and storage symptoms (13) and is often used as a self-reporting tool in both male and female PD patients in daily practice (4). However, the IPSS is not a perfect tool for evaluating OAB symptoms, as urgency has the same score weight as other questions and there is no assessment of urge incontinence (5). The OABSS is widely used to evaluate OAB symptoms (14), and it places more weight on the scores for urgency and urge incontinence (maximum score of 5) than on frequency or nocturia (maximum score of 2 or 3) (13). However, there is no assessment of voiding symptoms in OABSS.
Sex difference influences treatment options of OAB symptoms. Antimuscarinics (e.g., tolterodine) and β-3 adrenergic receptor agonists (e.g., mirabegron) are the most commonly used drugs to treat OAB symptoms (15). However, the side effect of urinary retention constrains antimuscarinics usage in men with BOO and DUA. The results of urodynamic evaluation exacerbated concerns about the usage of tolterodine in men in this study. Twenty-four (85.7%) male patients had BOO or DUA, while 2 (4.8%) female patients had BOO, with no DUA in female patients. However, there is no uniform standard for BOO and DUA in women (16).
Tamsulosin is a selective α-1a blocker and is recommended for voiding symptoms or combined symptoms in patients with prostatic hyperplasia. Currently, no specific well-designed PD studies have reported on the clinical use of tolterodine or tamsulosin for OAB symptoms. Two randomized placebo-controlled trials showed mirabegron was effective in treating OAB symptoms in PD patients with acceptable adverse events (17,18). In this study, tolterodine was used in 40 (95.2%) female patients, while tamsulosin was used in 25 (89%) male patients, including 7 (25%) male patients treated with tolterodine + tamsulosin. However, the therapeutic schedule was made at the urologists’ discretion, and the sample size was too small to compare the efficacy between tamsulosin alone and tolterodine + tamsulosin in male patients.
The study revealed that tolterodine and/or tamsulosin significantly improved OAB symptoms in PD patients. However, 28 (40%) patients still displayed moderate urinary symptoms, and nocturia and urinary urgency affected more than half of the patients after treatment. Psychological and lifestyle factors, such as anxiety, fluid intake, nocturnal polyuria, and sleep disturbances, should be analyzed in the management of persistent OAB symptoms after treatment (4). It is possible that an add-on therapy with other antimuscarinic drugs or β-3 receptor agonists, botulinum toxin bladder wall injection, or transurethral prostate resection could further relieve the OAB symptoms (4). Sacral neuromodulation is reportedly efficacious in selected PD patients with OAB symptoms (19).
This study has some limitations: small sample size, heterogeneous population based on gender and urodynamic findings, therefore heterogenous treatment patterns. In particular, we did not assess the effects of anti-motor disorder drugs on OAB symptoms, due to a lack of sufficient communication between the neurologists and urologists.
Conclusions
Tolterodine and/or tamsulosin can significantly improve OAB symptoms in PD patients. However, nocturia and urgency remain common after treatment, which requires further study.
Acknowledgments
The authors would like to thank Erhe Xu and Wei Mao from the Department of Neurology, Xuanwu Hospital Capital Medical University, National Clinical Research Center for Geriatric Diseases, Beijing, China for their assistance in patient enrollment.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-378/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-378/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-378/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-378/coif). C.J. receives grant from National Natural Science Foundation of China (Grant No. 82070787). T.O. receives grant from Capital’s Funds for Health Improvement and Research (Grant No. 2020-2-2015). The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet 2021;397:2284-303. [Crossref] [PubMed]
- Sakakibara R, Panicker J, Finazzi-Agro EParkinson's Disease Subcomittee, The Neurourology Promotion Committee in The International Continence Society, et al. A guideline for the management of bladder dysfunction in Parkinson's disease and other gait disorders. Neurourol Urodyn 2016;35:551-63. [Crossref] [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. [Crossref] [PubMed]
- Jia C, Cui X, Yoshimura N, et al. Assessment and Management of Urinary Dysfunction in 187 Patients with Parkinson's Disease. J Parkinsons Dis 2020;10:993-1001. [Crossref] [PubMed]
- Chuang FC, Hsiao SM, Kuo HC. The Overactive Bladder Symptom Score, International Prostate Symptom Score-Storage Subscore, and Urgency Severity Score in Patients With Overactive Bladder and Hypersensitive Bladder: Which Scoring System is Best? Int Neurourol J 2018;22:99-106. [Crossref] [PubMed]
- Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol 2005;7:S14-21. [PubMed]
- Rodrigues P, Hering F, Dias EC. Female obstruction after incontinence surgery may present different urodynamic patterns. Int Urogynecol J 2013;24:331-6. [Crossref] [PubMed]
- Liu Z, Uchiyama T, Sakakibara R, et al. Underactive and overactive bladders are related to motor function and quality of life in Parkinson's disease. Int Urol Nephrol 2015;47:751-7. [Crossref] [PubMed]
- Sakakibara R, Tateno F, Nagao T, et al. Bladder function of patients with Parkinson's disease. Int J Urol 2014;21:638-46. [Crossref] [PubMed]
- Yeo L, Singh R, Gundeti M, et al. Urinary tract dysfunction in Parkinson's disease: a review. Int Urol Nephrol 2012;44:415-24. [Crossref] [PubMed]
- Chapple CR, Artibani W, Cardozo LD, et al. The role of urinary urgency and its measurement in the overactive bladder symptom syndrome: current concepts and future prospects. BJU Int 2005;95:335-40. [Crossref] [PubMed]
- Temml C, Haidinger G, Schmidbauer J, et al. Urinary incontinence in both sexes: prevalence rates and impact on quality of life and sexual life. Neurourol Urodyn 2000;19:259-71. [Crossref] [PubMed]
- Shim JS, Kim JH, Choi H, et al. Diagnostic Tool for Assessing Overactive Bladder Symptoms: Could the International Prostate Symptom Storage Subscore Replace the Overactive Bladder Symptom Score? Int Neurourol J 2016;20:209-13. [Crossref] [PubMed]
- Homma Y, Kakizaki H, Yamaguchi O, et al. Assessment of overactive bladder symptoms: comparison of 3-day bladder diary and the overactive bladder symptoms score. Urology 2011;77:60-4. [Crossref] [PubMed]
- Sripad AA, Raker CA, Sung VW. Overactive bladder medication: Anticholinergics versus mirabegron by specialty. Urologia 2022;89:511-6. [Crossref] [PubMed]
- Hoffman DS, Nitti VW. Female Bladder Outlet Obstruction. Curr Urol Rep 2016;17:31. [Crossref] [PubMed]
- Moussa M, Chakra MA, Dabboucy B, et al. The safety and effectiveness of mirabegron in Parkinson's disease patients with overactive bladder: a randomized controlled trial. Scand J Urol 2022;56:66-72. [Crossref] [PubMed]
- Cho SY, Jeong SJ, Lee S, et al. Mirabegron for treatment of overactive bladder symptoms in patients with Parkinson's disease: A double-blind, randomized placebo-controlled trial (Parkinson's Disease Overactive bladder Mirabegron, PaDoMi Study). Neurourol Urodyn 2021;40:286-94. [Crossref] [PubMed]
- Martin S, Zillioux J, Goldman HB. Is sacral neuromodulation effective in patients with Parkinson's disease? A retrospective review. Neurourol Urodyn. 2022;41:955-61. [Crossref] [PubMed]


