
Nitro-oleic acid (NO2-OA) ameliorates erectile dysfunction in a rat model of diabetes mellitus via modulation of fibrosis, inflammation and autophagy
Highlight box
Key findings
• Inflammation, fibrosis and activating autophagy are involved in the pathogenesis of diabetes mellitus erectile dysfunction (DMED). Comprehensive laboratory detection verified nitro-oleic acid (NO2-OA) enhances erectile function within a rat model of DMED by inhibiting inflammation and fibrosis along with activating autophagy.
What is known and what is new?
• DMED is a complex condition for which there is no effective treatment despite the progress in our understanding of this condition over the last decade.
• This study tried to investigate the effect of NO2-OA in a rat model of DMED.
What is the implication, and what should change now?
• Our findings provide a foundation for the development of new treatments of DMED. Due to the relatively limited understandings of DMED, more in-depth and detailed research is needed.
Introduction
In recent years, there has been a sharp increase in diabetes. A chronic complication associated with diabetes in male patients, which has attracted increasing attention, is that of erectile dysfunction (ED) (1,2). The incidence of ED in diabetes mellitus (DM) patients is significantly greater (3.5-fold) than that observed in non-diabetic males (3), with the overall incidence of diabetes mellitus erectile dysfunction (DMED) accounting for 50–75% in diabetes male patients (4). Moreover, the morbidity of DMED increases with age and duration of diabetes. DMED patients are prone to severe anxiety, low self-esteem and a sense of failure, which not only affects their physical and mental health, but also seriously impacts their marital stability and family harmony (5). Accordingly, research directed at understanding the development and pathogenesis of this condition to enable the implementation of new interventions and treatments is sorely warranted.
While ED is closely related with diabetes, the specific underlying mechanisms involved is yet to be fully elucidated (6,7). It is generally believed that nerve damage, imbalances in hormonal metabolism, vascular and endothelial dysfunction as well as other factors may all contribute to DMED (8-11). Results from an increasing number of recent studies have suggested that chronic inflammation of the cavernosal body, due to a persistent increase in hyperglycemia, aggravates damage to the endothelium of the cavernous body, thus leading to a decrease in the diastolic function of this structure (12). In addition, a persistent endothelial injury leads to a proliferation of fibroblasts, which in turn results in excessive fibrosis to further aggravate the relaxation function of the corpus cavernosum (13,14). Such effects represent important factors which can then lead to decreased erectile function in diabetes patients. It has also been reported that the PI3K/AKT/mTOR and nuclear factor kappa B (NF-κB) related pathways play important roles in the progression of inflammation involved with DMED (15,16). The mechanisms of corpus cavernosum fibrosis are quite complicated. In general, the transforming growth factor-β1 (TGF-β1)/Smad signaling pathway plays a key role in regulating corpus cavernosum fibrosis (17), with results from several studies indicating that persistent hyperglycemia can upregulate this pathway, leading to the formation of corpus cavernosum smooth muscle fibrosis (18,19). Therefore, inhibiting inflammatory responses and progression of fibrosis of the cavernosal body along with reducing the damage to cavernosal endothelial cells would seem to be critical factors for improving the relaxation function and thus enhancing frontal erection function of the cavernosal body. In this regard, autophagic activation has been proved to be an effective method for treating DMED via its capacity to modulate detrimental conditions such as inflammation and oxidative stress (20). The underlying mechanisms for this beneficial effect appear to involve the recycling of damaged organelles and cellular components to maintain cellular homeostasis.
Nitro-oleic acid (NO2-OA) is one type of nitrated fatty acids (NO2-FA), which serves as an important endogenous signaling mediator (21). Interestingly, NO2-OA has been shown to exert effective anti-inflammatory and anti-fibrotic effects as demonstrated in several disease models (22,23), and is currently being tested in phase II clinical trials for the treatment of chronic pulmonary and renal diseases (24). However, its therapeutic effects in DMED are yet to be investigated. The purpose of this study was to assess the protective effects of NO2-OA in the treatment of streptozotocin-induced diabetes in a rat model as well as to identify some of the possible mechanisms for its effects. We present this article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-547/rc).
Methods
Animals and the DMED model
Eight-week-old male Sprague Dawley (SD) rats (N=60) were purchased from the Experimental Animal Center of Shandong University. Procedures involving animal subjects were approved by the Animal Care and Use Committee of Liaocheng People’s Hospital (Liaocheng, China; Approval No. 2023046). This study involving rats was conducted in accordance with the Laboratory Animal Care and Use Guidelines of the National Institute of Health. The protocol was prepared before the study without registration. DM was induced with a single intraperitoneal injection of 60 mg/kg STZ (Sigma-Aldrich, St. Louis, MO, USA). Three days later, blood glucose levels were determined and rats with a blood glucose of 16.7 mmol/L were considered as diabetic. Twelve weeks after DM induction, all rats were treated with apomorphine (APO) hydrochloride (80 µg per kg body weight; A4393; Sigma-Aldrich), an established method for evaluating erectile function in rats as described previously (25). APO was injected subcutaneously into the neck of all rats, after which the rats were placed in a dark chamber observation cage for 30 min. When the appearance of an engorged glans penis and distal penis shaft was considered standard for penile erection. DM rats demonstrating a positive APO test response were designated as DMED rats and were used for subsequent experiments. All DMED rats were randomly divided into three groups, with N=15/group: DMED, DMED + NO2-OA and DMED + Vehicle group. An additional untreated control group (N=15) was also included. Measures of erectile responses were performed in all four groups. The DMED rats in DMED + NO2-OA group and DMED + Vehicle were treated with the vehicle [polyethylene glycol (PEG)/ethanol, 90:10, v/v] or the vehicle with NO2-OA (7.5 µg per kg body weight per day) via osmotic mini-pumps (ALZET Model 2002, Durect, Cupertino, CA, USA) that were implanted subcutaneously into the flanks for a 4-week period. The specific regioisomer of 10-nitro-octadec-9 (E)-enoic acid (NO2-OA) was provided by Bruce Freeman, PhD, from the University of Pittsburgh (Pittsburgh, PA, USA) (Figure 1A).
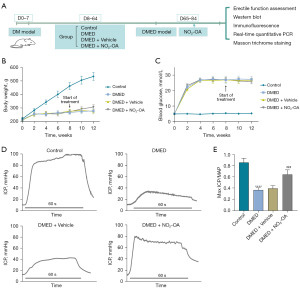
Erectile function assessment
Intracavernous pressure (ICP) and mean arterial pressure (MAP) were measured as described previously (26). Briefly, following anesthetization with pentobarbital sodium (35 mg/kg, Nembutal, St. Louis, MO, USA), a cannula was inserted into the isolated left carotid artery with a heparinized saline (250 IU/mL) filled PE-50 tube to monitor MAP. Next, after identifying and isolating the cavernous nerve (CN), a bipolar hook electrode connected to a signal generator (Taimeng, Chengdu, China) was placed to the left of the CN for electrical stimulation using monophasic rectangular pulses (5 V and 15 Hz with a pulse width of 1.0 ms for 1 min at 3-min intervals). ICP and MAP were measured continuously with use of a BL-420V pressure transducer system (AD instrument).
Western blot
Extraction of penile tissue and western blot assays were performed as described by Li et al. in 2020 (27). Penile tissue was carefully isolated and homogenized in radio immunoprecipitation assay (RIPA) lysis buffer. After determining the protein concentration with use of a BCA kit (Beyotime, Shanghai, China), the samples were diluted with 1× loading buffer, heated at 100 ℃ for 10 min for protein denaturation, and stored at −20 ℃. Proteins (30 µg) from tissue samples were loaded into each lane, electrophoretically separated on SDS-PAGE gels and then transferred onto PVDF membranes for overnight incubation at 4 ℃ with the following primary antibodies: PI3K (1:2,000; 4292S; Cell Signaling Technology, Danvers, MA, USA), AKT (1:5,000; 9272S; Cell Signaling Technology, Danvers, MA, USA), NF-κB (1:1,000; BS90940; Bioworld Technology, Nanjing, China), TGF-β1 (1:500; BS1361; Bioworld Technology), CTGF (1:500; BS7445; Bioworld Technology), α-SMA (1:1,000; BS70000; Bioworld Technology), mTOR (1:1,000; 2972S; Cell Signaling Technology, Danvers, MA, USA), P62 (1:1,000; 23214S; Cell Signaling Technology, Danvers, MA, USA), LC3I/II (1:1,000; 12741S; Cell Signaling Technology, Danvers, MA, USA) and GAPDH (1:5,000; 10494-1-AP; Proteintech, Wuhan, China). The secondary antibody was horseradish peroxidase-conjugated to mouse anti-rabbit/mouse IgG (1:5,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoblots were developed with use of enhanced chemiluminescence (ECL) and protein band densities, normalized to GAPDH, were quantified using Image J software (NIH, Scion Corporation, Frederick, MD, USA).
Confocal immunofluorescent assay
Frozen penile tissue serial coronal sections (30 µm) were incubated overnight at 4 ℃ with diluted primary antibodies including: TGF-β1 (1:200; BS1361; Bioworld Technology), CTGF (1:200; BS7445; Bioworld Technology), α-SMA (1:200; BS70000; Bioworld Technology) and CD63 (1:500, SC-5275, Santa Cruz Biotechnology) followed by the Alexa Fluor488-conjugated goat anti-rabbit IgG or Alexa Fluor594-conjugated goat anti-mouse IgG secondary antibody (all 1:200, Sigma-Aldrich). Images were captured with use of a LSM780 laser scanning confocal microscope (ZEISS, Germany). A minimum of 6 images were taken from each animal for analysis by Image-Pro plus 6.0 software.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from a 100 mg sample of cryopreserved penile tissue using the TRIzol kit and was reverse transcribed into cDNA using reverse transcriptase (GeneCopoeia, USA). GAPDH served as a loading control in each sample and targeted gene expression levels were evaluated using the 2−(ΔΔCt) method. The primers used are detailed in Table 1.
Table 1
| Gene | Forward (5'→3') | Reverse (5'→3') |
|---|---|---|
| IL-6 | CTCTCCGCAAGAGACTTCCA | TCTCCTCTCCGGACTTGTGAA |
| IL-1β | GGGATGATGACGACCTGC | CCACTTGTTGGCTTATGTT |
| IFN-γ | ATTCATGAGCATCGCCAAGTTC | TGACAGCTGGTGAATCACTCTGAT |
| TNF-α | TGATCGGTCCCAACAAGGA | TGCTTGGTGGTTTGCTACGA |
| GAPDH | AGTGCCAGCCTCGTCTCATA | GGTAACCAG GCG TCCGATAC |
PCR, polymerase chain reaction.
Masson’s trichrome staining
Slices of penile tissue (5 µm) were fixed in 4% paraformaldehyde overnight to assess the level of fibrosis as determined using the Masson’s Trichrome Staining Kit (D026-1-3; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions provided.
Statistical analysis
All data were expressed as means and standard error of the means (means ± SEMs). Group comparisons were performed using an analysis of variance. Two-way analysis of variance (ANOVA) was used for determinations of statistical significance. Tukey’s test was used to determine the subsequent pairwise differences. P<0.05 was required for results to be considered as statistically significant. The Statistical Product and Service Solutions (SPSS) 16.0 (SPSS, Chicago, IL, USA) software was used for these data analyses.
Results
NO2-OA improves basal metabolic parameters and erectile function
There were no statistically significant differences in initial body weights and blood glucose concentrations among the four groups. However, following the induction of diabetes, the three DMED groups all showed significantly lower body weights and significantly higher blood glucose concentrations as compared with the control group. Within the DMED + NO2-OA group there were some restorations of body weights and blood glucose levels (Figure 1B,1C) and significant increases in ICP and ICP/MAP (Figure 1D,1E) as compared with that of the DMED groups (DMED alone and DMED + Vehicle).
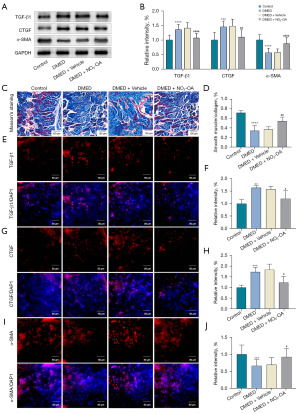
NO2-OA ameliorates fibrosis in penile tissue of DMED rats
Western blot results revealed that expressions of TGF-β1 and CTGF were significantly decreased in penile tissue after NO2-OA treatment, while that of α-SMA was increased. Such results suggest that an increase in smooth muscle tissue is present in response to this NO2-OA treatment (Figure 2A,2B). Masson trichrome staining was performed to assess the ratio of smooth muscle to collagen. A decreased smooth muscle/collagen ratio was observed in the vehicle-treated DMED and DMED group as compared with the control group, while 4 weeks of NO2-OA treatment significantly increased these ratios (Figure 2C,2D). Immunofluorescence assay results revealed that the expression levels of TGF-β1, CTGF and α-SMA were also significantly different among the four groups (Figure 2E-2J).
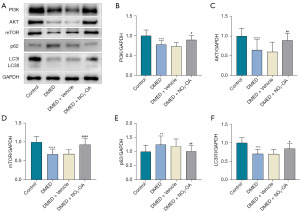
NO2-OA activates autophagy in penile cavernous tissue via the PI3K/AKT/mTOR pathway in DMED rats
As shown in Figure 3A, the PI3K/AKT/mTOR signaling pathway represents a key pathway for regulating autophagy and, in this study, we found that all three of these proteins were increased in the NO2-OA versus the DMED group (Figure 3B-3D). Elevated expressions of the autophagy marker, LC3, and decreased levels of the autophagy substrate, p62, were observed in the NO2-OA as compared with the DMED group (Figure 3E,3F). These results suggest that one of the means through which NO2-OA improves erectile function is through induction of autophagy via the PI3K/AKT/mTOR signaling pathway.
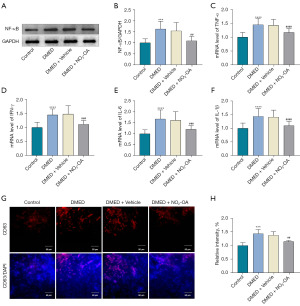
NO2-OA inhibits inflammation in penile cavernous tissue of DMED rats
It appears that NO2-OA treatment can effectively reduce inflammation in these DMED rats, as the expression of NF-κB (p65) was increased in the DMED versus control group, while NF-κB (p65) expression was decreased in the DMED + NO2-OA versus the DMED group (Figure 4A,4B). In addition, we found that mRNA expressions of several essential proinflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6, were all significantly increased in the DMED as compared to that observed in control group (Figure 4C-4F). However, the levels of IFN-γ, TNF-α, IL-1β and IL-6 were all decreased in the DMED + NO2-OA versus DMED group. Results from the confocal immunofluorescent assay indicated that the expression of CD63 was also increased in DMED rats, but decreased after NO2-OA treatment (Figure 4G,4H).
Discussion
Findings from a number of studies have indicated that fibrosis and autophagy play important roles in the pathogenesis of DMED (28-30). Our current results are in accordance with previous findings as we found that erectile function was decreased, penile tissue fibrosis was obvious and autophagy was diminished in DMED rats. While it has been reported that NO2-OA can exert a significant anti-inflammatory effect to inhibit ED (31), our current results provide novel evidence indicating that NO2-OA can also enhance autophagy. In this way, the significant improvement in erectile function of DMED rats treated with NO2-OA may be related to these anti-fibroses and enhanced autophagic effects of NO2-OA. Moreover, NO2-OA may restore the corpus cavernosum smooth muscle cell (SMC) and its structural integrity through inhibition of the TGF-β1/CTGF pathway.
Although oral PDE5 inhibitors can provide an effective and safe treatment of ED in non-diabetics, DMED patients often respond poorly to these drugs, mainly due to the chronic inflammation induced by long-term hyperglycemic stimulation, which then induces penile fibrosis and reduces penile diastolic function (32,33). Although in its initial stages fibrosis represents an adaptive response to unfavorable conditions such as tissue damage, its long-term consequences are irreversible and often more harmful than beneficial. Reductions in parenchymal cells are accompanied with an increase and loading of fibrous connective tissue, the direct result of which is a continuous decrease in extensibility and rigidity of the tissue or organ (34). Such processes will continue until the tissue or organ completely loses all functions. In diabetic rats, the expression of TGF-β1 and its downstream effectors, which regulate fibrosis, were significantly increased in penile tissue. TGF-β1 regulates fibrosis by activating receptor-associated Smad2 and Smad3, and there is evidence that TGF-β1 signaling promotes apoptosis and inhibits the regenerative capacity of penile SMCs (34). TGF-β1 may reduce penile elasticity and compliance by altering collagen types and increasing collagen synthesis. An additional pro-fibrotic molecule in tissues that is regulated by TGF-β1 is CTGF. Overexpression of CTGF induces fibroblast proliferation, migration and extracellular matrix overexpression as well as apoptosis in SMCs (18). In this study, α-SMA, which is the typical isoform found in SMCs, was used to assess smooth muscle content in the corpus cavernosum. Our results showed that, in DMED rats treated with NO2-OA, TGF-β1 and CTGF were significantly decreased, while α-SMA was increased.
Autophagy is a programmed process by which cells engulf and degrade cytoplasmic contents to replenish metabolic needs. It has been reported that enhancing autophagy can improve erectile function by alleviating the fibrotic process within the corpus cavernosum (35,36). Expression of the autophagic degradation-related protein, p62, increases when the degradation of downstream products of autophagy is blocked. The underlying mechanism for this effect is that ubiquitinated p62 binds to LC3-II and is degraded together in autophagolysosomes (37). LC3 represents the classic autophagy marker and, as a structural protein of autophagosome, exists mainly in the forms of LC3-I and LC3-II during the process of autophagy (38). In this study, the increased level of autophagy in the corpus cavernosum of the penis in DMED rats was increased in response to NO2-OA treatment. When considering the possible reasons for this effect, we speculate that the decreased autophagy in the penile corpus cavernosum of DMED rats may be due to the increase of damaging factors such as oxidative stress, inflammation and hyperglycemia. However, NO2-OA significantly improves the level of autophagy by inhibiting oxidative stress and inflammation. The P13K/AKT/mTOR pathway represents a classic signaling pathway involved with the ability to regulate the initiation of autophagy (39). Our current results demonstrated that the expressions of P13K, AKT and mTOR proteins and their corresponding mRNAs in DMED rats were all significantly decreased while NO2-OA treatment significantly increased these protein and mRNA expressions. In this way, the abnormal inhibition of the P13K/AKT/mTOR pathway in the corpus cavernosum of DMED rats was activated in response to NO2-OA. Finally, we have shown previously that the enhanced inflammatory response mediated by NF-κB represents an important pathogenic component of DMED (40). Here, we now report that NO2-OA reduced the expression of NF-κB and IL-6 as well as other important proinflammatory factors.
This study has some limitations. For example, we only studied the effects of a single dose. It is also necessary to study the effect of different doses of NO2 on DMED. In addition, we did not detect whether NO2-OA could improve the therapeutic effect of other conventional drugs on DMED. In our future research, we also need to improve this research.
Conclusions
In conclusion, in this study we demonstrated that NO2-OA can improve erectile function in DMED rats, effects which are related to activation of an over-inhibited P13K/AKT/mTOR pathway in the corpus cavernosum and through promotion of autophagy. In addition, there is an inhibition of TGF-β-related pathways with NO2-OA, which can then serve to diminish fibrosis. Taken together, these findings provide a foundation for the development of new treatments of DMED. This study provides a strong rational for future clinical studies of the therapeutic regime in DMED patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-547/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-547/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-547/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-547/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Procedures involving animal subjects were approved by the Animal Care and Use Committee of Liaocheng People’s Hospital (Liaocheng, China; Approval No. 2023046). This study involving rats was conducted in accordance with the Laboratory Animal Care and Use Guidelines of the National Institute of Health.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bajaj HS, Gerstein HC, Rao-Melacini P, et al. Erectile function in men with type 2 diabetes treated with dulaglutide: an exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinol 2021;9:484-90. [Crossref] [PubMed]
- Mazzilli F. Erectile Dysfunction: Causes, Diagnosis and Treatment: An Update. J Clin Med 2022;11:6429. [Crossref] [PubMed]
- Defeudis G, Mazzilli R, Tenuta M, et al. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab Res Rev 2022;38:e3494. [Crossref] [PubMed]
- Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med 2017;34:1185-92. [Crossref] [PubMed]
- Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med 2009;6:1232-47. [Crossref] [PubMed]
- El-Sakka AI. Pharmacotherapy for erectile dysfunction in diabetic males. Expert Opin Pharmacother 2018;19:1345-56. [Crossref] [PubMed]
- Zhang L, Bao B, Guo J, et al. Current status and prospects of diabetes mellitus induced erectile dysfunction: A bibliometric and visualization study. Front Endocrinol (Lausanne) 2023;14:1168744. [Crossref] [PubMed]
- Guven H, Durmus N, Hocaoglu N, et al. Protective effects of wheat germ oil against erectile and endothelial dysfunction in streptozotocin-induced diabetic rats. Int J Impot Res 2022;34:581-7. [Crossref] [PubMed]
- Wang JS, Feng JL, Li X, et al. Effect of leech-centipede medicine on improving erectile function in diabetes-induced erectile dysfunction rats via PDE5 signalling pathway-related molecules. Pharm Biol 2021;59:167-74. [Crossref] [PubMed]
- Gandhi J, Dagur G, Warren K, et al. The Role of Diabetes Mellitus in Sexual and Reproductive Health: An Overview of Pathogenesis, Evaluation, and Management. Curr Diabetes Rev 2017;13:573-81. [Crossref] [PubMed]
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014;65:968-78. [Crossref] [PubMed]
- Maiorino MI, Bellastella G, Giugliano D, et al. From inflammation to sexual dysfunctions: a journey through diabetes, obesity, and metabolic syndrome. J Endocrinol Invest 2018;41:1249-58. [Crossref] [PubMed]
- Liu K, Cui K, Feng H, et al. JTE-013 supplementation improves erectile dysfunction in rats with streptozotocin-induced type I diabetes through the inhibition of the rho-kinase pathway, fibrosis, and apoptosis. Andrology 2020;8:497-508. [Crossref] [PubMed]
- Castela Â, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol 2016;13:266-74. [Crossref] [PubMed]
- Sun T, Xu W, Wang J, et al. Paeonol ameliorates diabetic erectile dysfunction by inhibiting HMGB1/RAGE/NF-kB pathway. Andrology 2023;11:344-57. [Crossref] [PubMed]
- Landau D, Eshet R, Troib A, et al. Increased renal Akt/mTOR and MAPK signaling in type I diabetes in the absence of IGF type 1 receptor activation. Endocrine 2009;36:126-34. [Crossref] [PubMed]
- Ma TT, Meng XM. TGF-β/Smad and Renal Fibrosis. Adv Exp Med Biol 2019;1165:347-64. [Crossref] [PubMed]
- Qabazard B, Yousif M, Mousa A, et al. GYY4137 attenuates functional impairment of corpus cavernosum and reduces fibrosis in rats with STZ-induced diabetes by inhibiting the TGF-β1/Smad/CTGF pathway. Biomed Pharmacother 2021;138:111486. [Crossref] [PubMed]
- Cho MC, Song WH, Paick JS. Suppression of Cavernosal Fibrosis in a Rat Model. Sex Med Rev 2018;6:572-82. [Crossref] [PubMed]
- Zhang J, Li S, Li S, et al. Effect of icariside II and metformin on penile erectile function, glucose metabolism, reaction oxygen species, superoxide dismutase, and mitochondrial autophagy in type 2 diabetic rats with erectile dysfunction. Transl Androl Urol 2020;9:355-66. [Crossref] [PubMed]
- Zhou C, Su M, Sun P, et al. Nitro-oleic acid-mediated blood-brain barrier protection reduces ischemic brain injury. Exp Neurol 2021;346:113861. [Crossref] [PubMed]
- Braumann S, Schumacher W, Im NG, et al. Nitro-Oleic Acid (NO2-OA) Improves Systolic Function in Dilated Cardiomyopathy by Attenuating Myocardial Fibrosis. Int J Mol Sci 2021;22:9052. [Crossref] [PubMed]
- Arruebarrena Di Palma A, Di Fino LM, Salvatore SR, et al. Nitro-oleic acid triggers ROS production via NADPH oxidase activation in plants: A pharmacological approach. J Plant Physiol 2020;246-247:153128. [Crossref] [PubMed]
- Koudelka A, Cechova V, Rojas M, et al. Fatty acid nitroalkene reversal of established lung fibrosis. Redox Biol 2022;50:102226. [Crossref] [PubMed]
- Li X, Feng JL, Chen ZL, et al. Mechanism by which Huoxue Tongluo Qiwei Decoction improves the erectile function of rats with diabetic erectile dysfunction. J Ethnopharmacol 2022;283:114674. [Crossref] [PubMed]
- Jeong HC, Bae WJ, Zhu GQ, et al. Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes. Investig Clin Urol 2019;60:285-94. [Crossref] [PubMed]
- Li Y, Wang L, Wang P, et al. Ginsenoside-Rg1 Rescues Stress-Induced Depression-Like Behaviors via Suppression of Oxidative Stress and Neural Inflammation in Rats. Oxid Med Cell Longev 2020;2020:2325391. [Crossref] [PubMed]
- Song J, Sun T, Tang Z, et al. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol Med 2020;24:13289-302. [Crossref] [PubMed]
- Lin H, Wang T, Ruan Y, et al. Rapamycin Supplementation May Ameliorate Erectile Function in Rats With Streptozotocin-Induced Type 1 Diabetes by Inducing Autophagy and Inhibiting Apoptosis, Endothelial Dysfunction, and Corporal Fibrosis. J Sex Med 2018;15:1246-59. [Crossref] [PubMed]
- Zhang J, Li AM, Liu BX, et al. Effect of icarisid II on diabetic rats with erectile dysfunction and its potential mechanism via assessment of AGEs, autophagy, mTOR and the NO-cGMP pathway. Asian J Androl 2013;15:143-8. [Crossref] [PubMed]
- Schopfer FJ, Vitturi DA, Jorkasky DK, et al. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 2018;79:31-7. [Crossref] [PubMed]
- Kim S, Cho MC, Cho SY, et al. Novel Emerging Therapies for Erectile Dysfunction. World J Mens Health 2021;39:48-64. [Crossref] [PubMed]
- Langarizadeh MA, Salary A, Tavakoli MR, et al. An overview of the history, current strategies, and potential future treatment approaches in erectile dysfunction: a comprehensive review. Sex Med Rev 2023;11:253-67. [Crossref] [PubMed]
- Cui K, Tang Z, Li CC, et al. Lipoxin A4 improves erectile dysfunction in rats with type I diabetes by inhibiting oxidative stress and corporal fibrosis. Asian J Androl 2018;20:166-72. [Crossref] [PubMed]
- Yuan P, Ma D, Gao X, et al. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front Pharmacol 2020;11:1257. [Crossref] [PubMed]
- Wu CJ, Fu FD, Qin F, et al. Vacuum therapy ameliorates erectile dysfunction in bilateral cavernous nerve crush rats by inhibiting apoptosis and activating autophagy. Asian J Androl 2021;23:273-80. [Crossref] [PubMed]
- Jiang P, Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015;75:13-8. [Crossref] [PubMed]
- Matsuzawa-Ishimoto Y, Hwang S, Cadwell K. Autophagy and Inflammation. Annu Rev Immunol 2018;36:73-101. [Crossref] [PubMed]
- Li Z, Song Y, Hou W, et al. Atractylodin induces oxidative stress-mediated apoptosis and autophagy in human breast cancer MCF-7 cells through inhibition of the P13K/Akt/mTOR pathway. J Biochem Mol Toxicol 2022;36:e23081. [Crossref] [PubMed]
- Ma Z, Wang W, Pan C, et al. N-acetylcysteine improves diabetic associated erectile dysfunction in streptozotocin-induced diabetic mice by inhibiting oxidative stress. J Cell Mol Med 2022;26:3527-37. [Crossref] [PubMed]


