
Effects of electroacupuncture on chronic urinary retention after pelvic or lumbosacral tumor resection surgeries: a retrospective cohort study
Highlight box
Key findings
• Electroacupuncture (EA) could be a potential novel treatment option to decrease post-void residuals (PVR) and restore spontaneous urination for patients with chronic urinary retention (CUR) resulting from lower motor neuron lesions (LMNL) following pelvic or lumbosacral tumor resection surgeries, with long-term effects and limited adverse events. The optimal duration of EA treatment should be of 8 consecutive weeks or longer.
What is known and what is new?
• Patients with CUR caused by LMNL after tumor resection surgeries, have to rely on life-long catheterization to empty the bladder, despite complications of catheterization including recurrent urinary tract infection (UTI) and urethral stricture formation, negatively impacting on patients’ daily life, and bringing a heavy burden on family and society.
• EA is a promising therapy for patients with CUR resulting from LMNL secondary to pelvic or lumbosacral tumor resection surgeries, with long-term effects and safety.
What is the implication, and what should change now?
• EA should be considered as a promising therapy for CUR patients.
• A larger sample size and randomized controlled trials are needed to testify the feasibility of EA therapy for CUR patients.
Introduction
Chronic urinary retention (CUR) is defined as the consistent presence of a significant magnitude of the post-void residual (PVR) volume, generally set at 300 mL (1). The condition can be associated with morbidities such as hydronephrosis, chronic renal insufficiency and urinary tract infection (UTI) (2). CUR can be caused by lower motor neuron lesions (LMNL) secondary to pelvic or lumbosacral tumor resection surgeries, when nerves related to bladder, such as pelvic plexus and cauda equina, are accidentally damaged in the operation area (3-5). Nearly 30% patients undergoing pelvic surgeries, such as retropubic prostatectomy and hysterectomy, manifested detrusor and sphincter dysfunction and urinary retention, probably due to bladder denervation during surgery (6,7). It is generally believed that patients with acute postoperative urinary retention should re-establish their own normal voiding pattern with minimal residual urine by intermittent catheterization within 3 months (8), but there are still a group of postoperative patients with nerve lesions presenting with CUR, having to rely on life-long catheterization to empty the bladder (9). However, complications of catheterization including recurrent UTI and urethral stricture formation, negatively impact on patients’ daily life and bring heavy burden on family and society, together making it a less ideal option (10,11). Sacral neuromodulation (SNM) is an officially approved approach to relieving certain lower urinary tract symptoms including non-obstructive CUR (12,13). However, as a costly surgical invasive therapy, SNM can cause adverse effects that require reoperation when patients come up with technical faults and suffer complications of pain or discomfort (14). Especially for patients who have just gone through radical tumor resection surgeries, a more conservative therapy for postoperative CUR is needed.
Electroacupuncture (EA) is a type of therapeutic practice that inserts fine needles at specific acupoints and adds electrical stimulation, which can have positive impacts on diseases, according to traditional Chinese medicine (TCM) theory (15). In TCM theory, CUR is called Longbi. The major pathogenesis of Longbi in the patients after surgery is the deficiency of kidney qi and bladder function together with stasis of blood syndrome. So, the acupoints of the bladder meridian (BL) including Shenshu (BL23), Ciliao (BL32), Zhongliao (BL33) and Huiyang (BL35) are used for treating Longbi, following the TCM principle of “the meridian, the attending”. Sanyinjiao (SP6), as the connection point of three meridians which are Jueyin liver meridian of foot (LR), spleen meridian (SP) and the kidney channel of foot Shaoyin (KI), is often used to remove blood stasis as well as promote bladder function recovery together with acupoints in BL. Compared with manual acupuncture, EA added constant electrical stimulation at acupoints. It is believed that constant low amplitude electrical stimulation through the sacral nerve roots leads to ascending signals passed on to the micturition centers, which modulate efferent signals to the bladder as well as bowel (16). And EA stimulation at BL32 and BL33 can activate S2 and S3 afferent nerve fibers deep in BL32 and BL33 to promote detrusor smooth muscle contractions (17). The therapeutic effects and high safety of EA have been verified in patients suffering from certain lower urinary tract diseases including stress urinary incontinence and overactive bladder (18,19). However, few studies have examined the effect of EA on CUR secondary to pelvic or lumbosacral tumor resection surgeries. Therefore, in this retrospective cohort study, we present cases of CUR caused by LMNL after pelvic or lumbosacral tumor resection surgeries and discuss the potential role of EA therapy for these patients, providing practical results that could benefit further clinical decision and research. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-468/rc).
Methods
Study design and patients
This was a retrospective single-center cohort study performed at Guang’anmen Hospital, China, Academy of Chinese Medical Sciences from March 1, 2017 to June 30, 2020. As longer catheterization can result in more complications and negative impacts on patients’ life quality, this study was not designed as a randomized controlled trial (RCT) out of ethical considerations for patients with CUR after tumor resection surgeries. Patients were followed up in the outpatient setting monthly during the EA treatment of 2 to 12 weeks and once 6 months after the end of EA treatment. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Guang’anmen Hospital (No. 2018-102-KY-01). All patients had signed the written informed consent prior to participation. This study was registered at Chinese Clinical Trial Registry (registration number: ChiCTR1800020222).
Patients met all the following inclusion criteria to be eligible: (I) 18 years or older; (II) having a diagnosis of CUR resulting from LMNL (e.g., injury to the sacral plexus, cauda equina, sacral spinal cord or pelvic nerve) secondary to pelvic or lumbosacral tumor resection surgeries; (III) baseline PVR ≥300 mL; (IV) the course of CUR ≥3 months; (V) having bladder sensation; and (VI) utilizing clean intermittent catheterization (CIC) or indwelling catheterization (IC). Patients were excluded if any of the following criteria were met: (I) having a lower urinary tract obstruction, such as bladder neck contracture, urethral stricture, prostatic hyperplasia, or large urinary stones that can cause obstruction; (II) having any type of malignant tumors not removed; (III) diagnosed with peripheral neuropathy related to diabetes; (IV) having severe systemic disorders not controlled; (V) having implanted electrodes of cardiac pacemaker, pudendal nerve stimulation, bladder stimulation, or SNM; (VI) pregnancy or lactation; and (VII) treatment duration <2 weeks.
The PVR of patients undergoing CIC was averaged by two values measured by transurethral catheterization output following spontaneous urination, with an interval from 6 hours to 3 days. Spontaneous urination referred to urinating without catheterization or manual assisted voiding including putting pressure on abdomen. The PVR of patients undergoing IC was assessed when replacing with a new clean catheter, usually once a month. After the old catheter was removed, 300–500 mL normal saline was gently infused into the bladder according to patients’ bladder sensation. Then the patients would be asked to try urinating spontaneously, if satisfactory spontaneous urination was observed and catheterization was not needed, which was assessed by urologists, PVR was measured by ultrasound; otherwise, these patients’ PVR was obtained by the output after the new catheter was inserted.
EA treatment procedure
According to TCM criteria and modern acupuncture literature, acupoints including bilateral BL23, bilateral BL32, bilateral BL33, bilateral BL35 and bilateral Sanyinjiao (SP6) were selected and inserted with acupuncture needles (17,20). So, in total ten acupuncture needles were inserted per subject per session. The parameter of used needles at BL32, BL33 and BL35 was 0.30–0.40 mm in diameter with 75–100 mm in length, and at BL23 and SP6 was 0.30 mm in diameter with 40 mm in length (Hwato Brand, Suzhou Medical Appliance Factory, Suzhou, China). Bilateral BL32 and BL33 were needled to a depth of 70 to 95 mm with an angle of 60°–75° inward and downward, into the second and third sacral foramen. Bilateral BL35 were needled to a depth of 60 to 70 mm in a slightly superolateral direction. Bilateral BL23 and bilateral SP6 were vertically needled to a depth of 25 to 30 mm. After the Chinese needle sensation (de qi) was evoked individually, electric stimulators (SDZ-V electroacupuncture apparatus, Suzhou Medical Appliance Factory, Suzhou, China) with a 5-Hz continuous wave (5–10 mA intensity) were separately connected to bilateral BL32, bilateral BL33, and bilateral BL35, and stimulators with a 10-Hz continuous wave (1–2 mA intensity) were connected to bilateral SP6. Current intensity was adjusted according to the patients’ individual tolerance. During each session, EA was retained for 30 minutes. EA was performed by licensed acupuncturists with at least 5 years of experience. The patients were treated with EA three times a week for 2 to 12 weeks continuously. In general, if the patient acquired satisfactory spontaneous urination after several weeks of EA treatment, EA could be terminated depending on the doctor’s and participant’s decisions. Satisfactory spontaneous urination was defined as PVR <100 mL after spontaneous urination, and no complications of hydroureter, hydronephrosis or recurrent symptomatic UTI occurred (14). If the patient did not acquire satisfactory spontaneous urination, EA treatment was proceeded for at most 12 weeks. Meanwhile, CIC or IC was administered for all patients at baseline. UTI was controlled by antibiotics.
Outcomes
Responders were defined as patients whose PVR reduced by 50% or more from baseline (21). The other treatment outcomes included the change in PVR, the change in the proportion of patients reporting severe urinating difficulty and the proportion of patients having stool retention from baseline. In addition, the proportion of patients requiring catheterization, patients reporting 1 (much better) or 2 (moderately better) regarding the Patient Global Impression of Improvement (PGI-I) (22), patients having recurrent symptomatic UTI, hydroureter, or hydronephrosis were assessed. All the outcomes were assessed monthly during the EA treatment period and followed up at the 24th week since the end of EA treatment. In this study, the response rate was also seen as a time-to-event outcome as different patients could respond to EA treatment at different weeks. Time-to-event analysis was applied to investigate the association between response rate and EA treatment duration.
The patient’ s urinating difficulty was classified into four levels: severe, moderate, mild and none. The PGI-I assessment had scores of 1 to 7 corresponding to much better to much worse, with higher score representing less improvement (22). Recurrent UTI was confirmed by characteristic clinical signs, including painful, frequent, and urgent urination, pyuria (>10 white blood cell count/high power field), and a positive urine culture at least 2 times within the past 6 months (23). It was classified as persistent UTI when the UTI relapsed within 1 month or never resolved after antibiotic therapy (24). Hydronephrosis and hydroureter were assessed by ultrasound. Stool retention was diagnosed according to Rome IV criteria (25).
Safety assessment
Adverse events (AEs) were reported by patients and acupuncturists over the study period. All the AEs were categorized as either acupuncture related or not acupuncture related AEs, and closely monitored by acupuncturists and urologists. Any serious AEs would be immediately reported to the principal investigator (Z.L.) and Medical Ethics Committee within 24 hours.
Statistical analysis
Continuous variables were reported as mean [standard deviation (SD)] or median [interquartile range (IQR)] according to the distribution, which was assessed by skewness-kurtosis test. Categorical variables were summarized with frequencies and percentages. Paired t-test or paired Wilcoxon rank-sum test was used for comparing continuous variables in baseline and post-treatment data. Categorical variables in baseline and post-treatment data were compared by paired Fisher exact test. Two-sided P value less than 5% was reported to be statistically significant. Kaplan-Meier survival estimate was used to investigate the association between response rate as a time-to-event outcome and EA treatment duration. All the analyses were performed using STATA software 15.1 (Stata Corp, TX, USA) for Mac.
Results
Baseline characteristics
In total, 36 patients were screened for eligibility, and 16 of them were excluded (see Figure 1). Finally, 20 patients [mean (SD) age, 48.1 (15.5) years; 9 men (45.0%); 11 women (55.0%)] were included in the analysis. Two of the most common tumor etiologies for surgeries were cervical cancer (5/20, 25.0%) and lumbosacral teratomas (5/20, 25.0%). Baseline characteristics are shown in Table 1. All 20 patients were available for a median follow-up of 32 weeks (range, 26–36 weeks).
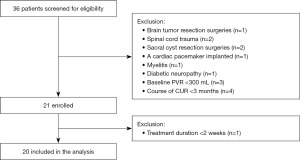
Table 1
| Characteristics | Participants (n=20) |
|---|---|
| Age (years), mean (SD) | 48.1 (15.5) |
| Male, n (%) | 9 (45.0) |
| PVR (mL), mean (SD) | 488.4 (192.3) |
| Course of CUR (months), median (IQR) | 5.0 (3.5, 9.5) |
| Etiology, n (%) | |
| Cervical cancer | 5 (25.0) |
| Prostatic cancer | 2 (10.0) |
| Endometrial cancer | 1 (5.0) |
| Colon cancer | 1 (5.0) |
| Sacral neurinoma | 3 (15.0) |
| Sacral lipoma | 2 (10.0) |
| Lumbosacral teratoma | 5 (25.0) |
| Lumbar vertebra osteoma | 1 (5.0) |
| Time after surgeries (months), median (IQR) | 5.5 (4.0, 10.3) |
| Damaged nerves, n (%) | |
| Cauda equina | 13 (65.0) |
| Pelvic nerve | 7 (35.0) |
| Catheterization methods, n (%) | |
| CIC | 10 (50.0) |
| IC | 10 (50.0) |
| Urinating difficulty, n (%) | |
| None | 0 |
| Mild | 0 |
| Moderate | 2 (10.0) |
| Severe | 18 (90.0) |
| Hydronephrosis, n (%) | 1 (5.0) |
| Recurrent UTI, n (%) | 2 (10.0) |
| Stool retention, n (%) | 10 (50.0) |
SD, standard deviation; PVR, post-void residuals; CUR, chronic urinary retention; IQR, interquartile range; CIC, clean intermittent catheterization; IC, indwelling catheterization; UTI, urinary tract infection.
Treatment outcomes
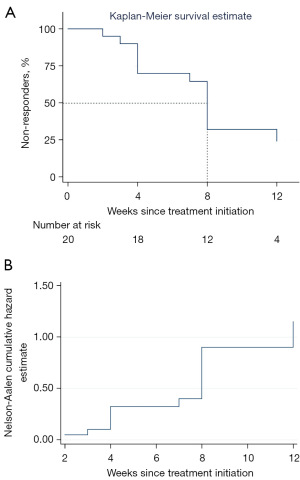
As shown in Figure 2A,2B and Table 2, of the 20 cases, 14 (70.0%) had responded to EA treatment within 12 weeks and stopped catheterization for achieving satisfactory spontaneous urination, 7 (35.0%) had complete resolution (90–100% reduction in PVR from baseline), and 13 (65.0%) scored 1 (much better) or 2 (moderately better) in the PGI-I assessment. Moreover, of the 14 responders, 13 (93%) had responded within 8 weeks, and 6 (43%) had responded within 4 weeks. According to Kaplan-Meier survival curve, we found that more than 50% patients could respond to EA treatment within 8 weeks or longer (Figure 3A). And Nelson-Aalen cumulative hazard estimate in Figure 3B also demonstrated a negative correlation between the proportion of non-responders and the duration of EA treatment. For all the 20 patients, PVR significantly decreased by 337.7 (95% CI: 230.5, 444.8) mL, with an average of 68.5% decrease from baseline at the 12th week since EA treatment initiation (P<0.001). Similarly, 35.0% (7/20) of the patients had severe urinating difficulty, with a 55% decrease from baseline (P<0.001). As to the proportion of patients having stool retention, it only decreased by 5% (P=0.625). None of the responders had ever experienced relapse in 24 weeks after EA treatment ended. None of the 20 patients manifested recurrent UTI, newly diagnosed hydronephrosis or hydroureter. One patient diagnosed with hydronephrosis at baseline recovered after 12-week EA treatment. Two patients with UTI at baseline were prescribed with antibiotics and did not present UTI again during the follow-up.
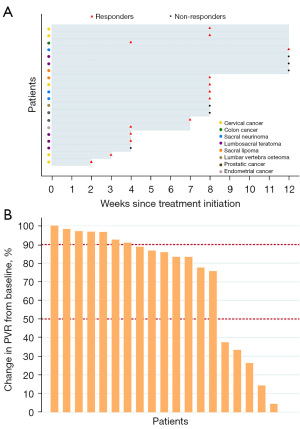
Table 2
| Outcomes at the 12th week | Outcomes (n=20) | Decrease from baseline | P value |
|---|---|---|---|
| Responders, n (%) | 14 (70.0) | – | – |
| Reduction of 90% to 100% in PVR, n (%) | 7 (35.0) | – | – |
| PVR (95% CI) (mL) | 150.8 (66.5, 235.0) | 337.7 (230.5, 444.8) | <0.001 |
| Catheterization withdrawal, n (%) | 14 (70.0) | – | – |
| Severe difficulty in urination, n (%) | 7 (35.0) | 55.0% (28.2%, 81.8%) | <0.001 |
| Stool retention, n (%) | 9 (45.0) | 5.0% (−13.5%, 32.5%) | 0.625 |
| PGI-I score of 1 or 2, n (%) | 13 (65.0) | – | – |
| Recurrent UTI, n (%) | 0 | – | – |
| Newly diagnosed hydronephrosis or hydroureter, n (%) | 0 | – | – |
Responders were defined as participants achieving a reduction of 50% or more in PVR from baseline. EA, electroacupuncture; PVR, post-void residuals; CI, confidence interval; PGI-I, the Patient Global Impression of Improvement; UTI, urinary tract infection.
Safety
Two (10.0%) AEs were reported. They were all mild and transient acupuncture-related AEs, including sharp pain in one patient and localized pigmentation in another patient. No patient discontinued EA treatment due to AEs. No episode of serious AEs was reported.
Discussion
As far as we know, this was by far the first cohort study focusing on the effect and safety of EA for CUR resulting from LMNL after pelvic or lumbosacral tumor resection surgeries. EA could be a potential treatment option to reduce PVR and facilitate spontaneous urination, with long-term efficacy and safety.
Lumbosacral and pelvic surgeries are found to be independent risk factors triggering CUR (26,27). Damages to sacral plexus, cauda equina, sacral spinal cord, or pelvic nerves during surgeries generally affect lower motor neurons, resulting in detrusor underactivity, a contractile detrusor, or detrusor areflexia, leaving patients unable to void the bladder spontaneously, resulting in a large amount of PVR (28,29). The elevated PVR is the primary problem to solve in case of complications including stones, hydronephrosis, and renal failure (30). Our cohort study demonstrated that EA could reduce PVR by 50% or more and restore satisfactory spontaneous urination (PVR <100 mL) in 70.0% (14/20) of the patients with CUR resulting from LNML secondary to lumbosacral or pelvic tumor resection surgeries. A retrospective study from China reported that after SNM therapy, 75.3% of patients with subspinal and peripheral nervous system lesions had PVR reduced by at least 50% (31), which was closely equal to EA’s efficacy presented in our study. In addition, according to time-to-event analysis we found that with EA treatment of 8 weeks or longer, more than 50% patients could respond. Compared to life-long catheterization or surgical electrodes impanation of SNM, EA treatment of 8 to 12 consecutive weeks probably represents higher safety and lighter economic burden. However, further research comparing the effect, safety, and cost-effectiveness of SNM and EA should be considered.
The treatment effects of EA are related to specific acupoints selected and electrical stimulation. According to TCM theory and modern studies on physiological and pathological mechanisms, specific acupoints have effects on specific symptoms (32,33) . For instance, Cao et al meta-analyzed that neostigmine could effectively improve the symptoms of postoperative urinary retention and neostigmine acupoint injection might achieve a better therapeutic effect (34). The mechanism of EA in our study may lie in electrical stimulation at BL32 and BL33, which can activate S2 and S3 afferent nerve fibers, promoting detrusor smooth muscle contractions via neural pathways, but more uncertain mechanisms of EA including anti-inflammation require further investigation (35).
The primary limitation of our study is that this was a retrospective cohort study of a small sample size derived from a single center, so future studies are needed to investigate EA in larger population in RCTs.
Conclusions
EA could be a potential novel treatment option to decrease PVR and restore spontaneous urination for patients with CUR caused by LMNL following pelvic or lumbosacral tumor resection surgeries, with long-term effects as well as a good safety profile. The optimal duration of EA treatment should be of 8 weeks at least. A RCT with a large sample size is needed to further confirm the feasibility of EA therapy.
Acknowledgments
The authors are grateful to patients who participated in this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-468/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-468/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-468/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-468/coif). Y.C. is an employee of Beijing Houpu Chinese Medicine Institute Company. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Guang’anmen Hospital (No. 2018-102-KY-01). All patients had signed the written informed consent prior to participation. This study was registered at Chinese Clinical Trial Registry (registration number: ChiCTR1800020222).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stoffel JT, Peterson AC, Sandhu JS, et al. AUA White Paper on Nonneurogenic Chronic Urinary Retention: Consensus Definition, Treatment Algorithm, and Outcome End Points. J Urol 2017;198:153-60. [Crossref] [PubMed]
- Groen J, Pannek J, Castro Diaz D, et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol 2016;69:324-33. [Crossref] [PubMed]
- Stoffel JT. Non-neurogenic Chronic Urinary Retention: What Are We Treating? Curr Urol Rep 2017;18:74. [Crossref] [PubMed]
- Agnello M, Vottero M, Bertapelle P. Sacral neuromodulation to treat voiding dysfunction in patients with previous pelvic surgery for deep infiltrating endometriosis: our centre's experience. Int Urogynecol J 2021;32:1499-504. [Crossref] [PubMed]
- Karani R, Mahdy A, Asghar F. Postoperative Urinary Retention in Patients Who Undergo Joint Arthroplasty or Spine Surgery. JBJS Rev 2020;8:e18.00040.
- Giannantoni A, Mearini E, Zucchi A, et al. Bladder and urethral sphincter function after radical retropubic prostatectomy: a prospective long-term study. Eur Urol 2008;54:657-64. [Crossref] [PubMed]
- Misal M, Behbehani S, Yang J, et al. Is Hysterectomy a Risk Factor for Urinary Retention? A Retrospective Matched Case Control Study. J Minim Invasive Gynecol 2020;27:1598-602. [Crossref] [PubMed]
- Anderson JB, Grant JB. Postoperative retention of urine: a prospective urodynamic study. BMJ 1991;302:894-6. [Crossref] [PubMed]
The European Association of Urology (EAU) Neuro-Urology Guidelines - Kowalik U, Plante MK. Urinary Retention in Surgical Patients. Surg Clin North Am 2016;96:453-67. [Crossref] [PubMed]
- Bos BJ, van Merode NAM, Steffens MG, et al. The Patient Pathway for Men with Chronic Urinary Retention: Treatments, Complications, and Consequences. Urology 2022;167:185-90. [Crossref] [PubMed]
- Moore CK, Rueb JJ, Derisavifard S. What Is New in Neuromodulation? Curr Urol Rep 2019;20:55. [Crossref] [PubMed]
- Fletcher N. An overview of sacral neuromodulation: a treatment for patients with symptoms of lower urinary tract dysfunction. Br J Nurs 2020;29:848-56. [Crossref] [PubMed]
- Saber-Khalaf M, Abtahi B, Gonzales G, et al. Sacral neuromodulation outcomes in male patients with chronic urinary retention. Neuromodulation 2015;18:329-34; discussion 334. [Crossref] [PubMed]
- White AEditorial Board of Acupuncture in Medicine. Western medical acupuncture: a definition. Acupunct Med 2009;27:33-5. [Crossref] [PubMed]
- Abello A, Das AK. Electrical neuromodulation in the management of lower urinary tract dysfunction: evidence, experience and future prospects. Ther Adv Urol 2018;10:165-73. [Crossref] [PubMed]
- Zhou J, Liu S, Jiao R, et al. Effects of electroacupuncture on patients with chronic urinary retention caused by a lower motor neuron lesion: An exploratory pilot study. Medicine (Baltimore) 2020;99:e18615. [Crossref] [PubMed]
- Hargreaves E, Baker K, Barry G, et al. Acupuncture for treating overactive bladder in adults. Cochrane Database Syst Rev 2022;9:CD013519. [PubMed]
- Liu Z, Liu Y, Xu H, et al. Effect of Electroacupuncture on Urinary Leakage Among Women With Stress Urinary Incontinence: A Randomized Clinical Trial. JAMA 2017;317:2493-501. [Crossref] [PubMed]
- Zhou B, Ma D, Yu H, et al. Acupuncture for acontractile bladder: a case report. Acupunct Med 2021;39:716-7. [Crossref] [PubMed]
- Chapman GC, Sheyn D, Slopnick EA, et al. Tamsulosin vs placebo to prevent postoperative urinary retention following female pelvic reconstructive surgery: a multicenter randomized controlled trial. Am J Obstet Gynecol 2021;225:274.e1-274.e11. [Crossref] [PubMed]
- Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol 2003;189:98-101. [Crossref] [PubMed]
- Kwok M, McGeorge S, Mayer-Coverdale J, et al. Guideline of guidelines: management of recurrent urinary tract infections in women. BJU Int 2022;130:11-22. [Crossref] [PubMed]
- Lee YK, Kuo HC. Effectiveness of Platelet-Rich Plasma Injections as Prophylaxis for Recurrent Urinary Tract Infection in Women. J Clin Med 2023;12:4129. [Crossref] [PubMed]
- Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016; Epub ahead of print. [Crossref] [PubMed]
- Alsaidi M, Guanio J, Basheer A, et al. The incidence and risk factors for postoperative urinary retention in neurosurgical patients. Surg Neurol Int 2013;4:61. [Crossref] [PubMed]
- Ghuman A, Dawidek MT, Athwal MS, et al. Prophylactic tamsulosin and urinary retention rates following elective colorectal surgery: a retrospective cohort study. Int J Colorectal Dis 2022;37:209-14. [Crossref] [PubMed]
- Krassioukov A, Biering-Sorensen CF, Donovan W, et al. International Standards to document remaining Autonomic Function after Spinal Cord Injury (ISAFSCI), First Edition 2012. Top Spinal Cord Inj Rehabil 2012;18:282-96.
- Burns ZR, Vishwanath VM, Ceballos B, et al. Evaluation and management of urinary retention after pelvic radiation therapy. AME Med J 2022;7:2. [Crossref]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-78. [Crossref] [PubMed]
- Masood I, Chen Q, Li J, et al. Sacral Neuromodulation in Patients With Neurogenic Lower Urinary Tract Dysfunction: A Multicenter Retrospective Study From China. Neuromodulation 2021;24:1278-83. [Crossref] [PubMed]
- Liu S, Wang Z, Su Y, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 2021;598:641-5. [Crossref] [PubMed]
- Wang GX, Zhou J, Chen YM, et al. Mechanism of electroacupuncture at acupoints of the lung meridian through PKA/PKC regulation of TRPV1 in chronic cough after lung surgery in guinea pigs. J Thorac Dis 2023;15:1848-60. [Crossref] [PubMed]
- Cao M, Wu X, Xu J. A systematic review and meta-analysis of neostigmine for urinary retention after surgeries. Transl Androl Urol 2022;11:190-201. [Crossref] [PubMed]
- Liu QY, Xu LC, Yi M. Anti-nociceptive mechanisms of electroacupuncture in inflammatory pain. AME Med J 2017;2:82. [Crossref]


