
Complicated artificial urinary sphincter insertion using the transcorporal cuff: keys to the tunical flap technique
Highlight box
Surgical highlights
• The tunical flap technique reinforces the urethra circumferentially using tunica albuginea from the corpora cavernosa.
What is conventional and what is novel/modified?
• This method provides extra urethral support, preventing cuff erosion, and preserves corporal volume.
What is the implication, and what should change now?
• Reliable haemostatic closure of the tunica albuginea after utilising the adjacent corporal tissue to place a transcorporal artificial urinary sphincter cuff.
Introduction
Urethral sphincter insufficiency following radical prostatectomy (RP) is a common cause of non-neurogenic stress urinary incontinence (SUI) (1). Artificial urinary sphincter (AUS) insertion remains the standard of care for fit patients with SUI refractory to non-operative interventions (2,3). Unfavourable outcomes associated with AUS implantation include erosion, mechanical malfunction, and infection which often necessitate explantation (4). Moreover, earlier revision is often necessary due to a combination of urethral atrophy and silicon fatigue (5). In a retrospective study of 158 patients who underwent AUS insertion, a significant portion of patients, 8% and 6% respectively, faced complications such as hematoma and urinary retention within the first 6–8 weeks (6). The proximal urethra is a common location for uncomplicated AUS placement, however, previous failed AUS insertion, urethroplasty or pelvic radiotherapy (RT) may compromise urethral tissue requiring technique modifications that optimise outcomes (4,7,8). In these situations, transcorporal cuff (TC) placement has been well described to facilitate continence restoration in men where there is no other feasible option other than urinary diversion or permanent incontinence (9). In the traditional TC approach, the procedure may be complicated by haematoma due to difficulty in completely closing the corporal defects behind the urethra (Figure 1). Although large-scale comprehensive studies specifically assessing hematoma formation rates post-AUS insertion are lacking, various reports suggest an incidence rate of approximately 2–3% (10,11).
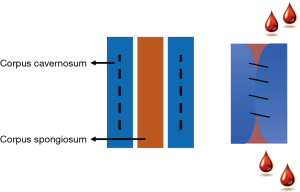
The indications for the TC modification are failed AUS placements secondary to urethral erosion particularly in the setting of a compromised urethra where the proximal urethra is no longer available for use. Common causes of a compromised urethra include urethroplasty or pelvic RT which require procedural alterations.
Multiple studies have assessed various technique modifications to improve short- and long-term outcomes. In a retrospective review assessing TC and standard AUS in patients with a fragile urethra, TC cuff placement was associated with lower revision and erosion rates as compared to the standard AUS insertion (12). Interestingly, there was no significant difference in functional outcomes such as continence patient satisfaction or the 90-day complication rate (12).
The gullwing modification utilizes bilateral raised bracket-shaped flaps on the ventral aspect of the tunica albuginea which are closed over the ventral urethra (9). Additionally, a similarly sized allograft segment is used to cover the tunical defect of the ventral corpora cavernosa. Functional outcomes secondary to closure of the tunica albuginea in the TC technique were also assessed in 39 patients. The study showed that pad usage was 1 per day while only one patient suffered from a postoperative hematoma while postoperative erectile rigidity was maintained (11).
In this narrated video, the tunical flap (TF) modification is demonstrated for the TC technique for AUS implantation. This is performed via a perineal and penoscrotal approach (Figure 2). The patients had previously failed AUS placements secondary to urethral erosion.
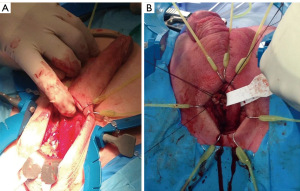
In reference to the penoscrotal approach, it is acknowledged that this is not a conventional method. Nonetheless, there are instances where the perineal region and anatomy present significant challenges, such as a history of multiple prior perineal continence procedures, complications from previous erosions, and the presence of perineal urinary fistulas. In these circumstances, opting for surgery through fresh tissue using a penoscrotal approach may prove to be a beneficial alternative.
The two patients featured in Video 1 underwent a RP 15–20 years earlier. Following the development of biochemical recurrence, both also underwent salvage RT. The patients suffered from SUI leading to AUS implantation 10–12 years ago. Initially achieving complete continence, they experienced a decline over the past 6 months, marked by moderate to severe SUI during most daily activities and a significant reduction in quality of life, necessitating the use of 4–5 heavy pads daily. We present this article in accordance with the SUPER reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-641/rc).
Preoperative preparations and requirements
The procedures were performed at Cabrini Hospital, Melbourne, Australia by Urologist Professor D.M. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from all patients for the use of information, images and accompanying video in this report. A copy of the written consent is available for review by the editorial office of this journal.
The procedure begins with the patient positioned in lithotomy and the AUS components prepared along with a Scott ring surgical retractor. The perineum is wet shaved followed by a 10-minute thorough chlorhexidine wash of the perineum and suprapubic area followed by a chlorhexidine and alcohol sterile scrub. The drapes are placed in situ with staples to exclude the anus. A 12-Fr indwelling catheter (IDC) is placed per-urethrally.
Step-by-step description (Figures 2,3)
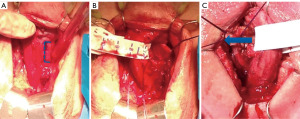
The perineal approach begins with a midline perineal incision and dissection of the subcutaneous tissues through Colle’s fascia and careful traction to create a space at the base of the corpora. A self-retaining retractor is placed, the bulbocavernosus muscle is incised, and the ventral surface of the bulbar urethra is exposed with a combination of sharp and blunt dissection. After careful dissection of the subcutaneous fascia, the existing AUS is located and subsequently removed along with all its components. In our technique, the pump is removed via the perineal incision. Identification of the appropriate site for cuff placement which includes selecting the healthiest portion of urethra separate from the previous cuff site. The corporal bodies are exposed on either side and stay sutures are placed. Two cm bilateral corporotomies are carried out between the two stay sutures on either side. On one side, horizontal incisions are made at the superior and inferior extent of the corporotomy to mobilise and raise a rectangular TF. Dissection of the area underneath the flap is performed to help mobilise it. Furthermore, dissection sharply through the midline septum between the corporal bodies; a transcorporal space is created. The raised TF is passed behind the urethra and a running 2-0 vicryl suture used to close the corpora. The rectangular shape of the flap ensures that a haemostatic closure can be achieved. This is best carried out with the measuring tape secured around the mobilized urethra and corporal tissue to prevent compromising the space for the cuff.
In the penoscrotal approach, a horizontal incision is made at the base of the penis. A space is created behind the urethra passing through the dorsal wall of the corpora cavernosa to minimise the risk of erosion. A corporal flap for advancement across the defect is created in the remaining tunica albuginea to permit tight closure.
After the AUS cuff has been placed around the reinforced urethra, the additional AUS components are inserted in the standard fashion. A 5 cm midline transverse suprapubic incision is made to facilitate balloon placement. This is performed to deliberately avoid the inguinal rings. Cystoscopic device cycling is performed to ensure appropriate device function. After haemostasis is achieved, skin incisions are closed in multiple layers using absorbable suture material. The total procedure time is approximately 2 hours.
Postoperative considerations and tasks
A compression dressing is applied to minimise scrotal and perineal haematoma formation and remains in situ for 24 hours. The IDC is left in situ for overnight urine drainage.
Tips and pearls
The TF technique for TC AUS implantation is a suitable alternative method for patients with compromised urethral tissue. Moreover, the rectangular TF shape allows for haemostatic closure which is best achieved with measuring tape around the mobilized urethra.
Discussion
Urethral sphincter insufficiency after RP commonly causes SUI. Inserting an AUS is the standard treatment for patients unresponsive to other methods with the TC placement being an alternative option for men with compromised urethral tissue (1). This manuscript and associated video (Video 1) provide a step-by-step demonstration of the TF modification of the TC technique through a perineal and penoscrotal approach. The TF modification provides circumferential urethral reinforcement with tunica albuginea from the corpora cavernosa. In cases of prior erosion, it is essential to reposition the cuff either proximally or distally as well as the incorporation of peri-urethral corporal tissue. This provides additional support over prior techniques to further prevent subsequent haematoma formation and cuff erosion. Both patients are pad-free. In our early findings, we have not observed any postoperative hematoma associated with this technique. Moreover, this technique preserves the corporal volume necessary for ease of subsequent penile prosthesis implantation.
Limitations of the study include lack of long-term follow-up data, including efficacy, durability, and long-term complications. Additionally, the findings are yet to be generalized and externally validated in diverse clinical settings. Recognizing these limitations, we are dedicated to further research and validation in future studies.
Conclusions
The TF technique for the transcorporal AUS insertion provides a method for reliable haemostatic closure of the tunica albuginea after utilising the adjacent corporal tissue to place a transcorporal AUS cuff. In this series of videos, we demonstrate this simple technique to provide additional urethral support in men with compromised urethral tissue who otherwise may not be able to undergo AUS insertion.
Acknowledgments
This work was presented at the Urological Society of Australia and New Zealand (USANZ) annual surgical meeting in February 2023.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-641/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-641/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-641/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from all patients for the use of information, images and accompanying video in this report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koch GE, Kaufman MR. Male Stress Urinary Incontinence. Urol Clin North Am 2022;49:403-18. [Crossref] [PubMed]
- Herschorn S. The artificial urinary sphincter is the treatment of choice for post-radical prostatectomy incontinence. Can Urol Assoc J 2008;2:536-9. [Crossref] [PubMed]
- Thüroff JW, Abrams P, Andersson KE, et al. EAU guidelines on urinary incontinence. Eur Urol 2011;59:387-400. [Crossref] [PubMed]
- Brant WO, Martins FE. Artificial urinary sphincter. Transl Androl Urol 2017;6:682-94. [Crossref] [PubMed]
- Bergeson RL, Yi YA, Baker RC, et al. Urethral atrophy is now a rare cause for artificial urinary sphincter revision surgery in the contemporary 3.5 cm cuff era. Transl Androl Urol 2020;9:50-5. [Crossref] [PubMed]
- Ruzhynsky VA, Hird A, Eapen RS, et al. Early Complications after Artificial Urinary Sphincter Insertion. J Urol Ren Dis 2017;2017:JURD-131.
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- Mock S, Dmochowski RR, Brown ET, et al. The Impact of Urethral Risk Factors on Transcorporeal Artificial Urinary Sphincter Erosion Rates and Device Survival. J Urol 2015;194:1692-6. [Crossref] [PubMed]
- Vasan R, Myrga J, Miller D, et al. The Gullwing Technique: A Novel Method of Transcorporal Artificial Urinary Sphincter Placement for the Fragile Urethra. Urology 2022;169:237-40. [Crossref] [PubMed]
- Zhang F, Liao L. Artificial urinary sphincter implantation: an important component of complex surgery for urinary tract reconstruction in patients with refractory urinary incontinence. BMC Urol 2018;18:3. [Crossref] [PubMed]
- Maurer V, Dahlem R, Howaldt M, et al. Transcroporal Artificial Urinary Sphincter Placement With Closure of Corporal Bodies-A Long-Term Analysis of Functional Outcomes. Front Surg 2022;9:918011. [Crossref] [PubMed]
- Redmond E, Tong S, Zemp L, et al. Improved artificial urinary sphincter outcomes using a transcorporal cuff placement in patients with a "fragile urethra". Can Urol Assoc J 2020;14:E621-4. [Crossref] [PubMed]


