
Treatment of male stress urinary incontinence at time of inflatable penile prosthesis placement a review of contemporary literature
Introduction
Male stress urinary incontinence (SUI) and erectile dysfunction (ED) are debilitating conditions that can greatly reduce the quality of life of elderly men. SUI occurs most often in men who have undergone prostate treatment and have suffered damage to both the internal and external urethral sphincters. Such damage can occur after surgical intervention or radiation therapy for prostate cancer, or after surgical treatment of benign prostatic hyperplasia (BPH) (1). Despite recent advances in robotic radical prostatectomy and continuous refinement in technique, about 8–21% of patients suffer from SUI after prostatectomy at 1 year post operatively (2). This morbidity can be significantly higher in patients who undergo salvage prostatectomy after radiation therapy (42–70%) or trans-urethral resection of the prostate in men who have previously received brachytherapy (25%) (3-5). Similarly, potency can be negatively affected by prostate treatment. In the general population, ED has been reported to be affecting about 20% of men of any age above 20 years. However, in men above 75 years old, ED can be as high as 77.5% (6). After radical prostatectomy, about 85% of patients suffer from some degree of ED (7). Radiation therapy usually exerts a negative effect on erectile function in a progressive manner over an extended time period due to the progressive obliterative endarteritis affecting both nerves and vasculature of the penis. About 70% of men in one study who underwent stereotactic body radiotherapy or external beam radiotherapy reported ED at 2 years follow up. Similar observations have been reported with brachytherapy. The percentage of ED was noted to be progressively higher with longer follow up at 5 years (8).
Surgical management of ED with inflatable penile prostheses (IPP) has been increasing since it was first introduced in 1973, with the vast majority (85%) being placed in the United States (9). With increasing numbers of patients suffering from both SUI and ED following prostate treatment, a significant portion of those patients will desire to undergo treatment of both issues simultaneously. Taken individually, well defined treatment algorithms exist with which many surgeons are comfortable; however, treatment of both in a single setting or staged fashion introduces complexity. The current literature review seeks to summarize the current treatment options for SUI as they relate to a combined surgical approach at time of IPP insertion, and a general summary is provided in Table 1. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-137/rc).
Table 1
| Treatments | Mild Incontinence | Moderate incontinence | Severe incontinence | Post-radiation | Safe at time of IPP | Safe to stage after IPP | Improve climacturia |
|---|---|---|---|---|---|---|---|
| PFPT | Yes | Yes | Yes | Less effective | NA | NA | Yes |
| External clamp/tension loops/catheters | Yes | Yes | Yes | Yes*—pressure injury and infections may be increased | NA | No—pressure injury and infections may be increased | Debatable, perhaps small improvement with tension loop |
| Urethral bulking agent | Consider only if unwilling/unable to undergo alternatives | No | No | Unclear but likely ineffective | Unclear, avoid after placing IPP | No | Unclear, poor overall efficacy |
| Male sling | Yes | Yes | No | No | Yes | Yes | Yes |
| Mini-Jupette | Yes | Yes** | Yes** | Yes | Yes | No | Yes, very good |
| Adjustable periurethral balloon | Yes | Yes | No | Unclear, known risk of decreased efficacy | Yes, avoid after placing IPP | No | Unclear |
| Artificial urinary sphincter | Yes | Yes | Yes | Yes | Yes | Yes | Yes, very good |
*, conditional—may increase complexity and/or complications; **, anecdotal—may improve but is not a primary indication for the procedure at this time. SUI, stress urinary incontinence; IPP, inflatable penile prosthesis; PFPT, pelvic floor physical therapy; NA, not applicable.
Methods
Using PubMed, a thorough literature review was performed to identify studies addressing contemporary treatment of SUI at time of IPP placement (Table 2). There were no strict inclusion or exclusion criteria used during literature review. English language research studies published prior to February 2023 were considered eligible for review and inclusion. Our attention was focused on the five major modalities used for treatment of male SUI: conservative management (specifically PFMT), urethral bulking agents, adjustable periurethral balloon, urethra slings/“mini” slings, and artificial urethral sphincters. We included landmark papers with an emphasis on more recent studies. In addition, anecdotal experience was added from high volume, subspecialty trained Men’s Health and Reconstructive Urologists.
Table 2
| Items | Specification |
|---|---|
| Date of search | February 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | Stress Urinary Incontinence and Penile Prosthesis, SUI, IPP, AUS, ProAct, urethral bulking agent, Male Urethral Sling, Mini-Jupette |
| Timeframe | Any studies published prior to the date of search |
| Inclusion criteria | Inclusion was limited to English language articles including meta-analyses, prospective, and retrospective clinical studies related to treatment of SUI at time of IPP |
| Selection process | Selection was performed independently by the primary/corresponding author and was reviewed by all authors. Source information was only included with unanimous consensus among the authors after independent review |
SUI, stress urinary incontinence; IPP, inflatable penile prosthesis; AUS, artificial urinary sphincter.
Patient evaluation
Thorough assessment including detailed focused history and physical examination are crucial at the initial patient evaluation. One of the main objectives during initial and follow up evaluation is to assess the severity and the degree of bother due to SUI. Often these are patients with whom multiple visits are required for workup, counseling, and working through a treatment plan. Identifying the type of incontinence and confirming that the patient is having SUI rather than urgency incontinence is very important before proceeding with any intervention, particularly in the post radiation setting. Urinary leakage with activities that increase intraabdominal pressure, such as physical exertion, laughing, coughing, etc., is suggestive of SUI and will most often be in the setting of prior prostate surgery or radiation. In patients with mixed symptoms, identifying the degree of bother of each component can help direct the treatment plan. Urgency and urge incontinence should be thoroughly worked up and treated if present. Detailed history of prostate treatment(s) should be obtained, including radiation therapy, which will guide options and counseling. Information regarding the time since treatment, the evolution of incontinence since treatment, the severity of current symptoms and the degree of bother should be collected.
Physical examination should be performed during initial evaluation with emphasis on redemonstration of the incontinence and evaluation of genital skin and scars of previous surgeries (1). The 24 hours pad weight test can be used to evaluate the severity of SUI. However, standing cough test such as the Male Stress Incontinence Grading Scale (MSIGS) was found to be more accurate in stratifying incontinence severity and is easier and more convenient to perform (10,11). Although not routinely indicated, urodynamic study can be useful in some situations such as patients with mixed incontinence. Exclusion of any anatomical abnormalities is necessary prior to any surgical interventions by performing cystourethroscopy to identify any post-prostatectomy urethrovesical anastomotic stricture, post-BPH treatment bladder neck contracture, or post-radiation urethral stricture or stenosis. Presence of any of any of these conditions will warrant adequate treatment with confirmation of durability of the treatment at an adequate follow up interval to diminish recurrence of obstruction (1). Extensive patient counseling regarding treatment options, expected outcomes and possible complications is essential to reach a shared decision that aligns with patient’s priorities.
Management
Pelvic floor muscle training (PFMT) and conservative measures
PFMT has been implemented in patient care both before and after radical prostatectomy to promote recovery of continence in the immediate postoperative period. Although PFMT was found to enhance continence recovery by decreasing time to continence after prostatectomy, it was found that patients who did not receive PFMT achieved delayed but similar continence rates and there was no benefit regarding overall continence rate at 12 months (12,13). New studies are suggesting different approaches to PFMT in male SUI than the ones used for female SUI, giving the different continence mechanisms. The more contemporary proposed protocols focus on strengthening the striated external urethral sphincter and maintaining tonic activation of bulbocavernosus and puborectalis muscles. They also include reeducation and retraining of those muscle groups to increase activity in response to predictable and unpredictable increase in intraabdominal pressure (14). While PFMT has an overall limited role in treatment of male SUI outside of early postoperative continence rehabilitation, it can be offered to our cohort of patients with mild SUI who are not willing to undergo any of the more invasive surgical intervention in conjunction with IPP. This can be offered in addition to other conservative measures such as penile clamp or external urinary catheter. This shared decision must be made after extensive counselling and understanding patient’s priorities. The patient must understand the limited efficacy of the mentioned conservative measures and the role of each of these measures. Penile compression clamp or occlusion band can be used intermittently for 2–3 hours periods to provide social continence and to provide comfort and confidence during physical activities. These devices are not without complications, as it may lead to edema, urethra pain, or urethral erosion (15). Although the relative risk is not widely reported, these complications may be of a higher risk when using a compressive device or external catheter with penile prosthesis in place. Urethral complications and risk of prosthesis erosion/infection are of particular concern, and patients should largely be counseled to avoid these options unless unwilling or unable to undergo more durable treatment.
Urethral bulking agents
Interestingly, one of the more widely used surgical interventions for the treatment of post-prostatectomy incontinence is urethral bulking agents. This technique was originally described in women suffering from stress incontinence, where various materials were endoscopically injected into the urethra in a circumferential matter to improve coaptation (16). Materials used today for men with post-prostatectomy incontinence include collagen, carbon coated zirconium beads, and silicone (1). Compared with other surgical therapies for post-prostatectomy urinary incontinence, urethral bulking agents provide the lowest cure rate. According to a systematic review by Choinière et al., the cure rate was found to be around 26% (17). Despite the known low efficacy, bulking agents are still one of the most used incontinence therapies utilized by urologists (18). This is likely attributed to the minimally invasive nature and low complication risk of the procedure (19).
Urethral bulking injections are relatively safe when performed by skilled hands in the setting of existing penile prosthesis, but extra care must be taken to avoid damaging the prosthesis with a misplaced injection needle (20). In addition, the use of indwelling foley catheter for greater than 48 hours must be avoided in the event of urinary retention, as this may result in a significant increase in implant infection and urethral erosion (21,22). Overall, the very low efficacy and potentially higher complication profile make urethral bulking agents a poor option for SUI treatment at time of IPP. If attempted, the injection should precede cylinder implantation and catheter dwell times should be limited as much as possible.
Adjustable periurethral balloon
Another therapy available for men experiencing SUI is an adjustable periurethral balloon, also known as the ProACT™ Adjustable Continence Therapy device (UroMedica, Plymouth, MN, USA). ProACT™ was Food and Drug Administration (FDA) approved in 2015 for treatment of SUI due to intrinsic sphincter deficiency (ISD) after prostatectomy failing conservative measures. Two ProACT™ devices are placed on either side of the urethra at the level of the bladder neck, with the injection ports placed in the subcutaneous tissue of the posterior scrotum. Through these palpable ports, a hypodermic needle is introduced directly through to skin to allow for sequential balloon volume adjustment, with maximum volume of 8 cc per balloon, often requiring 2–4 outpatient volume adjustments to achieve desired continence. One of the earlier prospective studies was conducted by Rouprêt et al., where 128 men suffering from urinary incontinence after prostate treatment underwent ProACT™ implantation (23). After a median follow-up of 56.3 months, they found that the mean number of incontinence pads had decreased to 1.46 from 4.2 at baseline. In addition, they found that those patients treated with radiotherapy had a lower success rate and higher rate of urethral erosion. Several updated studies and systematic reviews have replicated these results (17,24,25). Based on the available data, the American Urological Association (AUA) guidelines state that men with mild incontinence after prostate treatment can be offered adjustable balloon device; however, artificial urinary sphincter (AUS) is preferred in patients who underwent radiotherapy (1).
Yiou et al. evaluated the feasibility of combined ProACT™ and penile prosthesis implantation for treatment of post-radical prostatectomy ED and SUI (26). After mean follow-up of 22.7 months, they found that patients had significant improvement in urinary incontinence symptoms as well as decrease in mean pad use (2.8 to 0.3; P<0.001). With regards to penile prosthesis, patients were satisfied (n=4) or very satisfied (n=6) with their implant. As the ProACT™ device is implanted via transperineal approach, patients with 3-piece IPP may not be ideal candidates given significant risk of injury to corporal cylinders (20). While the dual implantation has been described, it should be noted that the adjustable balloon device requires passage of a metal trocar through the perineum; therefore, the ProACT™ should be implanted before the IPP as a clinical principle. Additionally, in-office adjustments of volume of the ProACT™ device would require special care in experienced hands to ensure no needlestick injury to the components of the IPP.
Male sling/mini-sling
Male suburethral sling is an alternative surgical option for treatment of mild to moderate SUI. It is recommended to avoid sling procedures in severe incontinence and in patients with history of pelvic radiation due to increased complications and decreased efficacy (1). There are two trans-obturator slings available in the US market for male SUI treatment: AdVance XP (Boston Scientific, Marlborough, MA, USA) and Virtue slings (Coloplast, North Minneapolis, MN, USA). The AdVance sling is a two-arm polypropylene mesh sling that was initially introduced in 2007 and the second-generation AdVance XP was introduced in 2010. The mechanism of action of the AdVance sling is by proximally relocating the bulbar urethra, leading to lengthening of the membranous urethra (27,28). Long-term success rate of AdVance sling was reported to be 62% and 64% for mild and moderate SUI (28).
The second available sling is the Virtue male sling, has four arms, two trans-obturator arms and two prepubic arms. This sling provides proximal relocation of the bulbar urethra like AdVance sling, and the two prepubic arms provide external compression to the bulbar urethra. Comiter et al. reported subjective success rate of 70% and objective success rate of 79% (29). However, other studies reported high failure rate with 68% failing to reduce the pad usage (30). A recent study with a 15-month follow-up period, reported objective success rate of 78% and subjective success rate of 85% in a cohort of 56 men (31). Rhee described the concomitant implantation of penile prosthesis and male sling in four patients with SUI and ED after radical prostatectomy. He reported no complications, and a 100% satisfaction rate with continence and sexual function (32). Another study by Gorbatiy et al. showed decrease pad usage to 1 pad per day (range, 0–2) in eight men who underwent dual implantation of IPP and sling (33). Technical considerations include the avoidance of components of the IPP during dual implantation particularly during passage of the prepubic components of the sling. Some experienced implanters advocate upfront IPP placement, then leaving the penoscrotal or infrapubic incision open to avoid tubing during tunneling of the prepubic arms of the Virtue sling; however initial placement of the sling followed by IPP has also been described. The transobturator arms of both AdVance and Virtue are typically well away from the components of the IPP. Overall, the dual implantation of AdVance/Virtue and IPP is a technically challenging endeavor that should likely be limited to high volume centers; however, it has been shown to be safe and effective.
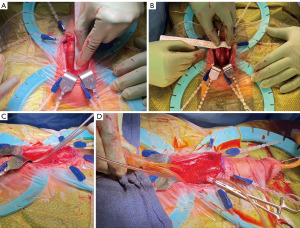
The “Andrianne Mini-Jupette” is a contemporary mini-sling that was developed by Robert Andrianne in 2005, and Yafi et al. (34) described the technique for the procedure and reported the initial outcomes in a cohort of 38 patients. It was described primarily for treatment of climacturia and/or mild SUI with no more than 2 pads used per day. In this procedure, a graft material, usually either macropore monofilament mesh (i.e., Restorelle, Coloplast, North Minneapolis, MN, USA) or biologic auto- or allograft is used as a mini sling and sutured to the medial edges of the corpectomies during the implantation of IPP. The Mini-Jupette applies some tension and compression of the urethra when the IPP cylinders are inflated which prevents climacturia and can improve mild urinary incontinence.
The main technical considerations are (Figure 1):
- Corporotomies should be extended to at least 3 cm in length to allow for sufficient sling surface area to have the desired effect.
- The mesh should be sutured to medial corporotomies in a running fashion with enough “slack” to allow passage of a right angle or Metzenbaum so as not to overtighten. Keep in mind that IPP placement will stretch the sling to a variable degree and placing too much tension initially can result in retention or having to take down the whole sling and redo it.
- IPP is then placed and surrogate reservoir test performed to confirm compression of the urethra at inflation. If the sling does not compress the urethra, then additional plicating sutures can fix the sling further.
- Corporotomies should be closed in a running fashion as a stay suture closure will bunch the sling causing malfunction.
- The IPP is not left partially inflated as this can lead to urinary retention.
- Early cycling is avoided to allow for incorporation of the mesh prior to activation.
Initial studies reported improvement from 1.6 pad per day to 0.3 pad per day, with 89% objective improvement. Complete resolution of SUI was reported in 75% of the patients with 0 pad used. Climacturia was also improved with 78% reporting improvement and 67% compete resolution. Complications were reported in 13% of the patients, including one urethra-corporal fistula which was likely related to graft suturing (34). Valenzuela et al. reported their preliminary outcome of a modified mini-sling combined with IPP in 36 patients. In their technique, they used the Virtue sling instead of a graft. Climacturia was resolved in 93% of patients and SUI was improved in 85%. They reported one case of urethral erosion that required urethral repair and explanation of the mesh (35). Overall, the Mini-Jupette has been gaining favor as a reliable, efficient, and effective solution for the treatment of climacturia and mild SUI at time of IPP insertion. Patient satisfaction has been excellent and it is a relatively easy to reproduce adjunct to a standard penoscrotal IPP.
AUS
AUS insertion is considered the gold standard treatment of male SUI after prostate treatment (1). A multi-component AUS was originally introduced in 1973 by Scott et al. (36). The cuff was then modified to be antibiotic coated in 1983 and to be designed with a narrow back in 1987, and the design has been largely stable since then (37). The device has three components, a cuff that is typically placed around the bulbar urethra, a control pump that is placed in the scrotum and pressure-regulating balloon (PRB) that is placed in the retropubic space (38). AUS was extensively studied in the last 4 decades and was found to have durable long-term results with a continence rate of about of 80–90% on long-term follow up and reported patient satisfaction up to 90% (39-41). In addition, AUS has been found to be more cost-effective when compared with male urethral slings (42). It is considered the preferred surgical treatment option for men with moderate to severe SUI and in men with history of pelvic radiation therapy suffering from any degree of SUI (1).
Dual implantation of AUS and IPP has been an established practice since it was first reported by Parulkar and Barrett in 1989. In the original report by Parulkar and Barrett, they used a two-incision approach for simultaneous implantation in 65 patients. They employed an infrapubic incision for the IPP components and the AUS pump and PRB, and a midline perineal incision for the AUS cuff (43). Since then, two approaches were reported, including single-stage or synchronous implantation in a single operation, and staged or asynchronous approach. Although multiple studies proved the feasibility of synchronous, there has been variable data regarding risk of infection and complications rate (43-50). Segal et al. retrospectively compared synchronous vs. asynchronous dual implantation and showed significant increase of operative time with synchronous implantation, but no difference between the two groups regarding infection, erosion, or device malfunction (47). The other studies reported results of retrospective evaluation of synchronous dual implantation in limited number of patients without a control group. The overall complication rate in those studies ranged between 6% to 30%. Those complications included urethral erosion, distal corporal extrusion, reservoir migration, device infection and UTI. The need for revision or removal of one or both implants was ranging from 6% to 41%. Functional outcome regarding continence was reported to be excellent with average of pad usage to be 1 or less pad per day (43-46,48,49). Sweigert et al. performed a retrospective analysis of claims databases from the two states of California and Florida to identify men who underwent implantation of IPP or AUS. They compared synchronous dual implantation to implantation of IPP or AUS alone and found that synchronous dual implantation had a higher risk of 90 days readmission. However, there were no differences in major complications. There was no similar data regarding asynchronous dual implantation (50). Based on a systematic review by Pyrgidis et al., the rate of reoperation rate was double for patient’s undergoing synchronous placement of IPP with AUS when compared with placement of only IPP or AUS alone (51). Despite the increased complexity and risk for reoperation, there are benefits to synchronous placement. They reported higher satisfaction rates with significant improvement of both ED and SUI (51). It is also well known that treatment of ED can be expensive, so the cost-effectiveness of treatments needs to be considered (52). In a single center study by Sellers et al., the authors found that concurrent placement of IPP with AUS decreased operative time and was associated with approximately $7,000 in cost savings when compared with asynchronous placement (53).
Additionally, various approaches were reported regarding the incision used during synchronous dual impanation (41). Two incisions approach was reported in the original series by Parulkar and Barrett as mentioned above (43). In 2006, Kendirci et al. published their technique of synchronous dual prosthetic implantation via a single transverse scrotal incision. In this approach, all the components of both devices are placed via the scrotal incision with adjustment of the retractor position towards the perinium for urethral dissection and cuff placement, and cephalad for the reservoir and PRB placement. In their series of 22 patient via single incision approach, no postoperative infection was reported (44).
Giving the complexity of operating both devices, careful patients’ selection should be considered to ensure intact cognitive function and manual dexterity (41). Patients should also be counseled regarding the likelihood of needing revision or replacement of the device in the future. Patel et al. found that patients who underwent dual implantation had a higher likelihood of undergoing IPP reoperation at 1 and 3 years when compared to patients who received IPP only. However, there was no difference when compared to patients who received AUS alone (54). Overall, dual implantation of IPP and AUS has been shown to be safe and effective. Whether performed synchronously or in a staged fashion, dual implantation is regarded as the gold standard for severe SUI and severe ED or in patients with SUI associated with radiation and severe ED.
Conclusions
Concomitant treatment of SUI at the time of IPP insertion is safe and effective; however, careful attention to patient selection, counseling, managing expectations, and technical execution are essential to ensure good objective outcomes and patient satisfaction. For mild to moderate incontinence with or without bothersome climacturia, male sling or mini-slings are safe and effective options at time of IPP insertion, particularly in a non-radiated setting. Data regarding periurethral balloon implants is not yet mature in this cohort despite some high-volume centers reporting success. For bothersome post-radiation or severe SUI, the dual AUS/IPP implantation is an excellent option and can be safely and effectively employed in either staged or synchronous fashion.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Gross, Jay Simhan, and David Barham) for the series “Complex Penile Prosthesis Surgery” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-137/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-137/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-137/coif). The series “Complex Penile Prosthesis Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol 2019;202:369-78. [Crossref] [PubMed]
- Kang SG, Shim JS, Onol F, et al. Lessons learned from 12,000 robotic radical prostatectomies: Is the journey as important as the outcome? Investig Clin Urol 2020;61:1-10. [Crossref] [PubMed]
- Ogaya-Pinies G, Linares-Espinos E, Hernandez-Cardona E, et al. Salvage robotic-assisted radical prostatectomy: oncologic and functional outcomes from two high-volume institutions. World J Urol 2019;37:1499-505. [Crossref] [PubMed]
- Mock S, Leapman M, Stock RG, et al. Risk of urinary incontinence following post-brachytherapy transurethral resection of the prostate and correlation with clinical and treatment parameters. J Urol 2013;190:1805-10. [Crossref] [PubMed]
- Polland A, Vertosick EA, Sjoberg DD, et al. Preoperative symptoms predict continence after post-radiation transurethral resection of prostate. Can J Urol 2017;24:8903-9. [PubMed]
- Saigal CS, Wessells H, Pace J, et al. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207-12. [Crossref] [PubMed]
- Emanu JC, Avildsen IK, Nelson CJ. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr Opin Support Palliat Care 2016;10:102-7. [Crossref] [PubMed]
- Dess RT, Hartman HE, Aghdam N, et al. Erectile function after stereotactic body radiotherapy for localized prostate cancer. BJU Int 2018;121:61-8. [Crossref] [PubMed]
- Baas W, O'Connor B, Welliver C, et al. Worldwide trends in penile implantation surgery: data from over 63,000 implants. Transl Androl Urol 2020;9:31-7. [Crossref] [PubMed]
- Henderson JW, Kane SM, Mangel JM, et al. A Randomized Comparative Study Evaluating Various Cough Stress Tests and 24-Hour Pad Test with Urodynamics in the Diagnosis of Stress Urinary Incontinence. J Urol 2018;199:1557-64. [Crossref] [PubMed]
- Yi YA, Keith CG, Graziano CE, et al. Strong correlation between standing cough test and 24-hour pad weights in the evaluation of male stress urinary incontinence. Neurourol Urodyn 2020;39:319-23. [Crossref] [PubMed]
- Anderson CA, Omar MI, Campbell SE, et al. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev 2015;1:CD001843. [PubMed]
- Fernández RA, García-Hermoso A, Solera-Martínez M, et al. Improvement of continence rate with pelvic floor muscle training post-prostatectomy: a meta-analysis of randomized controlled trials. Urol Int 2015;94:125-32. [Crossref] [PubMed]
- Hodges PW, Stafford RE, Hall L, et al. Reconsideration of pelvic floor muscle training to prevent and treat incontinence after radical prostatectomy. Urol Oncol 2020;38:354-71. [Crossref] [PubMed]
- Moore KN, Schieman S, Ackerman T, et al. Assessing comfort, safety, and patient satisfaction with three commonly used penile compression devices. Urology 2004;63:150-4. [Crossref] [PubMed]
- Elsergany R, Elgamasy AN, Ghoniem GM. Transurethral collagen injection for female stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:13-8. [Crossref] [PubMed]
- Choinière R, Violette PD, Morin M, et al. Evaluation of Benefits and Harms of Surgical Treatments for Post-radical Prostatectomy Urinary Incontinence: A Systematic Review and Meta-analysis. Eur Urol Focus 2022;8:1042-52. [Crossref] [PubMed]
- Poon SA, Silberstein JL, Savage C, et al. Surgical practice patterns for male urinary incontinence: analysis of case logs from certifying American urologists. J Urol 2012;188:205-10. [Crossref] [PubMed]
- Nguyen L, Leung LY, Walker R, et al. The use of urethral bulking injections in post-prostatectomy stress urinary incontinence: A narrative review of the literature. Neurourol Urodyn 2019;38:2060-9. [Crossref] [PubMed]
- Ajay D, Mendez MH, Wang R, et al. Treatment of Urinary Incontinence in Patients With Erectile Dysfunction. Sex Med Rev 2021;9:593-604. [Crossref] [PubMed]
- Vaidyanathan S, Soni B, Khadr R, et al. Erosion of urethra by malleable penile prosthesis in a spinal cord injury patient with diabetes mellitus and repeated misplacement of Foley balloon in the urethra: lessons we learn: a case report. Spinal Cord Ser Cases 2022;8:12. [Crossref] [PubMed]
- Hisasue S, Sato Y, Horita H, et al. Erosion of a penile prosthesis due to an indwelling urethral catheter as a late complication. Int J Urol 2002;9:525-7. [Crossref] [PubMed]
- Rouprêt M, Misraï V, Gosseine PN, et al. Management of stress urinary incontinence following prostate surgery with minimally invasive adjustable continence balloon implants: functional results from a single center prospective study. J Urol 2011;186:198-203. [Crossref] [PubMed]
- Larson T, Jhaveri H, Yeung LL. Adjustable continence therapy (ProACT) for the treatment of male stress urinary incontinence: A systematic review and meta-analysis. Neurourol Urodyn 2019;38:2051-9. [Crossref] [PubMed]
- Ricard H, Léon G, Branchereau J, et al. Adjustable continence balloons in postprostatectomy incontinence: Outcomes and complications. Neurourol Urodyn 2022;41:1414-22. [Crossref] [PubMed]
- Yiou R, Binhas M. Combined Implantation of a Penile Prosthesis and Adjustable Continence Therapy ProACT in Patients with Erectile Dysfunction and Urinary Incontinence after Radical Prostatectomy: Results of a Prospective Pilot Study. J Sex Med 2015;12:2481-4. [Crossref] [PubMed]
- Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol 2007;52:860-6. [Crossref] [PubMed]
- Rizvi IG, Ravindra P, Pipe M, et al. The AdVance™ male sling: does it stand the test of time? Scand J Urol 2021;55:155-60. [Crossref] [PubMed]
- Comiter CV, Rhee EY, Tu LM, et al. The virtue sling--a new quadratic sling for postprostatectomy incontinence--results of a multinational clinical trial. Urology 2014;84:433-8. [Crossref] [PubMed]
- McCall AN, Rivera ME, Elliott DS. Long-term Follow-up of the Virtue Quadratic Male Sling. Urology 2016;93:213-6. [Crossref] [PubMed]
- Abramowitz D, Sam AP, Pachorek M, et al. Virtue male sling outcomes and application to a contemporary nomogram. Can J Urol 2021;28:10625-30. [PubMed]
- Rhee EY. Technique for concomitant implantation of the penile prosthesis with the male sling. J Urol 2005;173:925-7. [Crossref] [PubMed]
- Gorbatiy V, Westney OL, Romero C, et al. Outcomes of simultaneous placement of an inflatable penile prosthesis and a male urethral sling through a single perineal incision. J Sex Med 2010;7:832-8. [Crossref] [PubMed]
- Yafi FA, Andrianne R, Alzweri L, et al. Andrianne Mini-Jupette Graft at the Time of Inflatable Penile Prosthesis Placement for the Management of Post-Prostatectomy Climacturia and Minimal Urinary Incontinence. J Sex Med 2018;15:789-96. [Crossref] [PubMed]
- Valenzuela RJ, Ziegelmann MJ, Hillelsohn JH, et al. Preliminary Outcomes of the Male Urethral "Mini-Sling": A Modified Approach to the Andrianne Mini-Jupette Procedure With Penile Prosthesis Placement for Climacturia and Mild Stress Urinary Incontinence. J Sex Med 2019;16:1310-7. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology 1973;1:252-9. [Crossref] [PubMed]
- Light JK, Reynolds JC. Impact of the new cuff design on reliability of the AS800 artificial urinary sphincter. J Urol 1992;147:609-11. [Crossref] [PubMed]
- Wilson S, Delk J 2nd, Henry GD, et al. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol 2003;169:261-4. [Crossref] [PubMed]
- Sacomani CAR, Zequi SC, Costa WHD, et al. Long-term results of the implantation of the AMS 800 artificial sphincter for post-prostatectomy incontinence: a single-center experience. Int Braz J Urol 2018;44:114-20. [Crossref] [PubMed]
- Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol 2000;164:702-6; discussion 706-7. [Crossref] [PubMed]
- Yafi FA, Powers MK, Zurawin J, et al. Contemporary Review of Artificial Urinary Sphincters for Male Stress Urinary Incontinence. Sex Med Rev 2016;4:157-66. [Crossref] [PubMed]
- Shamout S, Nazha S, Dragomir A, et al. A cost-utility analysis of artificial urinary sphincter versus AdVance male sling in post prostatectomy stress urinary incontinence: A publicly funded health care perspective. Neurourol Urodyn 2018;37:2195-203. [Crossref] [PubMed]
- Parulkar BG, Barrett DM. Combined implantation of artificial sphincter and penile prosthesis. J Urol 1989;142:732-5. [Crossref] [PubMed]
- Kendirci M, Gupta S, Shaw K, et al. Synchronous prosthetic implantation through a transscrotal incision: an outcome analysis. J Urol 2006;175:2218-22. [Crossref] [PubMed]
- Mancini JG, Kizer WS, Jones LA, et al. Patient satisfaction after dual implantation of inflatable penile and artificial urinary sphincter prostheses. Urology 2008;71:893-6. [Crossref] [PubMed]
- Rolle L, Ceruti C, Sedigh O, et al. Surgical implantation of artificial urinary device and penile prosthesis through trans-scrotal incision for postprostatectomy urinary incontinence and erectile dysfunction: synchronous or delayed procedure? Urology 2012;80:1046-50. [Crossref] [PubMed]
- Segal RL, Cabrini MR, Harris ED, et al. Combined inflatable penile prosthesis-artificial urinary sphincter implantation: no increased risk of adverse events compared to single or staged device implantation. J Urol 2013;190:2183-8. [Crossref] [PubMed]
- Martínez-Salamanca JI, Espinós EL, Moncada I, et al. Management of end-stage erectile dysfunction and stress urinary incontinence after radical prostatectomy by simultaneous dual implantation using a single trans-scrotal incision: surgical technique and outcomes. Asian J Androl 2015;17:792-6. [Crossref] [PubMed]
- Bolat D, Kozacioglu Z, Polat S, et al. Synchronous penoscrotal implantation of penile prosthesis and artificial urinary sphincter after radical prostatectomy. Arch Esp Urol 2017;70:367-72. [PubMed]
- Sweigert SE, Chuang E, Patel PM, et al. Synchronous Artificial Urinary Sphincter and Inflatable Penile Prosthesis Implantation: Short-Term Outcomes from a Statewide Claims Database. Urol Pract 2021;8:565-70. [Crossref] [PubMed]
- Pyrgidis N, Barham DW, Hammad M, et al. Synchronous Surgical Management of Erectile Dysfunction and Stress Urinary Incontinence: A Systematic Review and Meta-Analysis of Reoperation Rates. Sex Med Rev 2022;10:782-90. [Crossref]
- Rezaee ME, Ward CE, Brandes ER, et al. A Review of Economic Evaluations of Erectile Dysfunction Therapies. Sex Med Rev 2020;8:497-503. [Crossref] [PubMed]
- Sellers CL, Morey AF, Jones LA. Cost and time benefits of dual implantation of inflatable penile and artificial urinary sphincter prosthetics by single incision. Urology 2005;65:852-3. [Crossref] [PubMed]
- Patel N, Golan R, Halpern JA, et al. A Contemporary Analysis of Dual Inflatable Penile Prosthesis and Artificial Urinary Sphincter Outcomes. J Urol 2019;201:141-6. [Crossref] [PubMed]


