
UrethroNAV: the aetiology and extent of idiopathic urethral stricture in an Australian population
Highlight box
Key findings
• This study found microscopic evidence of lichen sclerosus (LS) proximal to the stricture site in 1 out of 15 patients with idiopathic urethral stricture disease (IUSD).
• Patients had disease pathology extending both proximal and distal to the site of stricture including scarring, inflammation, LS features and ulceration.
What is known and what is new?
• LS is considered a causative factor in 10% of IUSD cases.
• Histopathological changes extend beyond the stricture and may impact urethroplasty recurrence.
• Concomitant malignant disease may be present in patients with strictures.
• Diagnostic criteria of LS are variable and consensus guidelines are required.
What is the implication, and what should change now?
• Routine biopsy proximal and distal to the stricture should be considered in IUSD to assess for LS, inflammation, and potential malignant disease.
• Findings may guide surgical approach selection between anastomotic urethroplasty versus buccal graft substitution.
• Further research on the role of LS and inflammation in IUSD recurrence is warranted.
Introduction
Urethral stricture disease (USD) is characterised as scars in the subepithelial tissue of the corpus spongiosum, which is located between the membranous urethra and the external urinary meatus (1-3). This condition leads to the narrowing of the urethral lumen, resulting in luminal constriction. Over time, the constriction worsens, eventually causing urinary obstruction and micturition irritation. Patients commonly exhibit lower urinary tract symptoms, including hesitancy, a weak urine stream, terminal dribbling, and a sensation of incomplete voiding (4). Urethral strictures significantly contribute to morbidity, as men may experience symptoms that are debilitating and embarrassing in nature.
Stricture disease is a relatively common condition, with incidence rates of up to 1/1,000 reported in the literature and a prevalence between 0.2% and 0.9% (5). A similar prevalence rate has been researched locally in two regional centres (6,7). The occurrence of stricture disease tends to increase with age, likely due to a higher likelihood of iatrogenic injury resulting from urethral instrumentation in elderly individuals. The urethra is divided into two main segments: the posterior segment, which passes through the prostate and pelvic floor musculature (i.e., membranous urethra), and the anterior segment (bulbar and penile/glandular urethra). Among these segments, the bulbar urethra is the most frequently affected by stricture disease (8).
The aetiology of stricture disease varies depending on the geographical location and the specific site of the stricture along the urethra. In developed countries, iatrogenic injuries resulting from procedures such as urethral catheterisation, cystoscopy, and transurethral resection of the prostate (TURP) are the primary causes of stricture disease. In contrast, trauma is the leading cause in developing countries (4,9). A significant portion of stricture disease cases have an unknown cause, with up to 40% of strictures classified as idiopathic USD (IUSD) (2). Traditionally, biopsies are not taken in the workup or during the surgical management of USD.
Lichen sclerosus (LS) is increasingly recognised as an underlying cause of stricture disease, particularly in young and middle-aged adults. The disease tends to spread proximally from the meatus, affecting the urethra (2). Current literature suggests a causative rate of 10%, although there is a growing belief that this figure may be significantly underestimated due to missed cases of microscopic disease (10). It is essential to consider this factor, as the treatment approach for patients with LS-induced strictures differs from that for other types of strictures. The prevalence of LS in an Australian cohort undergoing circumcision was 63.6% with 22.1% subsequently developing USD (6).
The traditional surgical treatment for USD is transecting excision and primary anastomosis (EPA) which involves excising the stricture and reconnecting the two ends of the urethra. While EPA has excellent short-term success rates exceeding 90%, it is limited to strictures under 5 cm located to the bulbar urethra and carries risks of sexual dysfunction from nerve damage and vascular compromise (11,12). Substitution urethroplasty, utilising buccal mucosal grafts (BMGs), widens the urethral diameter without transecting it and offers an alternative, especially for longer strictures and in cases of macroscopic evidence of LS (1). Inflammation from LS can extend beyond visible stricture boundaries, predisposing EPA to recurrence, thus substitution urethroplasty is recommended for confirmed or suspected LS cases. However, there is limited prospective evidence characterising the microscopic extent of inflammation, representing a knowledge gap warranting further study to optimise surgical decision-making. Overall, while EPA remains the gold standard for short bulbar strictures without LS, substitution urethroplasty is emerging as the preferred approach for larger and inflammatory strictures to improve outcomes.
The primary objective of this pilot study is to investigate the aetiology of anterior strictures that were previously considered idiopathic. Specifically, we aim to determine the underlying cause for these strictures and explore the possibility of histological evidence indicating a predisposition of the surrounding tissue to develop strictures. The secondary outcome measured will be the freedom from stricture recurrence at a 1-year follow-up period after surgical intervention. Based on our research objectives, we hypothesise that LS will be identified as the causative agent for stricture disease in more than 10% of patients who meet the inclusion criteria. Furthermore, we expect to find histological evidence of inflammation occurring away from the primary site of stricture disease. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-549/rc).
Methods
A prospective cross-sectional study was performed on patients presenting for assessment and management of USD from January 2019 at Toowoomba, a regional centre specialising in urethroplasty, located in Queensland, Australia. Patients were enrolled based on a diagnosis of IUSD without history, examination, or investigation suggestive of trauma to the perineum, obvious macroscopic inflammatory disease (including a prior diagnosis of LS) or previous endoscopic instrumentation of the urethra which may have resulted from iatrogenic injury (including insertion of an indwelling catheter). Patients who had prior endoscopic management for their idiopathic stricture were included. Patients had to be 16 years or older, received no previous treatment for USD, and medically able to undergo urethroplasty. In addition, patients must not have had a history of hypospadias, radiation therapy, or concomitant posterior urethral disease. All urethroplasties were performed by a single fellowship-trained reconstructive urologist performing 50 cases per year with a total case volume of over 500. Pre-operative retrograde urethrograms were utilised to assess the location, extent and length of the stricture. These were performed by the operating surgeon. Ethics approval for this study was made through the Darling Downs Health Human Research Ethics Committee (No. EC00182) in accordance with the National Statement on Ethical Conduct in Human Research, Declaration of Helsinki (as revised in 2013), Australian Code for the Responsible Conduct of Research and Note for Guidance on Good Clinical Practice (Approval No. HREC/19/QTDD/49300). Informed consent was taken from all patients.
During the urethroplasty procedure biopsies were collected at specific points along the urethra. Methylene blue was injected into the submucosa to assist in the identification of damaged urothelium (13). After a urethrotomy was performed a full thickness biopsy was taken from the edge of the incision with the use of tenotomy scissors. For strictures located at a single site, biopsies were taken at the stricture itself, as well as one and 2 cm proximal and distal to the stricture (Figure 1). In the case of panurethral strictures, biopsies were obtained from the meatus, penile, penobulbar, proximal bulbar, and distal bulbar regions. Each biopsy was assessed by an independent external centralised single accredited uro-pathologist who provided detailed information regarding the macroscopic and microscopic findings. The biopsies were assessed utilising hematoxylin and eosin (H&E) stain. The histological diagnosis of LS as outlined by Fistarol & Itin 2013 was utilized (14). The presence of specific characteristics including hyperkeratosis, atrophy or thickening of the epidermis or squamous epithelium, upper dermal hyalinisation, lichenoid change, lichenoid infiltrate, ulceration and scarring was recorded. In cases that did not involve LS, the presence of inflammatory cells or other causes was noted.
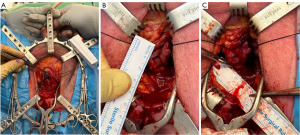
Standard post-operative care following urethroplasty was administered, with the typical duration of admission ranging from one to three days. The catheter was removed approximately four weeks after the procedure. Sequential reviews of all enrolled patients were conducted at one, three, six, and twelve months following the procedure. Follow-up continued yearly. The collected data included the type of repair performed, follow-up flow rates, and success or failure, as defined by Erickson and Ghareeb (15).
Stricture recurrence was defined as the requirement for procedures to dilate or bypass the urethra, including self-dilation, endoscopic dilatation, the placement of a permanent indwelling or suprapubic catheter, salvage urethroplasty, or urethrotomy. It also encompassed cases where there was a severe reduction in urinary flow rate indicative of a stricture (less than 10 mL/s), as well as macroscopic evidence of a visible stricture observed during flexible or rigid cystoscopy (15).
Results
Out of 109 urethroplasties performed over a 3-year period from 2019 to 2022, 15 male patients were identified and enrolled in the study. The average age of the patients was 58 years (range, 26–78 years), and their average American Society of Anesthesiologists (ASA) score was 2 (range, 1–4 years). Among the patients, five were active smokers, four were ex-smokers, and six had never smoked. Most patients had strictures located at the bulbar urethra (10), while two patients had strictures at the penoscrotal junction, one had a submeatal stricture, and one had a panurethral stricture. Four patients had stricture length below 2 cm, 10 patients had strictures between 2 and 7 cm and 1 patient had a stricture above 7 cm. The average length of stricture was 3.1 cm (range, 1–8 cm). All patients underwent BMG urethroplasty using various techniques, which were performed by a single urologist specialising in urethral reconstructive surgery (Table 1). The initial success rate was 80% (12 out of 15 patients), with 3 patients experiencing recurrence within one year of follow up. No patient exhibited macroscopic evidence of LS at the glans, foreskin or penile skin. The average follow-up time was 16 months with maximum of 36 months. Ongoing follow up continues with yearly clinic reviews.
Table 1
| Patient No. | Age, years | ASA | Smoker | Procedure | Site | Stricture length, cm | Recurrence | Subsequent treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 2 | Yes | Ventral onlay | Bulbar | 2 | No | – | Success |
| 2 | 31 | 1 | Yes | Dorsal onlay | Bulbar | 4 | Yes | BMG ventral onlay | Success |
| 3 | 42 | 2 | No | Dorsal onlay | Bulbar | 1.5 | No | – | Success |
| 4 | 52 | 2 | Yes | Double face | Bulbar | 4 | No | – | Success |
| 5 | 53 | 3 | No | Double face | Bulbar | 1 | No | – | Success |
| 6 | 59 | 3 | Yes | Transurethral ventral inlay | Submeatal | 1.5 | Yes | BMG dorsal inlay | Success |
| 7 | 60 | 2 | Yes | Dorsal inlay | Penile | 1.5 | No | Partial penectomy | Success |
| 8 | 61 | 3 | Ex | Dorsal onlay | Bulbar | 6 | No | – | Success |
| 9 | 62 | 2 | Ex | Dorsal onlay | Bulbar | 4 | No | – | Success |
| 10 | 63 | 2 | Ex | Dorsal onlay | Bulbar & penoscrotal | 5 | No | – | Success |
| 11 | 73 | 1 | No | Dorsal onlay | Bulbar | 2.5 | No | – | Success |
| 12 | 77 | 2 | Ex | Dorsal onlay | Panurethral | 8 | No | – | Success |
| 13 | 64 | 1 | No | Dorsal onlay | Bulbar | 2 | No | – | Success |
| 14 | 78 | 4 | No | Dorsal onlay | Bulbar | 2 | Yes | Focal soft ring stricture dilatation | Success |
| 15 | 74 | 2 | No | Dorsal onlay | Penoscrotal | 2 | No | – | Success |
ASA, American Society of Anesthesiologist Score; BMG, buccal mucosal graft.
Histopathological findings from biopsies at the stricture site and at 1 and 2 cm proximal and distal to the stricture are summarised in Figure 2 with additional details provided in Tables S1-S5. At the stricture site itself, no LS was observed, while 93.3% (14) of patients demonstrated scarring and 20% (3) showed inflammatory cells. Only one patient had histological evidence of ulcerations, one with hyperkeratosis, one with epidermal atrophy and two patients had epidermal thickening. 3 patients had denuded epithelium that some histological parameters were unable to be determined (Table S1).
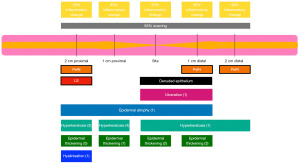
In biopsies taken 1 cm distally, no LS was found, 93.3% (14) had scarring, and 26.7% (4) exhibited inflammation (Table S2). Other features included ulceration in one patient and penile intraepithelial neoplasia (PeIN) in another. One patient had hyperkeratosis, one had epidermal atrophy and 3 had epidermal thickening. One patient had non diagnostic denuded epithelium.
At 2 cm distally, again no LS was seen, while 93.3% (14) had scarring, 40% (6) had inflammatory cells present and 33.3% (5) had epidermal thickening (Table S3). No ulcerations were observed at this location. One patient had hyperkeratosis. The same patient with PeIN at their biopsy taken 1 cm distal also returned histological evidence of PeIN at this site.
In proximal biopsies, at 1 cm, 93.3% (14) showed scarring and 33.3% (5) inflammatory cells present, 46.7% (7) had epidermal thickening, 26.7% (4) had hyperkeratosis and one patient had epidermal atrophy. No patients displayed ulcerative changes (Table S4).
Finally, at 2 cm proximally one of the patients demonstrated LS change at this site. Additionally, 93.3% (14) had evidence of scarring, 26.7% (4) had inflammatory cells present, and no patients exhibited ulcerations. Hyperkeratosis was present in 20% (3), 33.3% (5) had epidermal thickening, 1 patient had epidermal atrophy and 1 patient had upper dermal hyalinisation. Again, the patient with PeIN at other sites demonstrated PeIN at this site (Table S5).
Patients 2, 6, and 14 experienced recurrences. Patients 2 and 6 showed no evidence of LS. Patient 2 exhibited inflammation, ulceration, and scarring at the stricture site, scarring and inflammation distally, and scarring, hyperkeratosis, and epidermal thickening proximally (Figure 3A). Patient 6 had scarring throughout as well as epidermal thickening distally (Figure 3B). Patient 6 stricture site epithelium was too denuded to assess completely. Patient 14 had evidence of LS 2 cm proximal to the stricture site and demonstrated upper dermal hyalinisation (Figure 4A). Additionally, Patient 14 demonstrated evidence of inflammation, scarring, and ulceration distal to the stricture site. At the site of stricture, the histopathology of Patient 14 was unable to be fully analysed due to denuding of the epithelium (Figure 4B).
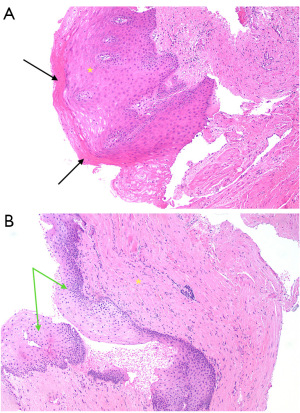
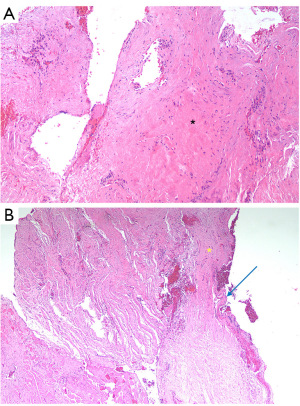
For Patient 2, a successful redo BMG ventral onlay urethroplasty was performed. Patient 6 underwent a BMG dorsal inlay redo urethroplasty, with subsequent ongoing obstructive lower urinary tract symptoms. No recurrence was observed on post-procedure flexible cystoscopy and flow was preserved on uroflowmetry with high post void residuals. This was suggestive of atonic bladder and the patient required to clean intermittently self-catheterise. Patient 14 underwent a urethral dilatation for a focal ring of recurrence, which was successful and demonstrated no recurrence during follow-up surveillance. Following recurrence surgery successful outcomes were at 100% (15/15). The patient in which PeIN was detected underwent a partial penectomy with clear margins.
Discussion
The histopathological incidence of LS in our cases was observed to be 6.7% (1 patient), which did not reach the anticipated rate according to our hypothesis. However, it is worth noting that the diagnostic criteria for LS in the urethra remain poorly defined, with different guidelines and literature presenting differing views (16). A recent survey conducted among pathologists demonstrated significant variability in their interpretations, without a consensus view being universally adopted (17). Diagnostic histopathological findings associated with LS include hyperkeratosis, thinning or thickening of the epidermis or squamous epithelium, attenuation or vacuolar degeneration of the basal cell layer, subepithelial hyalinisation/dermal collagen homogenisation, and lichenoid lymphocytic or plasmacytic infiltrate, although the specific diagnostic criteria can vary (14). In our study, LS was defined by our uropathologist based on standard criteria with diagnostic criteria being met if one of the following was found: lichenoid change and/or upper dermal hyalinisation. Notably, at the 2014 Annual Meeting of The United States and Canadian Academy of Pathology, new diagnostic pathologic criteria were introduced for LS. According to these criteria, cases exhibiting no or only one feature were classified as negative for LS, cases with two features were considered suggestive of LS, and cases displaying three or more features were deemed diagnostic for LS (18). If these criteria had been applied to our study, the suspected LS rate would have increased to 33% (5 out of 15 patients). This underscores the complexity and challenges associated with the diagnostic workup of LS in USD. Overall, our findings highlight the need for standardised diagnostic criteria and a consensus approach in diagnosing LS in the context of USD. Future research efforts should focus on establishing a more definitive and universally accepted diagnostic framework for LS, enabling more accurate identification and characterisation of this pathological condition.
Our data reveals significant histopathological changes in the underlying etiology of some idiopathic strictures, indicating the presence of an underlying inflammatory condition. While only one patient demonstrated LS, 20–40% of patients exhibited the presence of inflammatory cells. Importantly, these changes were observed both proximal and distal to the site of stricture. Notably, we observed pathological changes extending beyond the stricture site in the distal region, which is not consistent with the current understanding of the mechanism underlying stricture disease. The currently proposed pathophysiology of stricture disease suggests that an initial insult leads to ischemia and subsequent scarring, resulting in proximal pressure and urine extravasation into the tissue, leading to proximal changes (2). However, our findings indicate that similar changes also occur distally. This highlights the fact that stricture pathology extends beyond the immediate stricture site, which may have implications for the success rates of EPA as diseased segments of adjacent urethra may be joined together resulting in suboptimal outcomes. These findings should be taken into consideration in the management of patients with idiopathic stricture disease. In our study, the stricture recurrence rate within one year was observed in three out of 15 patients. Among these patients, one demonstrated LS proximal to the stricture site, while the other two exhibited histological changes throughout the biopsy sites, including inflammation, ulceration, epidermal thickening, and hyperkeratosis. This further supports the hypothesis that recurrence rates in stricture disease may be influenced by the pathophysiology extending beyond the immediate stricture site.
Performing a retrospective analysis of 36 urethroplasty patients, Samarska et al. compared histopathological features of excised urethral tissue between 10 recurrent and 26 non-recurrent stricture cases (18). They demonstrated that the presence of paucicellular fibrotic plaques with scarce nuclei was associated with higher recurrence rates post-operatively. They did not find inflammation predictive of recurrence however only performed histopathology on the diseased stricture segment excised. By assessing various inflammatory markers, they elucidated the compositional nature of immune infiltrates but found no prognostic utility. Their exclusive focus on excised segments for patients undergoing EPA differs from our biopsies spanning stricture and adjacent sites, enabling localised comparisons. Interesting, their recurrent EPA cases showed no improvement in uroflow post-operatively, implying potential incomplete stricture excision. This finding adds value to our hypothesis that joining inflamed or diseased edges during EPA may incorporate occult pathology yielding recurrence.
The literature has documented cases of isolated urethral stricture LS in the absence of LS at the glans. Liu et al. [2014] conducted a retrospective study demonstrating the presence of LS in isolated bulbar stricture disease (19). Using the newer pathological criteria, they reclassified 7.1% of patients as having LS, and an additional 18.6% showed features suggestive of LS, resulting in nearly 50% of the studied population exhibiting at least 2 LS features on histopathology specifically within the bulbar urethra, without evidence of LS elsewhere. Attia et al. [2021] also presented a case report of an isolated urethral stricture due to LS without macroscopic evidence of LS at the glans (20). These studies suggest that LS may not always follow the traditional pattern of proximal progression and can occasionally manifest as an isolated bulbar urethral stricture. Our finding of LS in one patient without a diagnosis of LS on the glans or foreskin further supports the notion of skip lesions associated with LS. This finding challenges the current understanding of LS pathophysiology and its proposed natural history.
Limitations of this study include the small sample size from a single center, heterogeneity in stricture location which may impact underlying pathophysiology, technical limitations in tissue handling leading to nondiagnostic pathology in some cases (denuded epithelium), lack of a control group for comparison, limited geographic diversity as an Australian population which may not represent global IUSD characteristics, and the relatively short follow-up period. The denuded epithelium of some samples was difficult to assess due to severe scarring present in the specimens, this may indicate burned-out LS and explain the absence of LS at the stricture site of Patient 14. By expanding the patient cohort and further refining the diagnostic criteria for LS, a more robust database can be established. It is important to note that since single biopsies were taken at predefined points at the edge of the urethrotomy, LS and other disease features may not have been captured comprehensively, as the geographic distribution of disease may extend beyond these specific points.
Future research directions include establishing a comprehensive histopathological database of IUSD biopsies to further characterise the extent and causes of idiopathic disease, utilising additional immunohistochemical staining to allow more complete pathological assessment as outlined by Samarska et al. in 2021 (18), utilising indocyanine green dye to evaluate stricture extent, expanding the study across institutions and geographic regions to improve the generalisability of findings, enrolling control groups without stricture disease or with known etiologies for comparison, and conducting long-term follow up to better understand disease progression and treatment durability.
Our recommendation is based on the histopathological findings obtained from our study involving 15 patients, which indicate that the diagnosis and management of USD is complex due to the histopathological changes observed both proximal and distal to the stricture site. These findings have significant implications for selecting the most appropriate surgical approach, such as EPA or substitution urethroplasty, as EPA may bring together segments of the urethra affected by the disease, potentially influencing the recurrence rate. The shift from EPA to BMG urethroplasty has already been noted in previous studies (21).
Performing biopsies on idiopathic strictures holds significance, as it facilitates the identification of inflammatory processes that can escalate to malignancy, a known outcome in instances of LS (22). While our patient with PeIN did not manifest LS characteristics, this case underscores the potential for malignancy within the spectrum of stricture disease and underscores the role of biopsies in enabling timely detection and initiating interventions.
We propose that considering the diagnostic variability of LS in the existing literature, LS should be viewed as a spectrum of disease. Based on this perspective, we hypothesise that patients diagnosed as non-LS despite exhibiting some LS histopathological features should be managed similarly to those with confirmed LS, adopting an expectant approach. Consequently, surgical techniques such as EPA and local skin flaps or grafts should be avoided due to their associated risk of recurrence.
Conclusions
In conclusion, this study introduces a novel technique for managing USD, contributing to a deeper understanding of the underlying aetiology and pathophysiology of IUSD. It acts as a pilot study so that future research can be conducted to deepen the insights through larger cohort studies These findings have important implications for optimising treatment strategies and improving patient outcomes in the field of urethral reconstructive surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-549/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-549/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-549/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-549/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval for this study was made through the Darling Downs Health Human Research Ethics Committee (No. EC00182) in accordance with the National Statement on Ethical Conduct in Human Research, Declaration of Helsinki (as revised in 2013), Australian Code for the Responsible Conduct of Research and Note for Guidance on Good Clinical Practice (Approval No. HREC/19/QTDD/49300). Informed consent was taken from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bugeja S, Andrich DE, Mundy AR. Non-transecting bulbar urethroplasty. Transl Androl Urol 2015;4:41-50. [PubMed]
- Mundy AR, Andrich DE. Urethral strictures. BJU Int 2011;107:6-26. [Crossref] [PubMed]
- Chapple C, Barbagli G, Jordan G, et al. Consensus statement on urethral trauma. BJU Int 2004;93:1195-202. [Crossref] [PubMed]
- Tritschler S, Roosen A, Füllhase C, et al. Urethral stricture: etiology, investigation and treatments. Dtsch Arztebl Int 2013;110:220-6. [PubMed]
- Flynn H, Ong M, De Win G, et al. Narrowing in on urethral strictures. Aust J Gen Pract 2021;50:214-8. [Crossref] [PubMed]
- Kwok M, Shugg N, Siriwardana A, et al. Prevalence and sequelae of penile lichen sclerosus in males presenting for circumcision in regional Australia: a multicentre retrospective cohort study. Transl Androl Urol 2022;11:780-5. [Crossref] [PubMed]
- Hong MKH, Murugappan S, Norton SM, et al. Male urethral stricture disease in a regional centre: 10 years of experience. ANZ J Surg 2019;89:747-51. [Crossref] [PubMed]
- Partin AW, Dmochowski RR, Kavoussi LR, et al., editors. Campbell-Walsh Urology. 12th ed. Philadelphia: Elsevier; 2020.
- Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol 2009;182:983-7. [Crossref] [PubMed]
- Erickson BA, Elliott SP, Myers JB, et al. Understanding the Relationship between Chronic Systemic Disease and Lichen Sclerosus Urethral Strictures. J Urol 2016;195:363-8. [Crossref] [PubMed]
- Eltahawy EA, Virasoro R, Schlossberg SM, et al. Long-term followup for excision and primary anastomosis for anterior urethral strictures. J Urol 2007;177:1803-6. [Crossref] [PubMed]
- Erickson BA, Granieri MA, Meeks JJ, et al. Prospective analysis of erectile dysfunction after anterior urethroplasty: incidence and recovery of function. J Urol 2010;183:657-61. [Crossref] [PubMed]
- Joshi P, Kaya C, Surana S, et al. A novel method in decision making for the diagnosis of anterior urethral stricture: using methylene blue dye. Turk J Urol 2017;43:502-6. [Crossref] [PubMed]
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol 2013;14:27-47. [Crossref] [PubMed]
- Erickson BA, Ghareeb GM. Definition of Successful Treatment and Optimal Follow-up after Urethral Reconstruction for Urethral Stricture Disease. Urol Clin North Am 2017;44:1-9. [Crossref] [PubMed]
- Thakare KV, Rasool TMM, Kucherlapati A, et al. Observational study on histopathology of male anterior urethral stricture: Toward better understanding of stricture pathophysiology. Asian J Med Sci 2023;14:172-7. [Crossref]
- Erickson BA, Tesdahl BA, Voznesensky MA, et al. Urethral lichen sclerosus under the microscope: a survey of academic pathologists. Can J Urol 2018;25:9328-33. [PubMed]
- Samarska IV, Dani H, Bivalacqua TJ, et al. Histopathologic and clinical comparison of recurrent and non-recurrent urethral stricture disease treated by reconstructive surgery. Transl Androl Urol 2021;10:3714-22. [Crossref] [PubMed]
- Liu JS, Walker K, Stein D, et al. Lichen sclerosus and isolated bulbar urethral stricture disease. J Urol 2014;192:775-9. [Crossref] [PubMed]
- Attia A, Morton A, Raveenthiran S, et al. Lichen sclerosus presenting as an isolated bulbar urethral stricture. Urol Case Rep 2021;39:101794. [Crossref] [PubMed]
- Cotter KJ, Hahn AE, Voelzke BB, et al. Trends in Urethral Stricture Disease Etiology and Urethroplasty Technique From a Multi-institutional Surgical Outcomes Research Group. Urology 2019;130:167-74. [Crossref] [PubMed]
- Fergus KB, Lee AW, Baradaran N, et al. Pathophysiology, Clinical Manifestations, and Treatment of Lichen Sclerosus: A Systematic Review. Urology 2020;135:11-9. [Crossref] [PubMed]


